In Part One we took a look at what data was available for “back radiation”, better known as Downward Longwave Radiation, or DLR. And we saw that around many locations the typical DLR was in the order of 300 W/m2, and it didn’t decrease very much at night.
In Part Two we looked at several measured spectra of DLR which clearly demonstrated that this radiation is emitted by the atmosphere.
In this article we will consider what happens when this radiation reaches the ground. The reason we want to consider it is because so many people are confused about “back radiation” and have become convinced that either it doesn’t exist – covered in the previous two parts – or it can’t actually have any effect on the temperature of the earth’s surface.
The major reason that people give for thinking that DLR can’t affect the temperature is (a mistaken understanding of) the second law of thermodynamics, and they might say something like:
A colder atmosphere can’t heat a warmer surface
There are semantics which can confuse those less familiar with thermal radiation.
If we consider the specific terminology of heat we can all agree and say that heat flows from the warmer to the colder. In the case of radiation, this means that more is emitted by the hotter surface (and absorbed by the colder surface) than the reverse.
However, what many people have come to believe is that the colder surface can have no effect at all on the hotter surface. This is clearly wrong. And just to try and avoid upsetting the purists but without making the terminology too obscure I will say that the radiation from the colder surface can have an effect on the warmer surface and can change the temperature of the warmer surface.
Here is an example from a standard thermodynamics textbook:
Probably this diagram should be enough, but as the false ideas have become so entrenched let’s press on..
Note that this topic has been covered before in: Intelligent Materials and the Imaginary Second Law of Thermodynamics and The First Law of Thermodynamics Meets the Imaginary Second Law
The First Law of Thermodynamics
This law says that energy is conserved – it can’t be created or destroyed. What this means is that if a surface absorbs radiation it must have an effect on the temperature – compared with the situation where radiation was not absorbed.
There’s no alternative – energy can’t be absorbed and just disappear. However, as a technical note, energy can be absorbed into chemical bonds or phase changes of materials. So you can put heat into ice without changing the temperature, while the ice turns into water. Of course, energy is still not lost..
Therefore, if your current belief is that radiation from a colder atmosphere cannot “change the temperature” of the hotter surface then you have to believe that all of the radiation from the atmosphere is reflected.
Or, alternatively, you can believe that the first law of thermodynamics is flawed. Prove this and your flight to Sweden beckons..
Bouncers at the Door – or Quantum Mechanics and the Second Law of Thermodynamics
One commenter on an earlier post asked this question:
But if at the surface the temperature is higher than in the atmospheric source then might the molecules which might have absorbed such a photon be in fact unavailable because they have already moved to a higher energy configuration due to thermal collisions in the material which contains them?
Many people have some vague idea that this kind of approach is how the second law of thermodynamics works down at the molecular level.
It (the flawed theory) goes like this:
- the atmosphere emits “a photon”
- the photon reaches the surface of the earth
- because the temperature of the surface of the earth is higher the photon cannot be absorbed – therefore it gets “bounced”.
Except it’s not physics in any shape or form – it just sounds like it might be.
Let’s review a few basics. It’s important to grasp these basics because they will ensure that you can easily find the flaw in the many explanations of the imaginary second law of thermodynamics.
“They all look the same to me” – The Energy of a Photon
This part is very simple. The energy of a photon, E:
E = hν = hc/λ
where ν = frequency, λ = wavelength, c = speed of light, h = 6.6 ×10−34 J.s (Planck’s constant).
You can find this in any basic textbook and even in Wikipedia. So, for example, the energy of a 10μm photon = 2 x 10−20 J.
Notice that there is no dependence on the temperature of the source. Think of individual photons as anonymous – a 10μm photon from a 2,000K source has exactly the same energy as a 10μm photon from a 200K source.
No one can tell them apart.
Wavelength Dependence on the Temperature of the Source
Of course, radiation from different temperature sources do have significant differences – in aggregate. What most, or all, believers in the imaginary second law of thermodynamics haven’t appreciated is how similar different temperature Planck curves can be:
Notice the similarity between the 10°C and the -10°C radiation curves.
Alert readers who have pieced together these basics will already be able to see why the imaginary second law is not the real second law.
If a 0°C surface can absorb radiation from 10°C radiation, it must be able to absorb radiation from -10°C radiation. And yet this would violate the imaginary second law of thermodynamics.
What determines the ability of a surface to absorb or reflect radiation?
Absorptivity and Reflectivity of Surfaces
The reflectivity of a surface is a measurement of the fraction of incident radiation reflected. It’s very simple.
This material property has a wavelength dependence and (sometimes) a directional dependence. Here is a typical graph of a few materials:
As you can see, the variation of absorptivity/reflectivity with wavelength is very pronounced. Notice as well in this diagram from a standard textbook that there is no “source temperature” function. Of course, there can’t be, as we have already seen that the energy of a photon is only dependent on its wavelength.
Just to be clear – radiation incident on a surface (irradiation) can only be absorbed or reflected. (In the case of gases, or very thin surfaces, radiation can also be “transmitted” through to the other side of the material or gas).
Conclusion from Basic Physics
So from basic physics and basic material properties it should be clear that radiation from a colder surface cannot be all reflected while at the same time radiation from a warmer surface is absorbed.
And if any radiation is absorbed it must change the surface temperature and therefore violate the (imaginary) second law of thermodynamics.
You have to ditch something. I would recommend ditching the imaginary second law of thermodynamics. But you can choose – instead you could ditch the first law of thermodynamics, or the basic equation for the energy of a photon (make up your own), or invent some new surface properties.
While considering these choices, here’s another way to think about it..
If All the “Back Radiation” Was Reflected..
So let’s suppose you still think that all of the radiation from the atmosphere, all 300W/m2 of it, gets reflected.
That presents a problem even bigger than the tedious physics principles articulated above. Why is that?
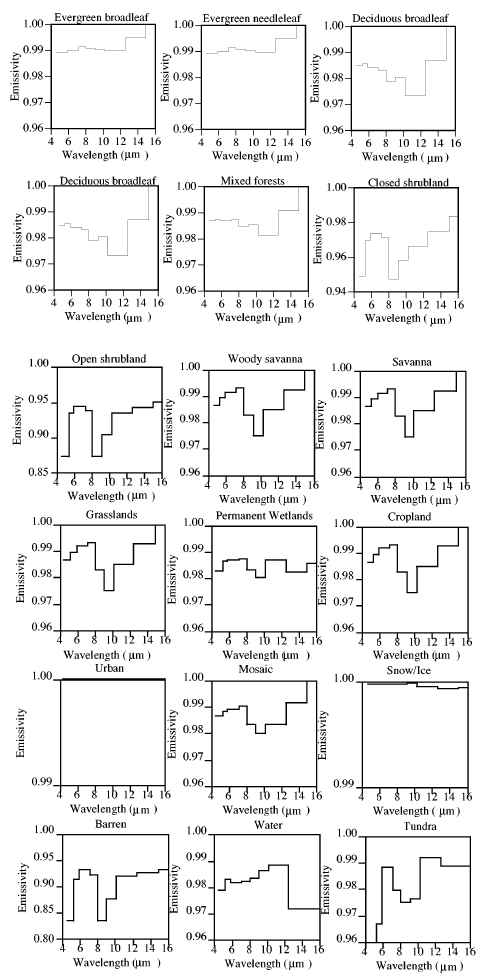
Well, let’s take the earth’s average surface temperature of around 15°C (288K) and the typical emissivity of various surface types:
As you can see, the emissivity is pretty close to 1. So for a temperature of 15°C the Planck curve will be pretty close to a blackbody, and the total surface emitted radiation (“flux”) is given by the Stefan-Boltzmann equation of σT4 – so in the typical surface case:
j = 390 W/m2
Now we have to add the reflected surface radiation of 300W/m2. So the upward radiation from the surface will be around 690 W/m2.
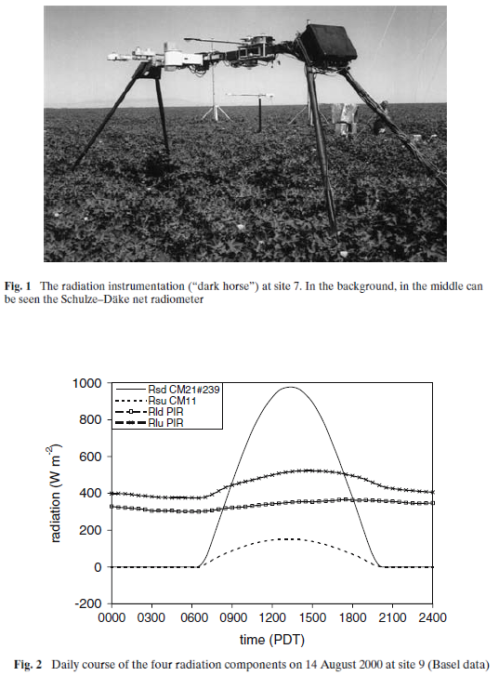
Here is one result from a very thorough experiment, The Energy Balance Experiment, EBEX 2000 (reference below):
The location was a cotton field of 800m × 1600m at coordinates 36°06’ N, 119°56’ W, approximately 20 km south-south-west of the town of Lemoore CA, USA. In this experiment, radiation measurements were taken at nine sites across the field along with wind and humidity measurements in an attempt to “close the energy budget” at the surface. Downward measurements were taken at a few of the sites (because the values wouldn’t change over a small distance) while upward measurements of both shortwave and longwave were taken at every site. Some sites measured the same value with two instruments from different manufacturers.
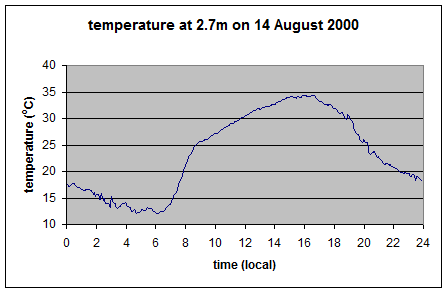
As you can see the upward longwave measurement is around 400-500 W/m2. The paper itself doesn’t record the temperature on that day, but typical August temperatures in that region peak above 35°C, leading to surface radiation values above 500 W/m2 – which is consistent with the measurements. [Update – the peak temperature measured at this location was 35°C on this day – thanks to Wim Kohsiek for providing this data along with the temperature graph for the day]
And so here it is the theoretical upward longwave radiation from the temperature graph (=σT4):
As you can see, this upward radiation calculation matches what was measured.
If the surface reflects all of the downward longwave radiation then the upward longwave measurement for these temperatures should be in the region of 700-800 W/m2.
There is a great opportunity for some enterprising people who still think that DLR is all reflected and not absorbed – buy a decent pyrgeometer and take some upward surface measurements to demonstrate that the whole science community is wrong and upward surface measurements really are 80% higher than everyone thinks. If you can afford an FT-IR to do a spectral analysis you will be able to prove your theory beyond a shadow of doubt – as the spectrum will have those characteristic CO2, O3 and water vapor peaks that were shown in DLR spectra in Part Two.
Conclusion
DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.
The physics behind this are very basic and have been known for around 100 years.
Proving that the surface doesn’t absorb DLR should be a walk in the park for anyone with a small amount of cash. But only if it’s true.
The world we live in does absorb DLR and adding 300W/m2 to the surface energy budget is the reason why the surface temperatures are like they are.
Further reading – Do Trenberth and Kiehl understand the First Law of Thermodynamics?
Darwinian Selection – “Back Radiation”
References
The Energy Balance Experiment EBEX-2000. Part III: Behaviour and quality of the radiation measurements, Kohsiek et al, Boundary Layer Meteorology (2007)