[Note: This article was significantly updated August 5th, 2010. Therefore, many comments became obsolete, or at least proved their worth, by encouraging an update]
If there’s one area that often seems to catch the imagination of many who call themselves “climate skeptics”, it’s the idea that CO2 at its low levels of concentration in the atmosphere can’t possibly cause the changes in temperature that have already occurred – and that are projected to occur in the future. Instead, the sun, that big bright hot thing in the sky (unless you live in England), is identified as the most likely cause of temperature changes.
Argument from Inconceivability
I personally find it hard to believe that we are hurtling through space at 67,000 miles per hour on a big spinning rock. It doesn’t feel like it. (Actually that’s just the speed that we orbit the sun, and the sun is moving as well, so its more complicated..)
And is this table (you can’t see my table, but any table will do) really made of tiny atoms but science claims it’s mostly space between the little balls? What? Not likely.
Satire over.
For science, personal experience and imagination are not the deciding factors. They lead you astray. Instead, investigation of phenomena lead to hypotheses, experiments and eventually “theories” – as well-established science “facts” are known. Your intuition might be great for understanding people’s motivations, or whether a person can run 100m in 3 seconds, but not so great for the energy absorption characteristics of invisible molecules.
Let’s look at the science.
How do we analyze the Earth’s Climate?
It’s a tricky problem. And like most tricky science problems we start with some simplications. We analyze a simplified model and see where that gets us. Like, how does this simple model compare to reality? And how do we verify the results from the simplified model if the reality is so much more complicated?
Read on, it’s a journey.
Energy from the Sun
The sun is our source of heat. We are 150 million km from the sun, so how does that heat energy get here?
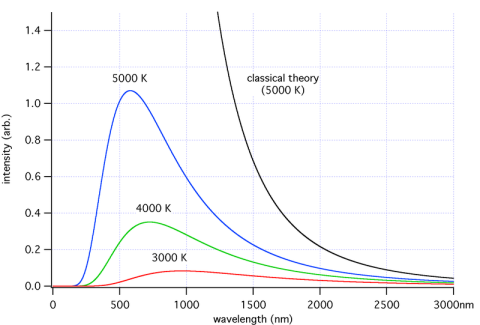
There are 3 mechanisms for heat transfer – conduction, convection and radiation. It’s a vacuum between the sun and the earth so energy from the sun can only arrive here through radiation. What does that radiation look like? A “body” emits radiation across a spread (a “spectrum”) of wavelengths, in a way that depends on that body’s temperature.
The fact that the wavelengths of the energy emitted vary with temperature is a key point, essential for understanding this aspect of climate science.
Here’s a few samples – each color represents a different temperature object. The blue line is a body at 5000K = 4727°C (8540°F).
For those new to the subject, K (“Kelvin”) is absolute temperature. It tracks degree Centigrade/Celsius one for one, but whereas °C starts at the freezing point of water, K starts at, well, absolute zero.
So 0°C = 32°F = 273K; and -273°C = -459°F = 0K
There are reasons why this temperature scale exists, but let’s just leave it at that.
So if you look at the graph you can see that the higher the temperature, the higher the total energy (which everyone would expect) and the lower the wavelengths of the peak energy.
At the end of the post I’ll show some maths, but many people don’t want to see any equations. Just as a preview, total energy is proportional to the 4th power of absolute temperature. Double the temperature and the energy goes up by 16 times.
And it’s worth stating as well at this point, none of this is in question. It’s reproduceable, non-controversial thermodynamics – a branch of physics. You can reproduce it in the lab and measure it everywhere in the real world.
Energy from the Earth
Now the earth also emits radiation according to the same formula. If the earth isn’t heating up or cooling down the energy absorbed will be equal to the energy emitted. (And we’ll leave discussions about how we know whether the earth is heating up and exactly what that means for another day).
If you do the maths (see the end of the post), you find that the equations say that the earth should be about -18°C (255K) when in fact it is an average +15°C across the globe. What’s going on?
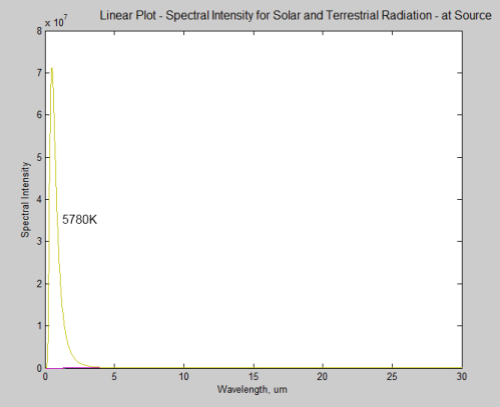
First let’s look at the energy from the earth and sun on the same graph. The sun has a surface temperature of 5780K:
What’s happened to the earth’s radiation? It can barely be seen on a linear plot, it is so small in comparison. However, this is at source – picture a spaceship parked just off the surface of the sun taking the measurements.
By the time the solar radiation has reached the earth it has reduced in intensity by a factor of around 46,000 (see the Inverse Square Law or The Sun and Max Planck Agree – Part Two):
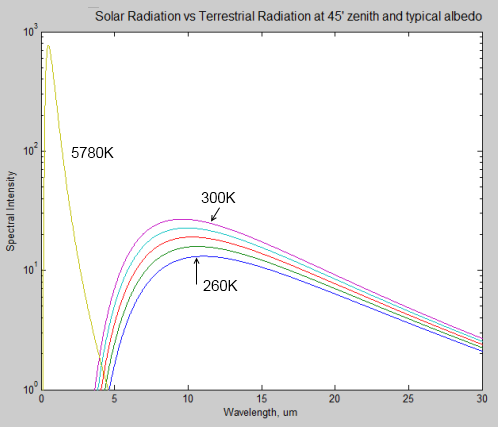
Here is a comparison of solar radiation (the 5780K curve) at the top of the atmosphere, compared with a few terrestrial radiation curves for 260K (-13°C) though to 300K (27°C). Note that it is a logarithmic plot.
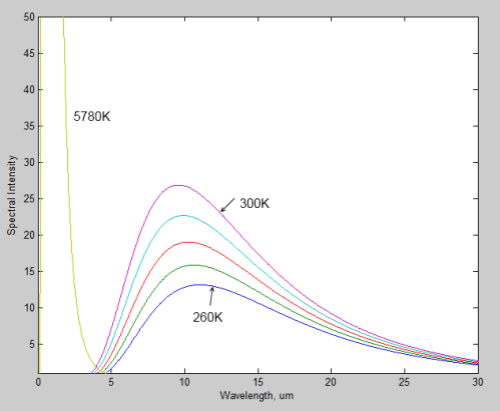
Here is a linear plot of the same for comparison:
Notice how the wavelength of the peak value of radiation shifts to the right as the source of the radiation gets colder. The typical value for the earth is 10μm, while for the sun it is 0.5μm.
What’s great about the graph is you can see clearly how the radiation from the sun can be easily discriminated from the radiation from the earth. There’s no complicated deductive work, if you measure radiation below 4μm, you know it came from the sun, no matter how many things it bounced off in the meantime. If you measure radiation above 4μm, you know it’s generated by the terrestrial system.
Check out The Sun and Max Planck Agree and The Sun and Max Planck Agree – Part Two for more on this subject.
What does this mean? It means that we can confident of the amount of energy:
- Arriving from the sun at the top of atmosphere and at the surface
- From the sun that is reflected back into space by the atmosphere or the earth’s surface
- Emitted by the earth
How do we work out 3)? We have satellites in space that look at the energy coming from the earth’s surface in the longer wavelengths that correspond to the lower temperatures of the earth’s surface.
And the climate science convention is to call the energy less than 4μm: short wave radiation and the energy greater than 4μm: long wave radiation.
Note that “infrared” is radiation greater than 0.7μm – a different term than “longwave”.
Energy Absorbed by Gases in the Atmosphere
Let’s look at some more standard science.
Each gas in the atmosphere has different absorption characteristics, which vary according to the wavelength of the radiation. In detail it is very complex, but here is a broad overview of total absorption:
Note that the horizontal axis is a logarithmic scale. The vertical axis shows “opacity” or what proportion of the energy is absorbed by the atmosphere. I picked this graph because you can easily see where the visible light fits in. What you should notice is how much radiation is actually absorbed by the atmosphere. This graphic is a bit too simplistic.
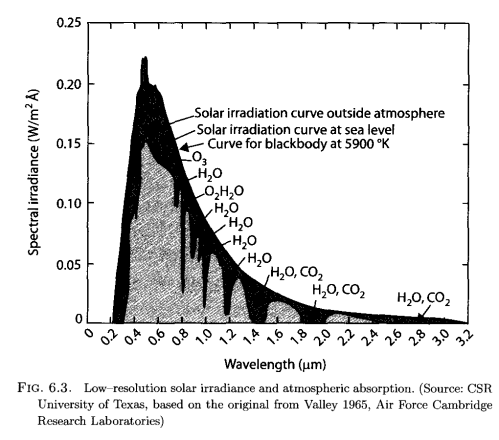
Here’s solar radiation at the top of atmosphere, and at the surface:
The lighter color is what we observe at the earth’s surface, while the darker surrounding is the observation of solar radiation by satellite. The difference is absorption by various molecules in the atmosphere and you can see from the annotation which gases absorb at which wavelength.
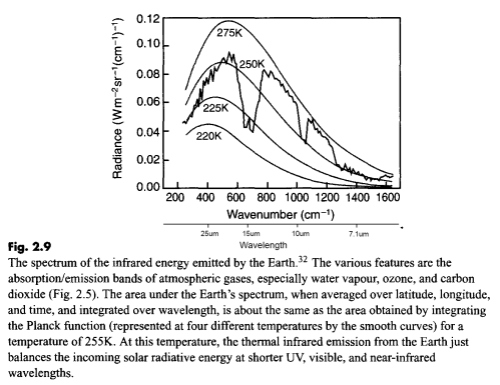
Here is a measurement of outgoing longwave radiation (terrestrial radiation) measured by satellite at the top of the atmosphere:
For those new to this kind of graph, they are usually shown in “wavenumber” rather than wavelength. It’s not important at this stage except to note that the longer wavelengths are to the left and the shorter wavelengths are to the right.
The reason for picking this measurement to show is that the emission curves for typical temperatures of the earth’s surface are shown overlaid. The highest one is 275K or 2°C. The surface of the earth emits radiation very close to the blackbody shape (see The Dull Case of Emissivity and Average Temperatures) but by the time the radiation leaves the earth’s atmosphere that isn’t what we see.
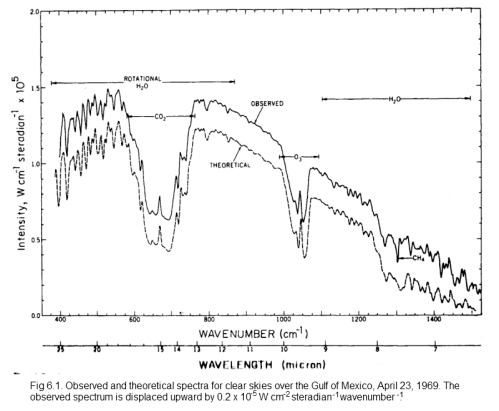
Here is another example, this time with a theoretical calculation (overlaid and displaced for comparison) which is something covered much later in this series:
Click for a larger view
On this spectrum, the authors have noted the reduced areas of outgoing radiation and marked CO2, H2O, O3 (ozone) and CH4 (methane).
How do they know these gases are the cause?
And what effect does it really have?
Measurements in the Lab
Scientists have been measuring the absorption characteristics of each gas in the atmosphere at different wavelengths for many decades.
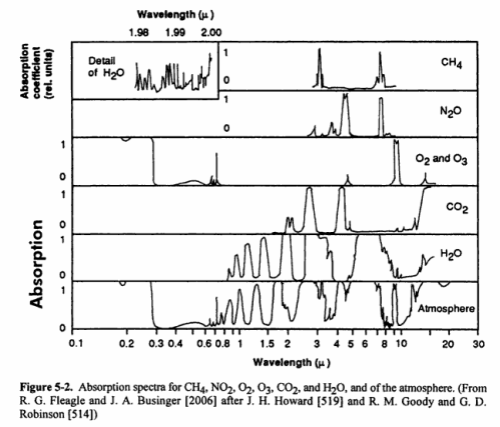
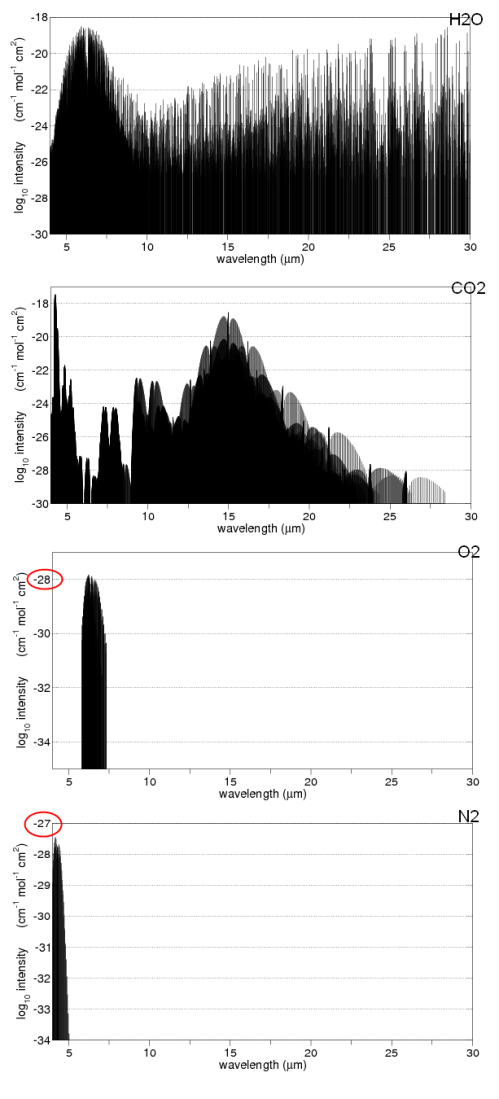
Here is a good summary of the main absorption bands:
The last bottom line shows the total in the atmosphere. You might notice that N2 (nitrogen) doesn’t show up. Is climate science ignoring this important gas? No – nitrogen absorbs almost nothing, for reasons that are touched on in Part Two. We can say that nitrogen is transparent to solar and terrestrial radiation.
You will also notice that O2 and O3 (oxygen and ozone) are shown. There is a chemical cycle in the upper atmosphere called the Chapman cycle which is responsible for generating ozone. In the very short wavelengths – below 0.3μm – oxygen and ozone both absorb solar radiation. In the longer wavelengths, ozone absorbs around 9.6μm. Oxygen doesn’t absorb at all in longwave – it is also (like nitrogen) transparent to terrestrial radiation.
What you can’t tell from the chart above is how influential each of the gases is in terms of total energy absorbed. That is a much more complex challenge – covered in later articles (but it isn’t as simple as the ratio of each of the absorbing gases in the atmosphere).
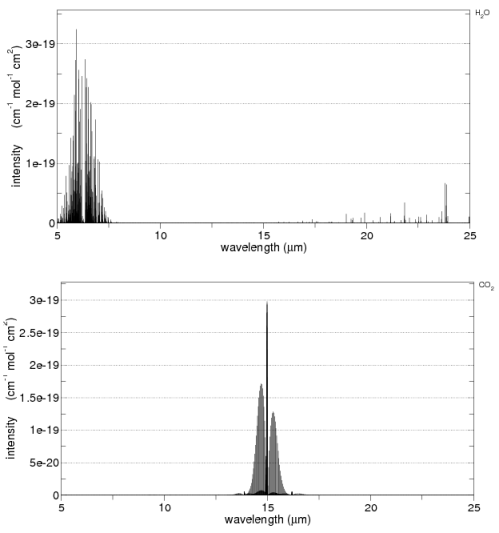
Before we leave the subject of absorption, it’s worth showing some lab measurements – from the HITRANS database. This might help see the main characteristics of CO2 and water vapor as well as the complexity.
First the main characteristics on a linear graph:
You can see that CO2 has high absorption around 15μm and water vapor around 6.3μm.
Now on a log plot – this shows the complexity – but note that each horizontal line represents a factor of 100. O2 and N2 are included at the bottom for comparison:
Note the vertical scale for N2 and O2 – even at their peak they absorb less than a billionth the radiation of CO2 and water vapor.
What Effect Does it Have?
Outside the world of atmospheric physics there is a lot of confusion about some thermodynamics basics. There are many articles on this blog that address those specific points (checkout the Roadmap) and there is no way to cover all of the misconceptions in this article – without it being 100 pages long..
As the surface of the earth heats up from the solar radiation absorbed, it in turn emits radiation – as shown in the 3rd and 4th graphs above.
If the atmosphere didn’t absorb any of this radiation then we would measure a spectrum like one of the Planck curves (as they are known). Instead we see large “chunks” (to use a non-technical term) of energy removed by the time the radiation leaves the atmosphere – “chunks” corresponding to water vapor, CO2 and ozone (as well as a number of other gases). And the larger the “chunk”, the more energy has been absorbed by the corresponding gas from the radiation.
When the atmosphere absorbs radiation it heats up. The absorbed energy is shared thermally via collisions with all other gas molecules, so the whole atmosphere in that region heats up. And the gases like CO2 and water vapor emit radiation – more emission as they increase in temperature.
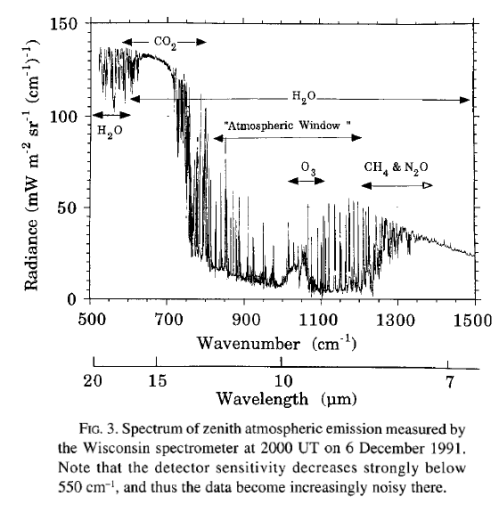
The atmosphere, once heated up, radiates equally in all directions. Some of this is downward. Here is a measured spectrum at the earth’s surface:
As you can see, the emission of radiation measured at the earth’s surface corresponds to the missing sections at the top of the atmosphere. See The Amazing Case of “Back-Radiation” and The Amazing Case of “Back Radiation” – Part Two.
Note: If a gas can absorb 15μm radiation it can also emit 15μm radiation. If a gas can’t absorb 15μm radiation it also can’t emit at that wavelength.
The energy radiated by the atmosphere which is received at the earth’s surface increases the temperature at the surface. (See The Amazing Case of “Back Radiation” – Part Three).
Although many people have become confused with imaginary second laws of thermodynamics to believe that this can’t happen, here is the easy way to understand the problem:
If we average the incoming solar radiation that is absorbed by the earth’s climate over the surface of the earth we get around 239 W/m2. (See The Earth’s Energy Budget – Part One).
If we average the outgoing longwave radiation from the top of atmosphere we get the same value: 239 W/m2.
If the atmosphere didn’t absorb any terrestrial radiation then the surface of the earth must also be emitting 239 W/m2.
The only way that the surface of the earth could emit this amount is if the temperature of the earth was around 255K or -18°C.
And yet we measure an average surface temperature of around 15°C – an emission of radiation of 396 W/m2. (See note 1).
If the atmosphere wasn’t absorbing and re-radiating longwave then the surface of the earth would be -18°C. This is the inappropriately-named “greenhouse” effect (and note that I haven’t used a greenhouse to demonstrate anything).
Conclusion
The question asked at the start was “Is CO2 an insignificant trace gas?” and the answer is no.
CO2 and water vapor are very significant in the earth’s climate, otherwise it would be a very cold place.
What else can we conclude? Nothing really, this is just the starting point. It’s not a sophisticated model of the earth’s climate, it’s a “zero dimensional model” or “billiard ball model” which takes a very basic viewpoint and tries to establish the effect of the sun and the atmosphere on surface temperature. It doesn’t look at feedback and it’s very simplistic.
Climate is a complex subject. Hopefully this explains some basics and we can start looking a little deeper in subsequent posts.
More in this series
Part Two – why different gases absorb different amounts of energy, why some gases absorb almost no longwave radiation
Part Three – the Beer Lambert model of absorption and the concept of re-emission of radiation
Part Four – band models and how transmittance of CO2 changes as the amount of CO2 increases under “weak” and “strong” conditions
Part Five – two results from solving the 1-d equations – and how CO2 compares to water vapor
Part Six – Visualization -what does the downwards longwave radiation look like at the earth’s surface
Part Seven – The Boring Numbers – the values of “radiative forcing” from CO2 for current levels and doubling of CO2.
Part Eight – Saturation – explaining “saturation” in more detail
CO2 Can’t have that Effect Because.. – common “problems” or responses to the theory and evidence presented
Other later series covering similar material
Visualizing Atmospheric Radiation – a lot more detail on how radiation travels through the atmosphere, and how it is absorbed and re-emitted by various “greenhouse” gases
Atmospheric Radiation and the “Greenhouse” Effect
The Maths
The Stefan-Boltzmann Law states:
j = εσT4
Where
j = total energy radiated per unit area per unit time
ε = emissivity, ranging from 0 to 1, where 1 is a perfect black body
σ = the Stefan Boltzmann constant, 5.67 x 10-8
T = temperature in K
The effective temperature of the sun is 5780K, its emissivity is quite close to 1, and so it radiates 6.3 x 107 W/m2
As the sun is a long way from the earth, its radiation by the time it reaches the earth is reduced according to Inverse Square Law.
The radius of the sun, rsun = 696 x 106m
Distance from the sun to earth, ao = 1.5×1011 m (150 million km)
Therefore the solar radiation is reduced by a factor of (1.5×1011/(696 x 106)2 = (215)2 = 46,225. Therefore, the solar radiation reaching the earth’s atmosphere = 6.3 x 107 / 46,225 = 1360 W/m2.
And from measurement by satellite we get 1367 W/m2.
Now we have to note two important facts:
- Some of the solar radiation is reflected
- The sun isn’t directly above all points on the earth at the same time
So how much energy is actually absorbed by the climate system?
The measured proportion of reflected solar radiation is 30% – we call this the albedo.
To work out the effect of the day and night and different angles of solar radiation sounds tricky but it’s actually an easy problem. The solar radiation from a long way away hits a disc of area = πr². But the surface of a sphere is 4πr² – (see The Earth’s Energy Budget – Part One for a fuller explanation). Therefore, to calculate the energy absorbed by the climate system averaged over the surface of the earth we can just divide by 4:
Esolar = 1367 x (1 – 0.3) / 4 = 239 W/m²
If the earth is not heating up or cooling down then the earth’s climate system must also be emitting radiation at the same rate. Note that these are global annual averages.
If there was no absorption of surface radiation by the atmosphere then the surface radiation would also be – on average – 239 W/m².
What temperature of the earth’s surface does this correspond to?
Remember the equation at the start of the maths section: j = εσT4
Rearranging to solve, T = (j/εσ)1/4
The emissivity of the earth is very close to 1 (see The Dull Case of Emissivity and Average Temperatures), therefore:
T = 255K or -18°C
Given that we actually experience much higher temperatures on the surface of the earth, we need an explanation. This can be found in the inappropriately-named “greenhouse gases”, which include water vapor, CO2 and methane (CH4).
When the earth emits its longwave radiation, these gases absorb energy and then re-emit, so that the earth’s energy doesn’t just fly off into space but instead it’s absorbed and re-transmitted, some of it back down to earth.
The “greenhouse gases” heat the earth’s surface up approximately 33°C higher than it would be otherwise.
Note 1:
There is a lot of confusion about the use of average temperatures in this approach to explaining the role of CO2 and water vapor in the atmosphere.
Calculating an average temperature has a lot of issues, as explained in Why Global Mean Surface Temperature Should be Relegated, Or Mostly Ignored. However, the explanation above doesn’t rely in any way on the arbitrary construct of average temperatures.
I simply used average temperatures to help newcomers visualize the issue more clearly. If I say that the earth’s average temperature should be -18°C everyone knows that I am wrong. If I say that the emission of surface radiation should be 239 W/m² who would know?
The use of energy per m² also confuses – the poles are colder, the equator is hotter – maybe the averages have lost something important. Once again, the averages just make it easier to understand. However, for those readers convinced that there is a problem in comparing average values, we can calculate the total energy:
Total solar energy absorbed globally annually = solar “constant” x (1 – albedo) x surface area the solar radiation irradiates x number of seconds in a year
Total energy = 1367 W/m² . (1 – 0.3) . πre² . 365.24.3600 = 3.8 x 1024 J
where re = radius of the earth = 6.37 x 106m.
How much energy does the surface of the earth radiate? Well, it can be calculated from the global temperature database and the Stefan-Boltzmann law.
This was done in Earth’s global energy budget, Trenberth and Kiehl, Bull. Amer. Meteor. Soc. (2009). They expressed the number as an global annual average – 396 W/m². We can simply multiply it back up the same way – using the surface area of the earth – to get
Total energy radiated from the surface = 396 x 4πre² . 365.24.3600 = 6.4 x 1024 J
Now there are no averages and no temperatures involved, but the same fundamental issue – the incoming and outgoing radiation balance at the top of the atmosphere, but the energy leaving from the surface of the earth is much higher than the incoming solar energy.
The absorption and re-radiation by “greenhouse” gases in the atmosphere is responsible.



As a physicist/geophysicist who has worked for the U. S. Naval Oceanographic Office and the U. S. Geological Survey for more than 30 years, and who has spent the last 8 years examining the Global Warming phenomenon (the first two of which were at the U. S. National Oceanic and Atmospheric Administration), I have come to two conclusions.
First Global Warming is real. Second, CO2 regardless of its origin (i.e., either natural or anthropogenic) does not drive Global Warming.
The CO2 Enhanced Greenhouse Effect Theory is totally irrelevant to the Global Warming phenomenon. Why? One finds on the secular time scale that both of the X- and Y- component temporal, annual-means profiles of the Earth’s Orientation mimic exactly the Global Temperature Anomaly (GTA) annual means profile On the decade time scale one finds that the GTA mimics the Geomagnetic Dipole variations and the variations in the Earths Anomalous Rotation Rate [i.e., Excess Length of Day (ELOD) Annual Means]. The Dipole Field, the GTA and the ELOD all have a 60 year period on the decade time scale. There are many other such correlations on both time scales.
Thus, if CO2 were driving the GTA, and given the geophysical parameters that change over time in sync with the GTA, CO2 enhancements would reasonably have to drive the Earth’s dynamo which creates the Dipole Field and somehow also affects the Earth’s orientation and its rotation rate. But CO2 cannot do this because it has no pondermotive force associated with it. Furthermore, CO2 on the decade time scale lags the GTA by about 9 years according to Mauna Loa, HI Observatory data collected since 1955, which is a period of time that is at the height of anthropogenic activity. Furthermore, on the millennium time scale the time lag averages about 800 years (Monin et. al., 2001). Therefore, if CO2 were the driver of Global Warming through the Enhanced Greenhouse Effect, then it would have to violate the Principle of Cause and Effect.
I have a short paperback book that explains this in more detail. It should be available in the book stores (e.g., Barnes and Nobel, Amazon.com, etc.) in late December 2009, or January 2010. Its title is:
GLOBAL WARMING: Geophysical Counterpoints to the Enhanced Greenhouse Theory
Publisher: Dorrance Publishing Co., Inc., Pittsburgh, PA, USA
ISBN: 978-1-4349-0581-9
While I do not know what precisely (though I know a little) causes Global Warming, I do know what does not cause it. CO2 and other greenhouse gases, anthropogenic or otherwise, are merely passive players that, like the GTA, are driven by other more dynamic forces associated with Earth’s core, the Sun, and even the Cosmos (referring to the Danish theory of cloud formation), all of which act, react, and interact in a very complex manner.
Note that the IPCC concentrates on Solar Irradiance, but ignores other solar energies such as that associated with Solar Magnetic Flux that has more than doubled since 1900. Gravity is another player in the Global Warming picture. Also note that Mars has global warming comparable to Earth’s without CO2 (Fenton, et. al., Nature, 2008). There are no Martians to either generate or enhance CO2 on Mars.
John M. Quinn
Lakewood, CO
USA
Mr. Quinn
I must get a copy of your book. Is the Chandler period part of what you are calling rotation and orientation? I firmly believe all this heating is caused by the sun (ha ha) with a variety of modulating functions that are not yet well understood. Of course CO2 and H2O are very important but our scientific understanding of the process must trump our beliefs. That is where I would like to see this discussion lead us. Thanks.
Bernie McCune
Hello John,
I am surprised your comments have been completely ignored (excluding Bernie’s request for book info) on this blog.
I commend you for speaking up, and I challenge science of doom to respond.
justcherrypicked:
Without buying the book I don’t know anything about this new theory.
And while John Quinn has posted a comment saying that CO2 has no effect on climate, what is really needed is for him – and everyone else interested – to deal with specific points in this seven part series. Then there’s something to “get my teeth into”.
I agree that CO2 does not cause global warming.
I have several major problems with this post.
First is the claim that the Sun is responsible for the temperature (heat) in the Earth.
1.Yes the solar radiation contributes, BUT also gravity and the transfer of energy by gravitons is the major source of energy to the Earth. The Sun’s gravity is responsible for the torque that spins the Earth to get its rotation. Friction of the various layers then results in heat.
Variations in the forces of gravity contribute to variations in the amount of energy coming into the Earth. Obviously the Earth’s eccentricity relativel to the sun (from .98AU to 1.02 AU) contibutes variation in both gravity and solar insolation.
There is also a 60 year cyclical variation in gravity from the Jupiter & Saturn resonance orbit, that is responsible for the Earth’s eccentricity that varies this energy input significantly. (see the http://www.scribd.com paper “Gravity Causes Climate Change” for further explanation.)
2. The equilibrium energy balance, says that at equilibrium the energy in equals the energy out. It does NOT say that the radiated energy in equals the radiated energy out. To be correct the energy balance MUST include the radiated E/M energy (ie solar insolation) plus gravity plus radiated magnetic fields etc . The measured 239W/m^2 is an incomplete measurement.
3. Finally the argument for more CO2 causing more warming is in itself self defeating in that it does not account for cooling. Whenever the temperature reduces and the temperature goes down, daily seasonally, yearly or in 30 year cycles, we find that the amount of CO2 has continued to go up (at least for the last 100 years.) The theory that CO2 causes the temperature variations FAILS.
Thank you, Mr. Quinn, for these writings regarding the ‘mechanics’ of atmospheric phenomena. I am going through them a second time as they are quite involved and my calculus is very rusty. I was wondering if you have furthered your quest to identify the causal agent with respect to the observed Global Warming. It appears that ‘politics’ has skewed the research into this very important event. So much so, that the necessary research is being devoted to furthering the CO2 narrative at the expense of identifying the true cause/s. If we dismantle our economy to reduce CO2 emissions only to realize later that net CO2 elimination does not achieve the Global Cooling that was promised. Also, thank you for your service!
Jay Coalson
Arvada, CO
USA
Nobody is promising cooling. Nobody. What is stated is that if CO2 and other greenhouse gas emissions are reduced, then the temperature won’t go up as much. It will still go up. That’s already baked in the cake.
And if you believe that Quinn is correct, I have a bridge in NYC for sale.
I am not a scientist, but the graph shown above labelled ‘From The Oceans and Climate, Grant Bigg, 2003’ has been the one most helpful piece of information in understanding the background to the debate on AGW. To understand better the debate itself I would like to know how the graph of observed radiation has changed since then.
Jeremy R Monson
( part time telephonist )
London
UK
That’s a great question, something I hope to cover in a later post on this subject, maybe part two or part three, after I get on top of the available data.
Firstly let me say what a great article this was especially the maths section. Ever since I first took an interest in global warming (probably around the time “An Inconvenient Truth” appeared) I have struggled to understand firstly what the core of the argument is and secondly to find science to support it. At this point let me also say I have a PhD in Physical Chemistry and have worked in R&D for the last 25 years. Once I had taken the time to cut through all the hysteria, hype, personal attacks, disinformation, claim and counter-claims I finally worked out that the heart of the matter is extremely simple. Does increasing the CO2 content of the atmosphere from todays levels (about 380 ppm) to higher levels cause the atmosphere to heat up to the degree predicted by The AGW community? If so this should be relatively easy to calculate. However due to demands of work, family, running a household etc. I never found time to go back to the basics and try and work it out for myself. Your explanation has saved me the trouble of digging out those old physics and chemistry texts on spectroscopy and radiation!
What I would like to see posted would be an extension of your maths section to show the methods used to calculate the effect of changing the concentration of CO2 in a model system. For the sake of simplicity lets use a mixture of N2 and O2 in the ratio it is in the atmosphere today and then add CO2 upto say 1 % vol (10,000 ppm) and plot the result out on a graph (increased absorption on the Y axis versus ppm CO2 on the X. This is one piece of data I have never seen anywhere despite hours of searching.
On another point I think the whole debate gives us a fascinating insight into the pyschology of the modern mind. Global Warming has clearly become a new religion complete with its high priests, its believe systems and its heretics.
The only way science can make progress is by constantly trying to prove a hypothesis is false. This is “The Scientific Method” pure and simple! To label people who don’t buy into the AGW hypothesis as a 100 % proven certainty as “Deniers” is dispicable and frankly extremely frightening. As such I’m proud to be in the “Deniers” camp and will be happy to change my mind when I see convincing data.
A question:
O2 and 03 make up 20% (versus .038% for C02) of the atmosphere, and has an absorption in the roughly 10um range (infrared band). Why is it not considered a GHG and one with a much larger influence than C02? It seems to me given the straight forward math, that it would have a far greater affect on GTA than CO2 – by 3 orders of magnitude.
kevoka: Different molecules absorb energy in different proportions to their concentration in the atmosphere. They also absorb at different wavelengths. But it’s a good question and one I will delve into in one of the next posts in this series.
Truth Hunter: Thanks for the kind words. I agree that it would be good to work through the ideas you describe especially the painful maths, so keep an eye out – subscribe on RSS or by email to the blog and you will see future posts.
As a quick note, the IPCC point of view is that doubling CO2 to 560ppm will cause around 1’C rise in temperature – for a first order forcing. The projections of much higher temperatures are due to computer models showing positive feedback. I expect to find the 1’C to be reasonable, although I haven’t worked through the maths myself.
kevoka:
Looking at this again, there is something I don’t understand. The chart of absorption shows “O2+O3” in the caption and yet O2 in the atmosphere is not a greenhouse gas. O3 (ozone) definitely absorbs in the ultraviolet section of the shortwave (solar) range and also at 9.6um in the longwave (terrestrial) range. However, the proportion of ozone in the lower atmosphere is very low – generally around 20 parts per billion (although occasionally much higher in big cities).
I believe that the caption “O2+O3” refers to the cycle of ozone creation from oxygen and back to oxygen (Chapman reaction).
I see that the same caption “O2 + O3” occurs in other graphics of spectral absorption.
In looking at the absorption spectrum, it appears that CO2 is responsible for the huge dip between 10 and 20 (microns?). If that is the case, how can increasing the amount in ppm of CO2 in the atmosphere make a significant change in the earth’s longwave radiation to space? I mean, whether a door is 1 inch thick or 1 foot — I’m still not getthing through it.
Tim
Tim, you are correct that CO2 is responsible for the 15um and above absorption.
There are 2 parts to absorption.
Firstly, the radiation in that wavelength is absorbed according to an e^-kz relationship.
(e to the power -kz), where z is height, and k is a constant for that gas for a given concentration and wavelength. This “constant”, k, doubles in value if the concentration doubles. This is the Beer-Lambert law.
Secondly, if a band is totally saturated increasing concentration won’t make any difference – your point is correct. But for CO2, as with other gases, the broad absorption spectrum is made up of many individual absorption lines. In the case of CO2, all bands are not completely saturated. The relationship then is more like a square root relationship – ie as the concentration goes up 4 times, the absorption through a given thickness only doubles.
The next post in the series will look at this in more detail.
Scienceofdoom,
Yes the graph has both O2 and O3. Which is what piqued my curiosity. O2 has absorption in the IR spectrum centered around 6.67um and then again starting at around 30um and beyond.
This comes from the HITRAN DB. A view of which can be found here:
http://www.atm.ox.ac.uk/group/mipas/atlas/index.html
You will see that O2 has at least as strong of absorption as N2O and CH4 – albeit in fewer bands. However, HITRAN does rank it as the 7th most absorptive molecule in the IR spectrum.
But I have not been able to find any calculations that treat it as the 7th most absorptive molecule in the atmosphere. I would think that if someone performed a relative forcing calculation for C2F6 (PFC-116) at 2.9 PPT – see IPCC AR4 ch.2 – then someone ought to have calculated RF for O2 at 20% of the atmosphere.
Yes it will be small. But is it more than C2F6 (PFC-116) with an RF of 0.0008 ?
Kevoka:
I checked a number of texts and it seems to be convention to represent O2+O3 as one group, for reasons that are probably tradition.
Nearly all of O2’s absorption is in shortwave – the UV absorption – where the high energy shortwave radiation separates O2 into O+O, then creation of O3, then destruction of O3 by UV back into O2. This is the Chapman cycle.
O3 absorbs in the 9.6um band but O2 has no part in this. Hence the confusion when O2+O3 is shown with all absorption spectra. I will add a note to the original blog article. For clarity, the SW absorption should be “O2+O3” and the 9.6um band should be on a separate line for O3 only.
Now, O2 shows up in the webpage you identified as absorbing at 6.67um. But it is not noted for anything in LW absorption in any texts I have encountered. I think this is because its absorption is actually very low.
Note that your webpage has Optical Thickness on the vertical axis. Optical Thickness is the integral of (concentration x absorption cross-section) by the vertical distance.
This means that Optical Thickness takes into account the concentration (as it changes through the troposphere and stratosphere), as well as its ability to absorb radiation of that frequency.
So comparing H2O and O2 around the 6.5-7um band –
O2 has an optical thickness of about 0.3 (reading by eye) at 6.7um
H2O has an optical thickness of about 3×10^3 at the same wavelength
So according to the webpage, H2O is about 10,000 more effective than O2 at absorbing radiation around 6.7um – this is taking into account the much higher concentration of O2.
If the vertical axis on the website you have been looking at showed absorption cross-section instead of optical thickness you would see O2 a million times lower than H2O in LW.
Does this make the subject any clearer?
Whilst I disagree with your basic premise, it is to your great credit that you allow others to post opposing views without censorship or offensive comments.
I tend to approach this from a historic perspective, and from that viewpoint there is nothing unusual about todays conditions. Also an examination of the temperature record-which I undertook long before ‘climate gate’-demonstrates the extraordinary and illogical manner in which ‘global temperatures are calculated even before any alleged ‘wilful’ manipulation is carried out.
Going back to basics, do we have an accurate base global temperature from which to work, commencing in 1850/80? No, certainly not. Do we have one today. Again no.
A thermometer only measures its immediate micro climate. If you move that thermometer-often to an airport- it starts measuring something completely different. Let it become urbanised -as very many of the records are- and it will be affected by uhi. The absurd disdain for calculating the real effects of UHI by the Ipcc, Real Climate et al -a very measurable effect known about since Ancient Rome- do nothing to help the case of the warmists.
So measuring temperature rise ’caused’ by co2 is based on a very shaky foundation and you really need to prove why- although we have been this way climatically many times before- this time its different.
The only person I have seen calculate the complex maths involved in any transparent manner is miskolczi;
Click to access ZM_v10_eng.pdf
Rather than do your own calculations it would be interesting to see you deconstruct the maths from this scientist.
Tonyb
[…] of “trace gases” cannot be deduced from our life experiences. Have a read of CO2 – An Insignificant Trace Gas? Part One to understand more about this […]
tonyb:
You said “I disagree with your basic premise”
I make no comment in the post about the temperature record. I also see nothing unusual in today’s conditions.
I make no claim that recent (last 100-150 years) temperature increases are caused by CO2.
The point of the post is to explain the basics of atmospheric physics.
I said in the conclusion: “..CO2 and water vapor are very significant in the earth’s climate, otherwise it would be a very cold place. What else can we conclude? Nothing really, this is just the starting point..”
What is it you disagree with?
I disagree with your basic premise that you state as;
“The question asked at the start was “Is CO2 an insignificant trace gas?” and the answer is no.”
Has it no affect at all? Of course it does. Is that effect wildly overstated-particularly when including the positive feedbacks-yes it is.
Creating a correlation of rising co2 to temp increase since 1850/80 is I believe flawed as the Global temperature record does not allow us to do this with the precision claimed.
You seem to agree that we have been this way before without the help of added co2 (although I am inclined to believe Beck) so why is it different this time?
Will be interested to see your dissection of miskolczi.
Tonyb
tonyb:
Let me explain. Why the post in the first place? Many “skeptics” of the IPCC make the comment, “CO2 is an insignificant trace gas and, therefore, can’t have any significant impact on global temperatures”.
Not true. It can. Even though it is 0.04% of the atmosphere (by volume) it absorbs and re-emits a dis-proportionate amount of long-wave energy.
I don’t claim anything else for CO2, but for many, even allowing CO2 a significant role in the radiation budget of the planet is a controversial point.
Whether or not its current effect and future effect is wildly over-stated isn’t covered in the post. The climate is way too complex.
If I have helped people understand the basics of what CO2 does – then the post has succeeded.
If many people who read it conclude that I am claiming that CO2 is – or isn’t- responsible for the last 100 year’s temperature rise – then I haven’t done a good job in this post.
To: scienceofdoom; get a real name for starters. It does not matter if carbon dioxide emits a dis-proportinate amount of long-wave energy. As you have stated, 98% of the atmosphere, nitrogen and oxygen, are transparent to long wave energy. Meaning, this long wave energy is not captured by 98% of the atmosphere; where the temperature is set for humanity and all living things
and the only place it matters for measuring and /or aggregating global temperatures.
Further, the atmosphere is a thermodynamics heat transfer problem. With energy in from the sun and its interior 5000 deg K core. And energy out
mostly by radiation, however, as much as 20% of escaping energy
is simply highly energized atmospheric molecules; which furthers vertical convection all the way out to the inside of Earth’s magnetic bottle, and the Van Allen radiation belt.
The atmosphere is a completely dynamic system and can only be seen
through flows and movements of atmospheric heat and gases;
a heat transfer problem. Nothing you have presented addresses the
actual problem of atmospheric heat transfers and the resulting temperatures. Or you have present a really good distraction from reality.
You have missed the obvious reason in front of your face why carbon dioxide and all trace gases can never be significant with the 98% nitrogen
oxygen earth atmosphere, the transparent long wave energy you
have described just travels on through the nitrogen and oxygen.
So your conclusion that carbon dioxide and other rare atmospheric gases
have a significant effect on the 98% nitrogen oxygen earth atmosphere is false or not true.
And the facts are as strait forward as you have presented them supporting
the fact the rare atmospheric gases can not have an impact on the
98% nitrogen oxygen atmosphere.
Nothing you have listed here as summary is in my 65th edition of the Handbook of Physics and Chemistry. While some of your data is there,
your conclusions are clearly not there. The Handbook of PHysics and
Chemistry is the pre eminent source of reality; with my 65th edition having
139 authors.
Be responsible. Get a credible peer review with the authors of the
Handbook of Physics and Chemistry and stop hiding behind offensive
blogging names.
Maybe then, responsible people might start taking you seriously instead
of this criminal hiding you seem to have penchant for. Fancy and plausible
explanations not tied to the real problem are not only not helpful; they
provide false conclusions most attorneys would label as fraud if widely
publicised.
Avoid trouble. Make sure what you are publishing is actually true; especially
your conclusions, if you are drawing any.
tdwelander,
You have missed the whole point of this discussion.
The small amount of green house gases can absorb most of the IR radiation and also emit a similar amount of IR radiation. The GHG’s transfer immediately (within one nanosecond) the energy they have absorbed to the main atmospheric gases N2 and O2.
Simultaneously the main atmospheric gases excite other GHG molecules to a state where they can emit IR. Only a very small fraction (like one in billion) of the excited molecules emit IR before they’re brought back to ground state in other collisions.
The excitations of CO2, H2O and other GHG’s are always nearly in thermal equilibrium with N2 and O2. The energy is stored by N2 and O2, but the GHG’s are needed to transfer energy between the radiation and the atmospheric gases.
Dynamics of the atmosphere is very important, but the atmosphere is close enough to a stationary system to make discussing its stationary properties very useful.
THe GHE is more about how atmosphere affects IR radiation than how IR radiation affects atmosphere.
tdwelander,
The CRC Handbook is a useful resource. It isn’t the fount of all knowledge. If the atmosphere were indeed transparent to IR radiation from 10-2000 cm-1, then the IR emission spectrum as observed from high altitude would look like a black body spectrum. It doesn’t. Similarly, the emission spectrum as observed at the surface would be essentially non-existent. It isn’t.
The snide remarks about the blog host’s nom de plume don’t help your case either.
Pekka,
“The small amount of green house gases can absorb most of the IR radiation and also emit a similar amount of IR radiation. The GHG’s transfer immediately (within one nanosecond) the energy they have absorbed to the main atmospheric gases N2 and O2.”
This seems to be the conventional wisdom, but out of curiosity what is the evidence claimed to be in support of this? This implies the probability of ‘stimulated’ emission upon absorption of a photon by a GHG molecule is very low or near zero.
This kind of questions are fundamental in our understanding of physics. Quantum Mechanics and Quantum Electrodynamics give clear answers to those. Our belief that QM and QED are good theories are based on numerous very accurate empirical tests.
What happens to a molecule when a photon hits it cannot be described in simple terms of classical mechanics, it’s deeply a quantum process. It has nothing to do with stimulated emission unless another photon gets involved. Using my rough numbers billion molecular collisions occur before a spontaneous emission would take place based on average rates. The influence of stimulated emission is orders of magnitude less than that of spontaneous emission – as long as we are not looking inside a laser.
RW,
Another reason we think the theory is correct is that we can calculate atmospheric emission spectra that are in very good agreement with observed spectra using the assumption of spontaneous emission and local thermodynamic equilibrium. We can also calculate the parameters of nearly all of the CO2 and other small molecule emission lines from first principles or ab initio as the phrase goes.
Pekka & Dewitt,
So it can be verified or deduced that virtually all the emission at the TOA is broadband and not narrow band? I’m curious specifcally how this is established, as it seems to me much of the GHGs would be in a high enough energy state for stimulated emission to occur, especially in the clear sky, low humidity atmosphere that is infinitesimally ‘thin’ thermally.
Just to clarify, ‘stimulated’ emission is the absorption of a photon by a GHG molecule of a certain wavelength that results in the immediate emission of another photon at the same wavelength, right?
Broad band and narrow band are properties of the source of radiation (or of mathematical models used in calculations) not of the radiation itself. Every photon has a specific energy, not a band of energy.
Solids and liquids emit at all frequencies. The emissivity is not constant but typically a rather smooth function of wavelength.
Gas molecules absorb and emit mostly at specific wavelengths. Not at strictly fixed values, but with a sharply peaked spectrum. I posted recently links to graphs that tell, how narrow the peaks and droughts are. Gases do have also some continuum emission and absorption that’s linked to situations where two molecules happen to be close enough for interacting with each other at the moment of absorption or emission.
Because the surface emits at all frequencies, all frequencies are present also at TOA. Gases cannot stop radiation at frequencies they don’t emit themselves.
If the continuum absorption and emission happens to have a suitable strength, it may affect significantly the GHE. Continuum is important for water vapor, but of relatively little importance for CO2.
(As discussed recently in another thread the overly simplistic model of SoD would predict that continuum type absorption and emission by CO2 would get important at very high concentration, but that’s due to the unrealistic line shape used for CO2.)
On the stimulated emission.
The case where a molecule absorbs a photon and emits soon another is normally not stimulated emission. It’s either scattering or a combination of absorption and spontaneous emission. It gets stimulated emission only if another photon gets involved in the emission.
In lasers coherent light (or IR) bounces back and forth between two mirrors and creates an exceptionally strong electromagnetic field in the space between the two mirrors. That strong field influences excited molecules and induces them to emit a photon in the same direction and in the same phase the other photons maintain. That’s stimulated emission.
Pekka,
What do you have to say about what is in this paper? In particular, pages 8 & 9 (especially pg 9 “Line Strength Expressions”). The information given here would seem to contradict what you’re saying.
“Absorption Line Physics”:
Click to access week4.pdf
RW,
There’s nothing contradictory to what I have written on those pages. What made think that there would be.
Pekka,
That stimulated emission was not a factor or not something that occurs in the atmosphere.
The formula can be used to calculate the strength of the stimulated emission in the atmosphere. That turns out to be negligible. Only in lasers and some other situations of very high intensity of radiation that strength is large enough to be observable.
To Mr Pirila. The point of the discussion is whether rare earth atmospheric gasses, carbon dioxide in particular, affect the rest of the atmosphere to the point of adding or subtracting sufficient heat which would change global temperatures. Carbon dioxide being in the parts per million in the atmosphere, can not change global temperatures. The volume of carbon dioxide is too insignificant to change anything noticeably in the atmosphere.
Your explanations do not address this fundamental issue as suggested in the title of the article. Carbon dioxide is in fact insignificant on global temperature based on the less than tiny volume alone; no matter what
indirect effects may exist, which are distractions.
To Mr. Payne. Whether or not carbon dioxide affects global temperature
is fundamentally a thermodyamics and heat transfer question. Is there enough volume of carbon dioxide to move heat into earths nitrogen oxygen
atmosphere to change temperature? There is not; not even close.
My 65th edition of the Handbook of Chemistry and Physics has 139 authors.
None of them hide their names. The Handbook of Chemistry and Physics
is the pre eminent source of reality. If the info is not in the Handbook of Chemistry and Physics, it is someone or some groups speculation or theory.
Publishing speculation or theory as fact is fraud. Why the Handbook of Physics and Chemistry is critical to at least minimizing all of the sophisticated fraud floating around masquerading as fact.
Supposed well trained people for over 20 years have been saying carbon dioxide has some kind of undue influence on earths atmosphere. It does not, has never, and will never based on the above info and the Handbook of Physics and Chemistry. Why confronting fraudsters has become so important.
This is a heads up for you from my end. If your information is not accepted as fact in the Handbook of Chemistry and Physics, and you publish theory as fact and do not make it clear that it is theory, these days there is a reasonable probability you could be confronted by law enforcement for committing these sophisticated kinds of fraud. I thought it worthwhile to at least try to keep you out of trouble.
tdwelandar,
Thanks. I needed a good laugh today.
tdwelander,
The starting post of SoD and much of this thread explains, how a very small amount of CO2 can have a strong effect on temperature, when it interacts strongly with IR. That’s a very similar observation as observing that a very small amount of strong dye can give a strong color for a glass of water Your declaration that it’s not so, has zero weight in comparison with the valid arguments presented here.
tdwelander
This is another version of the argument from incredulity rather than an argument from physics.
Let’s take your last statement cited above: “The volume of carbon dioxide is too insignificant to change anything noticeably in the atmosphere”
And contrast it with some measurements of the atmosphere (shown in the article):
Given that the climate system and the sun/rest of the universe only exchange energy via radiation the actual emission of thermal radiation from the climate system is very significant. The “notch” in the emission of thermal radiation around the CO2 band is very significant in energy terms.
There is no law of physics which says that atmospheric radiative effect is linearly proportional to atmospheric concentration.
In fact, the carefully studied subject of radiative physics has determined that the equations of radiative transfer are the relevant equations. These are shown in Atmospheric Radiation and the “Greenhouse” Effect – Part Six- The Equations.
You can join the (so far) unanimous crowd of people determined to claim that physics says something different from standard atmospheric physics -while never actually stating whether:
a) these equations are wrong, or
b) the result of applying these equations to the real atmosphere with real boundary conditions produces a different result from standard atmospheric physics
I realize that many lurkers without maths background cannot understand that article.
Well, the passionate masses who believe something different from this article are equally unable to understand or engage with the maths written down in physics textbooks.
That should be sufficient to demonstrate the point.
Physics is a technical subject.
Followup to Mr. Pirilla. When I see or find your spectra charts posted in the Handbook of Physics and Chemistry, is when I and most other realists will take them seriously. If you would like to quote the edition and pages of where your spectra chart or charts are shown in the Handbook of Physics and Chemistry, I would likely then look them up. Until then, all you have presented is theory. Which puts you in a very onerous position.
Assuming what you have presented is factual and carbon dioxide does absorb and re emit substantial energy, at 400 parts per million in the atmosphere, it could not possibly be enough to show any measurable
change in global temperature.
As previously stated, earths nitrogen oxygen atmosphere is fundamentally
a thermodynamics and heat transfer question for temperature changes, if any. There are not any significant temp changes due to the long wave transparency of nitrogen and oxygen, even with your notch spectra for carbon dioxide considered, due to the less than small source of carbon dioxide at 400 parts per million in the atmosphere.
Give me your responsible sources and locations within the Handbook of Physics and Chemistry and then we will have a starting point. Until then, you are just blowing in the wind. And worse, people will take you to legal task for your expoundments if you publish these assertions as fact.
Oh, and I will put my factual understanding (which is the definition of physics) of the atmosphere up against yours based on the Handbook of Physics and Chemistry, probably any time.
Referring to a handbook in the way you do is one of the most ridiculous arguments I have ever seen in climate debate (and there have been plenty of ridiculous arguments.)
The Handbook has it’s own goals (i.e. collecting a wide variety of numerical data and some other straightforward factual data.
How can anyone propose that only those things are true that fit in one handbook.
Whatever your specialty you are likely to trust your knowledge on many issues not found in the Handbook of Physics and Chemistry. It’s not the ultimate repository of all knowledge.
tdwelander,
It’s called a Handbook, not an Encyclopedia for a reason. It contains a lot of useful data, but nowhere near all. The HITRAN database of spectroscopic parameters (2,713,968 lines) of 39 small molecules comes to mind. There isn’t room to put this in the Handbook and a printed version would not be very useful anyway. Your view that the Handbook is the equivalent of inerrant holy writ is quaint and amusing. Do you only handle it while wearing clean gloves?
tdwelander,
Oh, awesome parody! Nice work.
Just in case (oh no!!) you are not..
Don’t start with this blog. You urgently have a long list of journals to contact.
Start with these guys: Journal of Quantitative Spectroscopy & Radiative Transfer.
They have published literally tens of thousands of papers on the subject without so much as an Imprimatur from the Handbook of Physics and Chemistry.
They have papers like this one: The HITRAN 2008 molecular spectroscopic database. All out in plain sight, sucking in academics, researchers, bloggers..
Let us know how you go and I can give you a long list of other journals.
Then there’s university courses, university libraries..
Hey I have reviewed your information and it ignores completely the fact that the absorption and emission spectra for Carbon Dioxide are the same. The only molecules that are not transparent like nitrogen and oxygen are other Carbon Dioxide molecules. That is why a trace gas like CO2 can only have a minimal impact on global temperature changes.
No, it doesn’t. Did you bother to look at the atmospheric spectra in the main article? Apparently not, or you wouldn’t have posted what you did. Here’s the spectrum of radiation emitted by the Earth to space:

See the big dip centered at 667 cm-1? That’s CO2. That missing radiation must be compensated by increased emission at other frequencies for energy in to approximately equal energy out. That can only happen if the surface is warmer than it would have been without CO2.
I forgot to mention that I read your post over at WUWT and thought your criticism of the video pretty fair.
Skeptics have to put over a proper acurate case and it doesnt help to resort to the tactics of the warmists by making unsubstatiated or dubious claims.
Whether co2 is an impoprtant trace gas or not is the subject of a debate, but there is no doubt that many individuals believe it is present in far greater quantities than it actually is.
By the way where is the scene pictured on your blog photo at the top?
Tonyb
tonyb:
Thank you for your kind words.
The picture is just part of one of the themes that the wonderful people at WordPress provide. So I don’t know. But it looks good.
Hi scienceofdoom,
I also came here after reading your review of the John Coleman video at WUWT.
While the fact that CO2 lags temps in the ice cores doesn’t prove that CO2 has no effect on temps it doesn’t show that CO2 is amplifying those temps either as often claimed by climate alarmists.
The claim is made that once some other mechanism starts to raise temps CO2 kicks in and accelerates the warming. I don’t see any evidence in the record that shows that CO2 is accelerating the rate of warming. In fact as the CO2 level is peaking the temps take an abrupt downward turn despite the fact that CO2 is at its highest level and still increasing!
To ignore this anti-correlation and insist that CO2 displays an accelerating effect on temps is not supported by the evidence.
Do you have evidence to support their contention that CO2 is shown to be amplifying the warming when the data from the ice cores shows no such thing? If not perhaps you shouldn’t perpetuate these false claims.
A good read into the basic first layers of absorption. However, the true question that most skeptics have ( unless I am totally off my rocker which may be the case ) is does an increase in CO2 cause the kind of response that has been predicted by the IPCC. Not whether there is a spectrum absorption of CO2 ( any kid that has taken a physics class should be able to say “well duh” ) and that it is not needed for the stable temperature values that we have.
Nevertheless a good read. Thanks for the post.
Lance:
There were 2 parts to the claims in the first section of the Coleman video.
The second part commented that CO2 was a trace gas, the clear inference was, “therefore, it can’t be significant in affecting temperatures”
The reason for providing the link to this article was to show that even though CO2 is a trace gas it is influential.
That is, it is a mistake to ignore CO2 because it is only 0.04% of the atmosphere.
This article doesn’t claim anything else. I claim CO2 is “influential” because the science demonstrates it. I haven’t claimed that it is the reason for amplified warming during past warming periods.
So no I don’t have evidence to support this point, because I’m not claiming it.
Forrest:
You are right, the article is basic – anyone who has taken a physics class will agree. Most haven’t and this is the motivation for the article.
As to the final effects of CO2 and the claims of the IPCC, well that’s a lot more complicated. Watch out for later articles.
Science of doom
My original motivation for contacting you was that I had just come from delivering an address on ‘man made climate change’ to a group of verry bright 11 year olds at a school.
The amount of co2 and the effect it has (bearing in mind the logarithmic curve and lag times) is of course a subject of great debate. Howevere I was particularly struck by;
1) How doomed the youngsters felt by the science-it was accepted completely that we will cause the planet to fry unless we completely change our ways.
2) The knock on effect that had with their world view-very pessimistic about the future.
3) How completely mistaken they were about the quantities. They believed that between 50-100% of the atmosphere was comprised of co2 AND that 90-100% of that co2 was created by man-so virtually the whole atmosphere is comprised of co2 ‘we’ have created.
Now I’m sure I don’t remember what I knew of physics at that age, but the point is that we are scaring our youngsters without giving them the knowledge to put it into context.
I had a long conversation with a member of the UK green party a couple of months ago and his view of the atmosphere and our effect on it was not dissimilar to that of the youngsters. The difference being he was actively campaigning to do something about it-in his case he had been to a coal fired power station to try to close it down and taken part in a protest at an airport to do the same there.
So there is an awful lot of misconceptions about concentrations even BEFORE we start to talk about its actual effect.
Not sure there is anything you can do about it as you are aiming at a levelk of knowledge far beyond those examples. However if anyone reading this comes across a really basic AND objective physics for beginners I would be interested in pointing people towards it.
I do think we assume the average person has far more knowledge of physics than they actually do.
All the best
Tonyb
Thank you for this very illuminating article and the posts that follow. Very helpful for non-scientists like me. I came to it from the Coleman story, which in turn came from the UK’s AGW-sceptic blogger James Delingpole. My own AGW scepticism has been fueled by the lack of calm, reasoned debate on the issue – particularly the labelling of “Deniers” etc
Your article and the following posts are what the debate should be like. It is also the first time some one has properly explained to me why a gas being 0.04% of the volume of the atmosphere might have any effect on temperature. All the supposedly illuminating programmes on the BBC have consistently failed to do this.
As bit of info (without any statistical validity at all): of my 4 most intelligent friends (all of whom graduated from top UK universities) one is a strong advocate of AGW, while the remaining three are sceptics. Their sceptic reasoning is mainly due to the lack of certainty of data and the relative youth of climate science against mature scientific disciplines. Three of the four are scientists by degree (Physics, Chemistry and Medicine) and one is a lawyer – guess which one?
British Diogenes, Is it the lawyer who is the AGW advocate?
The foundational principles of climate science appear to be very strong.. in fact, climate science is mostly physics with some chemistry. But there are a lot of unknowns in climate science.
Glad you found the articles useful, I will be covering more on CO2 in Part Two soon.
Science,
Thanks.
I know that H2O is 1e5 to 1e6 times more absorptive than O2 around the 6.67 um range. However, the IPCC AR4 choose to ignore H2O in its forcing discussions and listing in chapter 2 table 2.1. So that makes me wonder then what is O2’s RF.
The H2O in this range is not 100% opaque, so the O2 absoption – albeit small – plays a part. By some really rough calculations the RF of O2 appears to be on the order of
HFC-152 at RF = .0004 and C2F6 (PFC-116) RF= .0008, which both get listed in the IPCC AR4.
At least I have seem to have gotten past the “its not a GHG, cause its a diatomic” argument. By definition, it is a GHG.
[…] Part One of the series started with this statement: If there’s one area that often seems to catch the imagination of many who call themselves “climate skeptics”, it’s the idea that CO2 at its low levels of concentration in the atmosphere can’t possibly cause the changes in temperature that have already occurred – and that are projected to occur in the future. Instead, the sun, that big bright hot thing in the sky (unless you live in England), is identified as the most likely cause of temperature changes. […]
[…] Part One of the series started with this statement: If there’s one area that often seems to catch the imagination of many who call themselves “climate skeptics”, it’s the idea that CO2 at its low levels of concentration in the atmosphere can’t possibly cause the changes in temperature that have already occurred – and that are projected to occur in the future. Instead, the sun, that big bright hot thing in the sky (unless you live in England), is identified as the most likely cause of temperature changes. […]
Thanks ScienceOfDoom,
Just found your site today and will return to learn more about CO2 and its effect on climate change (if any).
I do appreciate your effort.
I don’t quite follow why you say there is no solar radiation at the longer wavelengths. You say the graph was scaled down a million times to show the curve of the i/c (black body) radiation. Looking at the first graph there is a long tail into longer wavelenghts. Without working it thru it would seem that the sheer quantity of solar radiation would mean some at above 5 mu?
However, many thanks for this tutorial. I can see more easily how feedback mechanisms, +ve and -Ve, are so vital.
Still wonder tho’ about the ice core “CO2 lags temperatures, even when cooling” finding….
For Keith:
Good question. The two graphs compare the blackbody radiation in W/m^2 from the surface of those blackbodies at the 2 different temperatures.
Suppose the Sun and the Earth were the same size and near each other, then if we parked ourselves a little distance way from both then the sun’s radiation in the 5-30um would be a lot more than the earth’s in the 5-30um band.
Sun’s radiation in 5-30um spectrum = 329,000 W/m^2
Earth’s radiation in 5-30um = 339 W/m^2
So the Sun’s is 1000 times higher in this band if both Sun and earth were comparable distances away. And the Sun is a lot bigger, so the total energy would be higher again.
However, the Sun is a long way away from the earth.
We only receive 1 in two billionth of the sun’s energy that it radiates out (see the maths section at the end of the post).
That’s what makes the difference and is the reason why the Sun’s energy in the 5-30um band is so much less than the earth’s.
Hope this helps.
[…] Part One opened up the topic and introduced the simple “billiard ball” or zero-dimensional analysis of the earth’s climate system. The sun radiates “shortwave” energy which is absorbed in the atmosphere and the earth’s surface. This heats up the earth’s climate system and it radiates out “longwave” energy. […]
Science of Doom – thank you for a very detailed explanation of the reason why a gas which comprises .04% of the atmosphere CAN have a disproprtionate warming effect. I have to admit that I started to glaze over through the maths – I am but a retired mechanical engineer who struggled with the maths at degree level, and try to avoid it as far as possible..!
However, since (recently) retiring I have taken an avid interest in the Skeptic viewpoint – which I share – and spend much time following the collapse of the AGW propoganda (to my wife’s undisguised despair).
The point which I WOULD like to make (Oh – at last) is this – none of the ‘warmists’ seem to have read the Kyoto Protocol, which talks quite clearly about reducing CO2 EQUIVALENT. NOT reducing CO2 itself..! Why, then, is the political/environmental establishment so keen to portray CO2 as a sort of horrid black cloud hanging over the country (that’s water vapour, that grey thing up there) – instead of the life-blood of plants which we all know about from basic biology..?
David:
Thanks for the kind comments. The maths is always painful. The challenge is to present the science in a way that can still shine through without any maths. I fear that I may have not succeeded in the later posts in the series.
On your first point
I haven’t read the Kyoto Protocol either. But if it refers to CO2 equivalents the same way as the IPCC..
The idea is to be able to compare the effect of all the “greenhouse” gases in a comparable way with CO2.
Looking at the change in ppm in the atmosphere – or absolute ppm – of different gases isn’t a good comparison as there are other factors. So the change in ppm of each gas is turned into “radiative forcing” at the top of atmosphere in W/m^2. This is a unifying concept so that the different gases can be compared.
Therefore, when the IPCC talks about CO2 equivalent it is with the idea that the increases in concentration of different “greenhouse” gases can be compared.
So – if the IPCC uses the same terminology as the Kyoto protocol – the idea is to be able to compare the effect of increases in different gases – CO2, methane, N2O and a whole raft of other CFC-**
And on the second point
In part I agree with you. The US EPA has denounced CO2 as a pollutant. This is not really true and perhaps this is more about the politics of the subject, rather than the science.
Leaving aside the politics. If CO2 – wonderful plant food that it is, and necessary for all life – if doubling CO2 will increase the temperature of the planet by a few degrees then it makes sense for people/governments to be concerned about it.
Therefore, the AGW community wants to reduce “greenhouse” gas emissions when they talk about “CO2 equivalents”. But what they are saying is “well, reducing CO2 emissions by 10ppm is the same as reducing CH4 (methane) by 1ppm so either one will be ok..”
I have a question regarding calculating the radiation balance. One uses the the “disc” area of the earth to calculate how much radiation the earth receives but the entire surface area for how much it emits. If we’re calculating a strict radiation budget in the absence of other mechanisms like convection, shouldn’t those areas be the same?
Dave:
Excellent question and one which is worth attention. In fact, I am thinking about doing a quick post on this specific topic, including a nice graphic – because when you start looking at this subject it’s all the little points – and the different numbers that get quoted – that easily trip you up.
So watch out for the next post..
The first question is, how much radiation does the earth receive? That’s easily calculated and since 1978 we have satellites measuring it from outside the earth’s atmosphere.
The number, the Solar “constant”, S = 1367 W/m^2
What area does that radiate over?
If we want to work out the total energy received by the earth, does it radiate over:
1. the entire surface area of the earth (4 x pi x r^2)? or
2. over the “2d disc” which is pi x r^2.
The answer is number 2.
Easy to understand in a conceptual way because the sun is not radiating equally over all points on the earth at the same time. Half the earth is in darkness at any one time, and some of the earth has the sun low down on the horizon.
I’ll show a nice graphic in the post on this topic.
So total energy received = S x pi x r^2
(where r is the radius of the earth)
So the second question, what about the radiation out? Your first instinct is a good one – shouldn’t we compare like with like?
But the earth doesn’t just radiate out from one side of the planet in one direction. It radiates out energy from every point on earth at all times (in proportion to T^4).
And the earth’s surface area is 4 x pi x r^2.
Energy is received from the sun and then the climate system spreads it all around.
Hope this answers the question, watch out for the next post..
HI Scienceofdoom,
I came across your lovely post and would like to ask a question if possible. If the radiative forcing of CO2 is 1.6 Wm-2, then how do we compare it to the solar constant which is 1367 Wm-2?
Looking forward to hearing your answer.
Best
If you’re considering the 1.6 W/m^2 of incremental GHG absorption to be equal in its intrinsic surface warming ability as post albedo solar power entering power the system, it would be (1367/4)*(1-0.3), because the Earth is a sphere you divide the 1367 by 4 and to account for the Earth’s albedo of about 0.3, i.e. the fraction of incident solar energy reflected back into space, it comes to around 239 W/m^2. The 1.6 W/^2 would be equal to +1.6 W/m^2 of post albedo solar power entering the system. Or equivalent to an increase from 239 W/m^2 to 240.6 W/m^2.
Very clear explanation but somehow unsatisfying to me. Still not sure how x number of watts received in an average square meter can be emitted by some greater number of square meters. It really seems like the conditions of the initial equation have been altered.
It seems as though the calculations assume the earth is static when it receives the energy, but dynamic when it radiates that energy.
I look forward to an additional post on this vexing riddle
Hello,
If “Energy received from the sun = Energy emitted by the earth (if the temperature of the earth’s surface stays the same).” then there appears to be no energy left to be used (stored) by life on Earth. The biosphere should be part of the view. The surface of the oceans stores energy in algae, fish, and mammals. Same thing for most of the surface of the continents.
Also, there is the energy used to move the ocean water around the continents (oceanic currents, winds) this is kinetic energy that arrived as radiation from the Sun.
But if you look at the Earth from low orbit, you see clouds covering most of the planet. Water and water vapor seem to be the primary features of this planet.
Cheers!
[…] 6, 2010 by scienceofdoom In the first post about CO2 I included a separate maths section which showed the energy budget for the earth and also derived […]
Dave:
Check out the new post:
The Earth’s Energy Budget – Part One
I might post a comment over there on your question above.
[…] Part One of the series introduced the shortwave radiation from the sun, the balancing longwave radiation from the earth and the absorption of some of that longwave radiation by various “greenhouse” gases. The earth would be a cold place without the “greenhouse” gases. […]
Thanks for an illuminating article : clear, lucid and informative.
I am not a scientist, but ‘common sense’ tells me that the planet must have many ‘negative feedback’ systems – since almost any parameter we look at seems to oscillate back and forwards either side of a mean – within a fairly narrow range.
Just a personal anecdote: last February I was hiking in the Cheviot Hills, Northumberland here on the Scottish Border.
It had been bitterly cold with sub-zero temperatures for weeks on end, and the ground was still frosted every morning. That particular day we had a high-pressure system and the clear blue sky was cloudless, with no wind at all. The temperature climbed rapidly to about 20 degrees C – so hot that I removed my fleece and walked in a T shirt. I met a farmer and said that this looked good for the lambing season; he laughed and said ‘I wouldn’t count on it’. That night, the cloudless skies sent the temperature plummeting to minus 8 degrees C, and a few days later we were back to snow and frost until well into March.
My point is that there was no ‘heat storage’ on the land nor in our local atmosphere that day. The temperature range on that February day varied by a full 28 degrees C – but as soon as the clouds disappeared and the sun set – temperatures plummeted by 28 degrees within 8 hours.
My ‘common sense’ observation is that the heat must have re-radiated into space as infra-red – since none remained in the rocks or the air above them.
You could go to any desert in the world – and experience temperatures of 40-50 degrees C at noon – but the same night you could freeze to death if not suitably protected.
It seems clear that the Earth does not hold onto heat – and can radiate it into space in vast amounts very quickly. I realise that the oceans store heat much more than the land – but it still seems unlikely that there can be any runaway global warming, since – if my local hills can lose 28degrees of heat in justa few hours, so can the planet?
If a CO2 molecule can cause a warming of an spot on the earth can the earth warm the sun?
The pot cannot warm the burner.
The earth at a higher temperture than the CO2 molecule will not be warmed by a lesser energy emission. No where in radiative heat transfer does a cooler object warm a hotter object. At best they can be the same temperature and that would be what the sun caused.
Also using vector math the earth sends a photon that is intercepted by a CO2 molecule goes from a plus one to zero. The CO2 goes from zero to plus one then emits a photon (not the same one) out. So at best the earth would go from a zero where it was after emitting back to a one. So the two cancel each other and no warming of the earth takes place except that which comes from the sun to begin the process.
Also nowhere did you account for PV= nRT.
If the Second Law of Thermodyanmics means anything there is a increase in entropy during the exchange of photons between the earth and CO2 molecules. So there could not be any extra warming. Again at best it would stay even with what the sun caused.
Finally, you are the only site that doesnot show a H2O absorbtion line at the 15 spot.
William of Ockham:
Thanks for the kind words.
Your common sense observation is more or less correct. The earth – the rocks, soil, sand plus the atmosphere are very good at radiating heat away.
So is the ocean – although its specific heat capacity is much higher and so it will store heat for a lot longer.
However, although the overnight temperature in your example dropped to -8’C, it didn’t drop to say -50’C or -100’C. There is still heat stored in the climate system. But under a clear night sky the heat is effectively conducted, convected and radiated away. (Under a cloudy sky less so). Then when the sun rises the next morning that part of the earth warms up again.
In the absence of any positive feedbacks – and “all other things remaining equal” – the extra radiation because of the current level of CO2 simply moves the average temperature up “a notch”.
Remember if there were no greenhouse gases the average temperature at the earth’s surface would be -15’C. Clearly this absorption and re-radiation of longwave energy has a noticeable effect at the surface!
The extra longwave energy from higher CO2 doesn’t cause an immediate temperature rise, because the oceans take time to warm up. They have a large specific heat capacity and store around 1000x the energy of the atmosphere.
As the oceans warm up, they radiate more heat. Eventually a new balance is reached at a new higher temperature. (This is negative feedback – a new equilibrium is reached).
This certainly isn’t a “runaway” scenario. And the maths is fairly simple.
And of course – “all other things are not equal”, as so many other climate effects also have their impact on the global mean surface temperature.
Mike Kelly:
If I follow your reasoning correctly, putting a roof over a house wouldn’t warm the house because it’s not creating any extra heat?
What happens is the earth radiates out energy from its surface. With no greenhouse gases that energy would all be radiated out to space. That’s nice and simple
(Well, there might be some reflection and scattering on aerosols and other particles but we will leave out that extra complication).
With greenhouse gases in the atmosphere, energy is absorbed, the gas heats up and then re-emits radiation both upwards and downwards.
If you like you can think of it as heat reflected – like putting a roof on a house. That’s not what happens of course, it’s just an analogy that might help.
As we’ll see in Part Six !! – we can measure the downward longwave radiation at the earth’s surface. If the “greenhouse” effect didn’t happen there would be no downward longwave radiation at the surface.
Also what we see is the radiation coming back to the earth’s surface is in the energy bands (like the 15um CO2 band, the 9.6um O3 band, etc) that match the absorption we measure at the top of the atmosphere.
(And of course we can also measure the same absorption in the lab by taking each gas and shining radiation of various wavelengths through).
“Your common sense observation is more or less correct. The earth – the rocks, soil, sand plus the atmosphere are very good at radiating heat away.
So is the ocean – although its specific heat capacity is much higher and so it will store heat for a lot longer”
I would note that there is stored heat in the evaporated water vapour on that warm Scottish day.
The heat loss from the water vapour is due to net outbound radiation. As the temp drops, dew forms(condensation), and there is a heat transfer to the air and ground.
I think the maths of water vapour heat gain and retention would show it overwhelms CO2.
With greenhouse gases in the atmosphere, energy is absorbed, the gas heats up and then re-emits radiation both upwards and downwards.
Your quote.
Then following the second law entropy must increase and a less energetic photon is sent out. Since has less energy it cannot raise the temperature of the earth higher than what the sun did.
Also givenPV= nRT the earth’s temperture is roughly 0 deg C. So any effect by gases is only 15 deg not 33.
Two discs radiating at each other can only get to the higher of the two in temperture. So again that is what the sun causes not gases. If you snuggle with your wife at night does your temperture go up to 98.6+98.6= 197.2? No you both stay at 98.6. Even with covers on you stay at 98.6. Even with CO2 inside the covers you stay at 98.6.
CO2 cannot cause the earth to have increased.
Water vapor also has absorbtion line in the 15 range but you don’t show that was my point.
Don’t get me wrong I like your work it is very readable and I enjoy it. I just find no evidence that CO2 can cause an increase in the temperature of the earth and stay with the laws of physics and gases.
If CO2 could increase temperature and it has been known for so many years why hasn’t someone developed a blanket, coat, house wrap, anything that takes advantage of this and make money? Because it cannot be done.
EdB:
No one could doubt your point.
All that this first post in the series attempts to demonstrate is that the greenhouse gases in total have a significant effect on the surface temperature of the earth. And also that CO2 is one of these significant greenhouse gases.
How that compares with latent heat (movement of heat through evaporation and condensation of water) and “sensible heat” (due to convection) isn’t covered in this first part.
In the lower part of the atmosphere sensible and latent heat dominate heat transfer – you are correct.
Untangling the relative effects and getting to a point where some of the numbers are explained first requires an understanding that the effect exists.
In subsequent posts I try to work through some more specific issues.
Mike Kelly:
There is no violation of thermodynamic laws. There is no increase in total energy.
Let’s consider a simple comparison.
Case 1. Due to energy received from the sun, 2W are radiating up from the ground out to space.
Case 2. Exactly like case 1 but for whatever reason 1W keeps going out to space and 1W is “sent” back to earth.
(Case 2 could be reflection or absorption and re-radiation or any other phenomenon, doesn’t matter)
My questions:
1. Does this violate any thermodynamic principles?
2. In case 2 is the ground warmer?
[…] By the way, if you are new to this subject and think CO2 is an insignificant trace gas, then at least take a look at Part One. […]
Part Six -Visualization of the series has just been added.
It shows the downwards longwave radiation at the earth’s surface, might be interesting for some people.
I did not say there was an increase in energy. I said that there must be an increase in entropy. Given a increase in entropy the photon emitted from the CO2 molecule must be of less energy than the one received from earth. That being true then the CO2 molecule cannot heat up the earth to a temperature as hot or hotter than what the sun did.
I do not disagree that gases in the atmosphere delay the exit of photons from the earth to space, but they are of less intensity and energy and cannot heat the earth up. Blankets keep me warm at night but not hotter than my body is.
There is no known example of a gas being used for anything other than heat dissipation. (Not counting burning gas for heat that is a different subject.) Gases dissipate heat they do not generate heat. Your car radiator, fins on electronic components, fans in the summer, etc.
Please address my inquiry about PV=nRT.
Mike Kelly:
Not wanting to press the point if you don’t want to.. but the blanket analogy is kind of what happens with CO2.
The photon emitted from the CO2 molecule is of less energy that the one received from the earth. But because a proportion get emitted back towards the earth they increase the surface temperature.
Take a look at Part Six – you can see the longwave radiation downwards at the earth’s surface. What is generating that downward longwave radiation? And why does it match absorption spectra of the gases that we know – CO2, methane, O3, etc?
Well onto your question about PV=nRT.
There were 2 questions and I didn’t really understand what you were getting at with either of them, I’m sorry to say.
First: “Also nowhere did you account for PV= nRT.
In the “billiard ball” model or zero dimensional model of the earth’s energy balance we just balance energy in from the sun with energy radiated out from the earth’s surface – using the well-known blackbody radiation according to Planck’s function. There is no need to introduce the ideal gas equation as we don’t need to work out the temperature, pressure or volume of any gases.
Second: “Also given PV= nRT the earth’s temperature is roughly 0 deg C. So any effect by gases is only 15 deg not 33.”
Can you elaborate?
[…] background is the series CO2 – An Insignificant Trace Gas? and especially the last post – which maybe should have come earlier! – CO2 – An […]
Second: “Also given PV= nRT the earth’s temperature is roughly 0 deg C. So any effect by gases is only 15 deg not 33.”
Can you elaborate?
If gas laws are valid (which I belive they are) then the atmosphere of the earth all by itself must cause a certain temperature. By using a square meter up to 100 km one can figure what the temperature of the earth should be based on the pressure of air. I figure 0 deg C. If so then radiation only accounts for 15 deg C not 33 deg C.
“The photon emitted from the CO2 molecule is of less energy that the one received from the earth.”
Also if entropy is increased which you agree that it is then that photon cannot raise the temperature to a point as high or higher than what the sun did. Down hill flow of energy will not allow that.
Mike Kelly:
I’m not ignoring you, just thinking.
Well, not about entropy. Quoting a law doesn’t mean it applies to this situation although I’m sure you believe it does! Just that I don’t think I can help.
A roof on the planet would increase warming? Yes. But wouldn’t break the laws of thermodynamics? No. And this is different fundamentally from the “greenhouse” effect because… it’s a roof! Analogies often solve nothing!
10,000 physicists don’t think the greenhouse effect breaks the basic laws. The “skeptic” scientists (Lindzen, Spencer, Christy..) don’t think it breaks the laws.
So.. A test of any theory is the experimental evidence. So maybe a different approach – take a look at Part Six and explain where that downward longwave radiation is coming from.
According to your interpretation of thermodynamics, it can’t be from the “greenhouse” gas effect. So where is it coming from? Your turn.. You have the floor.
– I was thinking about the ideal gas laws. My first reaction was – A gas under pressure doesn’t create heat all by itself.
But anyhow, I will mull on it for a day or two and consider whether it is possible that the changes in pressure can account for some/any of the temperature gradient.. watch this space..
In the meantime, don’t forget to come up with your theory!
You should include why the absorption of upwards longwave radiation that we measure at the top of atmosphere is similar in spectral shape to the downward longwave radiation at the earth’s surface..
[…] a discussion on another blog when I commented about CO2 actually creating a “radiative forcing” – shorthand for “it adds a certain amount of W/m^2 at the earth’s surface” […]
I don’t believe I have expressed myself correctly. You seem to think I disagree that there is IR being radiated both up and down in the atmosphere from GHG’s. I do not. As I stated earlier, gases delay the loss of heat but do not add to it.
What I disagree with is that the IR can raise the temperature of the earth as high as or higher than what the sun already did. The intensity is lower and the energy of the IR is less, as you agreed, so increase in heat is not possible.
Further two bodies radiating at each other can only get to the max temperature of the highest body which is the earth and the temperature comes from the sun.
Given the IPCC formula of dF=5.35*ln(C/Co) where C in the present level of CO2 and Co is a past level of choise. They say roughly 1.6 W/m2 is from CO2.
One Watt= 1 j/s (juole per second)
Given this and placed into the standard specific heat formula (Q=c*m*dT) there should be a warming of nearly 5 deg C. That is not happening. So something is awry. Even if you want to cut that in half because only half the earth receives sunshine at a time something is still wrong.
Of your 33 deg C as much as 95% of that is from water vapor. Three per cent for CO2.
So let’s do the math
33 X .95 = 31.35
33 x .03 = .99 for CO2
Human CO2 = roughly 4 per cent of total CO2
.99 x .04 = .0396 for human influence this is not mearsureable nor important.
But CO2 can only absorb approximately 8% of IR in its 14-16 mirco range. So the 33 x .03 should be really 33 x (.03 x .08) = or .0792 Then .04 X .0792 is .003 degree from human. That is not measureable nor important.
You may quibble with some of the percentage, but CO2 cannot warm the earth as you seem to be portraying.
[…] Part One of this series, in the maths section at the end (to spare the non-mathematically inclined), we […]
Nicely done, I am in the “skeptic” camp but the foundation of the AGW theory are indeed solid, and the “0D” simplification is not really challenging mathematically, at least for who like math ;).
If you welcome suggestions, I would modify slightly the presentation of the blackbody emisions of earth and sun: instead of scaling the sun emissions just to get it in the same graph (which raise the problem that a blackbody of higher temperature emit more at all wavelength than one at lower temperature – the “tail” of the sun emissivity is indeed much higher than the peak of the earth one as was mentioned in some comments), why not introduce immediately scaling due to distance, which would measure the emission right a the earth – keeping the earth curve as it is but scaling the sun by 1/r^2.
And another image that, imho, makes the 0D model more clear (and hightlight the simplifications) would be to say that it assume perfect conduction in the non-radial directions. It means that the earth is a perfectly conducting sphere, hence have only one temperature that does not vary between night and day, or places to places. This makes the area to consider for absorption (the disc for solar radiation, the sphere for longwave greenhouse back-radiation) and emissions (the sphere for earth surface blackbody radiation) quite clear….
Now time to go read the following parts 😉
Thanks for those,
regards,
kai.
kai:
Thanks for the comments and the suggestions. Definitely a great idea to scale the two graphs and explicitly state that – also have a second graph with a zoom in on the overlap and add a bit of text specifically about that – or a reference to another post that has those numbers..
On the second idea I’m not sure whether that then confuses the non-mathematicians/scientists i.e., those that aren’t used to seeing models. Who say “oh well that doesn’t really happen so the model isn’t true.. and not valid..” – but anyway something for me to think about..
Comments from people who don’t get understand the theory here and on other blogs have been very illuminating, but clearly there is a lot more that can be done. So all suggestions about how to improve the material are good.
[…] 23, 2010 by scienceofdoom In the series CO2 – An Insignificant Trace Gas? we concluded (in Part Seven!) with the values of “radiative forcing” as calculated for […]
Mike Kelly:
I’ll break it up into 2 comments..
First, you said:
Well, without any “greenhouse” gases, the earth’s (average surface) temperature would demonstrably be:
-18’C.
It’s not, so your interpretation of the 2nd law of thermodynamics is off.
And I don’t understand because you agree with that absorption and re-radiation of longwave energy happens – which raises the temperature above what it would be without these gases..
-Perhaps we can leave this part of the debate here and others can form their own opinions.
Mike Kelly
You raise an interesting question with a huge flaw..
What the radiative forcing increase of 1.6W/m^2 means is actually that the top of atmosphere longwave increase since pre-industrial times is 1.6W/m^2.
Suppose we take 1.6W/m^2 and heat the oceans – the major heat store – and ignore melting ice and warming of air..
What happens?
Let’s look at the oceans:
-Mean depth = 4km (4000m)
-70% of earth’s surface is covered by ocean so let’s say 1.6/0.7 = 2.3 W/m^2 going into the oceans
-Density is approximately 1000 kg/m^3
So each square meter of ocean has a volume of 4000 m^3, and therefore a mass of 4×10^6 kg.
Q = mc x dT
Q is energy, m is mass, c is specific heat capacity = 4.2 kJ kg-1 K-1,
dT = change in temperature
We have energy per second (W/m^2), so change in temperature per second, dT = Q/mc
dT per second = 2.3 / (4×10^6 x 4.2×10^3)
= 1.4 x10^-10 ‘C/second
dT per year = 0.004 ‘C/yr
—-
Let’s suppose – more realistically – that only the top “well-mixed” 100m of ocean receives this heat, so we would get (just scaling by 4000m/100m):
dT per year = 0.17 ‘C per year.
An interesting result.
So after 100 years the temperature increase will be 17’C
And after 1000 years the temperature increase will be 170’C.
Clearly radiative forcing can’t exist…
Or maybe there is a misunderstanding about how to apply it?
What we can do is use W/m^2 to look at how quickly that will warm the planet – we can investigate the very important subject of thermal lag
What we can’t do, is just use the formula for heat capacity and work out a new temperature – because as the climate warms up, it radiates more heat.
Which you can see in Part Seven, at the end.
Isn’t that 1.6 * 0.7 ?
Dan Hughes:
No. Here’s the reason why.
If the energy is divided into land and sea and ice – equally all around the world, the energy is 1.6W/m^2.
Now we are saying – just for the sake of this thought experiment – that all of the energy goes into heating the oceans.
If the oceans were half the surface area of the globe (50%) then the energy going into the ocean would be double = 3.2W/m^2. The energy flowing into every square meter of land diverts/flows on instead into a square meter of ocean.
Because the oceans are about 70% of the earth, 0.7, we multiply up by less than 2, by 1/0.7=1.4.
No wanting to labor the point.. but another way to look at it for sake of completeness:
Total energy,T (in this thought experiment):
T = 1.6 * S (S= surface area of the earth)
This total energy is all absorbed by the oceans. How much energy per m^2 of oceans?
E(total ocean) = T (because all the energy flows into the ocean, so this is just the definition)
E(per m^2 of ocean) = T/area of ocean
Area of ocean = S*0.7
E(per m^2 of ocean) = T/(S * 0.7)
What’s T? T= 1.6 * S (above)
So E(per m^2 of ocean) = (1.6 * S)/(S*0.7)
=1.6/0.7
[…] The IPCC, drawing on the work of many physicists over the years, states that the radiative forcing from the increase in CO2 to about 380ppm is 1.7 W/m2. You can see how this is all worked out in the series CO2 – An Insignificant Trace Gas. […]
[…] yet there is a whole series on CO2 – An Insignificant Trace Gas? where the answer is “no, it’s not insignificant”. Doesn’t that support AGW? […]
[…] an earlier series, CO2 – An Insignificant Trace Gas we delved into simpler numerical models. These were 1d models. They were needed to solve the […]
[…] For a detailed explanation of these points, see the CO2 – An Insignificant Trace Gas? series at The Science of Doom https://scienceofdoom.com/2009/11/28/co2-an-insignificant-trace-gas-part-one/ […]
[…] the CO2 series for a little more on this if you wonder why it’s longwave getting radiated out and not […]
[…] This article will cover the first paper which appears to be part of a conference proceeding: Changes in the earth’s resolved outgoing longwave radiation field as seen from the IRIS and IMG instruments by H.E. Brindley et al. If you are new to understanding the basics on longwave and shortwave radiation and absorption by trace gases, take a look at CO2 – An Insignificant Trace Gas? […]
[…] These numbers are global annual averages under a clear sky. Under a cloudy sky the numbers are different but similar – and still the radiation from the surface of the earth is a lot greater than that leaving through the top of atmosphere. For more on this take a look at CO2 – An Insignificant Trace Gas? – Part Six – Visualization and the followup CO2 Can’t Have That Effect Because.. as well as the start of the series on CO2. […]
[…] of longwave radiation by trace gases – the “greenhouse” effect. See the CO2 – An Insignificant Trace Gas? series, and especially Part Six – Visualization and CO2 Can’t Have that Effect […]
[…] are a few basics. For newcomers, you can take an extended look at the theory in CO2 – An Insignificant Trace Gas? It’s in seven parts! Actually it’s a compressed […]
[…] radiative transfer equations using line by line calculations. For more on these equations, see the CO2 – An Insignificant Trace Gas series, especially Part Three, Four and […]
Question: how can we be sure that we have accounted for all the elements of the energy balance equation?
I think radiation is the easiest mode to model. But I would imagine that orbital asymmetries deform the earth and those deformations result in heat. Also, we have an iron core spinning, precessing, orbiting and wobbling in the presence of a varying magnetic field — producing inductive coupling, which also trasfers energy and results in heat.
Given this, why do we say that the only mode of inbound heat transfer is EM wave radiation?
[…] Anyway, it’s just a mental picture I wanted to create. It’s not a perfect mental picture and it’s just an analogy – a poem, if you will. If you want real science, check out the CO2 – An Insignificant Trace Gas Series. […]
gcv:
Orbital asymmetries cause small fluctuations in the solar energy received – or at least the location (latitude and time) of maximum and minimum energy. And the % variation during the year of the solar energy received.
Energy received from internal processes – mainly heat – is miniscule by comparison with the approx 240W/m^2 of solar energy absorbed over the surface of the earth (averaged globally and annually).
There is much more uncertainty about how much energy is actually absorbed by the earth’s climate system – as 30% “or so” of the incident solar radiation is reflected.
“Orbital asymmetries cause small fluctuations in the solar energy received – or at least the location (latitude and time) of maximum and minimum energy. And the % variation during the year of the solar energy received.”
I’m not about about changes in solar energy, I’m asking about inductive heating of the core. Do you know (or could you point me to) the amount of heat generated by this inductive effect?
gcv:
You are asking if inductive heating makes its way out to the earth’s surface to affect climate?
Geothermal energy is very small, believed to be 0.06W/m^2.
If you are asking about inductive heating just heating the core and not affecting climate then it’s not really a topic of interest for me unfortunately.
It is my understanding the the 1365 W/msq value is at the top of the atmosphere and that even in the solar spectrum there are significant water vapor and dust “blocks” to this flux.
I ran a solar furnace for several years and we used a calibrated normal incident pyrheliometer NIP to measure surface solar flux. The best we ever saw here in the southern NM desert was about 1100 W/msq during very low humidity days in the fall. Summer NIP readings (on clear days) were rarely above 950 W/msq. During one period we watched the NIP readings slowly drop to a steady 100 W/msq below normal as the cloud of ash that was blown into the stratosphere spiraled around the earth to finally block the view of the sun. This drop lasted for months and did slowly dissipate. Is there something wrong with “my” reality or is there something wrong with your theory (of insignificant solar [SW] atmospheric blockage)? Especially by water vapor (not clouds).
Bernie
Bernie McCune
You are spot on. The earth’s albedo, made up of clouds, certain types of aerosols and reflections from the earth’s surface is around 30%. From the article:
I think your average 30% figure is good for solar noon on many days of the year here in NM but I would guess that much less of the 1365 W/msq found at the top of the atmosphere gets to the surface here primarily due to components of water (vapor and clouds).
From my experience of a couple of years (1980-2) the local and regional solar surface flux averaged less than 70% from flux at the top of the atmosphere (and for some periods of months even much less). During solar noon surface flux was on most days 70% (of TOA irradiance) but early in the morning until about 11 am and from 2 pm to sunset it probably averaged 40 to 60% (more atmosphere including water to penetrate?). Maybe 20 days in the Fall at solar noon it was 80%. The volcano I referred to in the previous post was Mt. St. Helens.
Anyway the point is – the average regional solar surface flux is drastically variable throughout the day, the year and from year to year – primarily due to water vapor and clouds. Throw in a volcanic eruption and at least a drop of 100 W/msq for many months can almost be assumed.
Bernie
Bernie McCune
Take a look at Earth’s Energy Budget – Part One and a new part, to be added shortly, will cover albedo in some more detail.
The 30% is a global annual average. There’s a little more about albedo already, but in a post on a totally different topic, Positive Feedback, Albedo and The End of All Things
I have been looking deeper into the material here and welcome a clear relatively simple approach to explaining these concepts. My initial effort was to get a clearer explanation of the greenhouse theory. I will spend some time working here on this site and get back to you in a month or so – ha ha.
I am also trying to reconcile my earlier experience with surface solar irradiance values with these other more complex issues of atmospheric thermodynamics. Rather than continue into this discussion which may too quickly drift into opinion, I will go away and study this more. Thanks for having a place to do this without all the smoke and mirrors.
Bernie
[…] This concept can be found in CO2 – An Insignificant Trace Gas? Part One […]
[…] where most of the longwave radiation takes place from the earth’s surface. Check out CO2 – An Insignificant Trace Gas? for more on […]
[…] affect anything in climate – this post isn’t for you – check out the CO2 – An Insignificant Trace Gas? […]
gives a fairly comprehensible explication of
Miskolczi’s work with an emphasis on the
way recent data accords with it. In particular,
the point is made that the climate is homeostatic
with respect to
g = 1/3
I will elaborate around that simple result:
g = 1/3 in the actuality of a vast amount of data
g = 1/3 in the theory of Ramanathan.)
Overall, the climate is a homeostatic system
which maintains g = 1/3.
The climate’s primary business is to maintain
a particular energy balance. The “name”
of that energy balance is “g = 1/3”.
The role of CO2 is incidental to maintaining
the energy balance at which g = 1/3. CO2 is
a relatively minor player in the overall climate
picture from a thermodynamic standpoint.
The temperature sensitivity of the global temperature
to a doubling of CO2 is way less than the IPCC
predictions.
I found Miskolczi’s paper hard going and it
may well have an error or two in it. One should not,
however, IMHO, get too hung up on Miskolczi’s slip ups
because it seems to me that there are alternative
ways to arrive at Miskolczi’s intermediate results. The thing to remember is that Miskolczi
is a physicist who works from data towards theory.
His constant optical depth theory was the result of
his long study of actual atmospheric data that had
been accumulated over quite a span of time.
The central result of all this data was that
g = 1/3, exactly what Ramanathan said it
must be. Miskolczi say that as being more than
coincidence.
IMHO, if one can really get a solid grasp of
Ramanathan’s
g = 1/3
, one can then extend to Miskolczi’s
central result
that
2 = tau + ( exp ( -tau ) )
(where tau = optical depth of the atmosphere)
g = 1/3 and
2 = tau + ( exp (-tau ))
go together
G.N. Plass in 1956 worked out the effect of increasing CO2 concentrations in the atmosphere. He found that doubling the CO2 concentration in the atmosphere would cause a 3.6 oC rise in the earth’s temperature. The temperature increase is much like we have been observing. See http://www.aip.org/history/climate/co2.htm –
I have a couple of question that I’ve yet to see answered. In the 1940’s, a nice fellow named Hottell did a bunch of work on radiant heat absorption by water and CO2 in the atmosphere. In the 1970’s, Hottell’s curves were updated by another nice fellow named Leckner. If one uses these curves to ascertain the emissivity of the atmosphere, and then calculates q, one comes up with a curve that is remarkably similar to the simplification of F=5.35lnCO2. In reading Ramanathan, there is no reference to any of this. He seems to have reinvented the wheel. Can anyone explain two things:
1: When I was taught heat balances (3rd year engineering), we were taught that radiative and convective transfers were independent. Did Ramanathan provide a justification for choosing to attempt to couple these?
2: Why was the HUGE amount of work on radiant heat transfer in the atmosphere, done prior to Ramanathan, never referenced by Ramanathan?
Just found your site today via CA. Enjoyed the radiation emission/absorption background. It was a well done presentation; however, you didn’t provide the maths to support the contention that LW radiation back to the earth was 55 – 75% H2O and 25% CO2. Are those numbers empirical? If they are, wouldn’t some if not all of the 25% CO2 LW be the result of H2O reradiating at a longer CO2 wavelength?
The answer to this connundrum will determine whether CO2 is a significant contribution to the GH effect as you claim. The ppm saturation point of CO2 is critical to determining the effect of increasing concentrations of CO2.
E.F. Zeamba
The explanation is in Part Five of the series.
Feel free to post a question there on any specifics. The numbers are calculated by solving the radiative transfer equations, which you will see in Part Three
John Eggert
He doesn’t really couple them. How do you work out the radiation of each layer in the atmosphere? It depends on temperature of that layer – as well as the concentration of each trace gas. How do you work out the temperature?
If you calculate the radiative equilibrium it isn’t what you find in the atmosphere because the temperature profile (lapse rate) is determined by convection (see, for example Tropospheric Basics )
So his justification for using the “Radiative-Convective Model” is that when the calculated lapse rate is lower than what is found (more negative) then the lapse rate is set to the known convective profile. And when the radiative lapse rate is high than what is found the temperature profile is set to the radiative calculation.
This allows a calculation of the absorption and emission within the atmosphere without needing to solve convective fluid calculations. You can see more about this paper in Part Five
I took a look at a paper by Leckner: Spectral and Total Emissivity of Water Vapor and CO2 (1972). In it his curves for emissivity go down only to 300K, whereas the atmosphere is mostly below 300K
He says:
and cites RM Goody, Atmospheric Radiation, vol 1, Theoretical Basis
Ramanathan cites RM Goody including this same volume. Goody does seem to be the source for this kind of work.
Perhaps some coincidence – because the solution to the radiative transfer equations requires emission and absorption using the temperature profile and concentration profiles for each trace gas. You can see the source of this 5.35lnCO2 equation in Part Seven
Holy smoke, someone actually answered a good many of my questions. Steve M. at climate audit was right, this is an excellent site.
As for your questions, I have written a paper outlining a method for calculating the path length (as defined by Hottell and Leckner) of the atmosphere. e-mail me if you want it.
Regarding temperature / lapse rate, I ignore it (well … consider it constant at 300K). Here is why.
The temperature variations in the atmosphere are relatively small compared to the ranges considered by Leckner and Hottell. 10s of degrees for the atmosphere, thousands of degrees for Leckner and Hottell. Also, the projection from Leckner’s data is very small. The critical aspect of Leckner’s work is that he showed that there is a point (a path length of about 200 barXcm) where further increases in CO2 concentration have no further impact on radiative heat transfer. This is consistent over a huge range of temperatures. The absolute emissivity at various temperatures varies a little, but the fact that there is no change from 200 to 500 barXcm is consistent over all temperatures. The change is insufficient to match the increases hypothesized by Ramanathan. Unless of course there is some huge deviation in the absorbance characteristics of CO2 that occurs from 200 K to 300 K compared to 300 K to 3000 K. This is possible and indeed provable (and disprovable, so to speak). I believe Hottell’s curves go to 0 F (255K). They do not show such a deviation.
Finally, no coincidence. This is exact the point of Hottell’s work. Provide a robust an simple solution to the radiative transfer problem without requiring a solution to the equations.
Once again, thanks for taking the time to indulge me.
I would appreciate receiving your paper
via email.
John Eggert
Which paper by Hottell should I be looking at to see his solution to the radiative transfer problem?
Firstly – great site! You have obviously put a lot and time and effort into this and I for one greatly appreciate this – it explains the science (to me at least) in a many which I can understand and make sense of.
I have a couple of question in order to help my understanding (points of clarification really).
Referring to the graph showing the theoretical “earth radiation curve” and the measured actuals (the one referenced from Grant Brigg 2003). You conclude that the difference between the observed and the theoretical curves (the shaded part) is a measure of the energy absorbed by the atmosphere. Inaddition, as we can work out which gases absorb at which frequencies we can determined the relative proportions each gas contribute to the “Greenhouse Effect”.
1) Based on the above is the “conclusion” that ~20% of the effect is due to CO2. i.e. 20% of the shaded area is at wavelengths where CO2 is the main / only absorbing gas? N.B. I have heard the 20% figure “banded around” on many sites and wondered if this is the scientific basis for this?
2) At ~15mico-meters there is total absorbtion. I assume this means that the CO2 blocks all energy at this wavelength (e.g. the door is shut!). By looking at the wavelengths that CO2 absorbs the energy and seeing how much energy currently gets through can we not determine the “maximum” effect CO2 can ever have? E.g. simply work out the amount of energy current not being absorbed at these wavelengths and this will give us the maximum impact etc? If this is correct has this been calculated?
Would love to be able to get a copy of the data for the graph to be able to do some simple analysis on it e.g. workign out the areas etc. Don’t suppose you know if this is in the public domain?
Once again many thanks for any help / insight you can give.
Regards
Neil:
Not really – this is just Part One of the series. By the time you hit Part Five you will see that the calculation depends on properly solving an equation which is complex. But don’t skip the parts in between..
Yes we can.
But if you work through the series you will see that even though CO2 might absorb strongly, each layer of the atmosphere also re-radiates (according to temperature and concentration of each trace gas).
The effect of doubling and quadrupling CO2 is outlined in Part Seven and is calculated by solving the radiative transfer equations.
Much better to properly understand the theory and how it is applied. However, you can see one example of the changes from doubling CO2 in the second set of graphs in CO2 in the Solar Spectrum
Science of Doom:
You asked where you could find Hottell’s papers. I can’t tell you for sure. My reference is a text book written in the 1950’s. The references are generally Hottell and ??Sarafin??. The work was done at MIT (I believe) in the 1940’s. Sorry I can’t give better at the present time. I’m working out of town and don’t have access to my hard copy notes.
I’ll post again when I get back home on Friday.
Science of Doom:
A friend has a heat transfer text, so was able to find the reference for Hottel.
Hottel H.C. & Egbert R.B.: Radiant Heat Transmission From Water Vapour: Trans AIChE, Vol 38, p531, 1942.
John Eggert
I tried to find this paper, but unsuccessfully. If you have a copy I would be very interested to see it. It would be interesting to see the method and results vs the later methodology.
Science of Doom:
You asked about the paper, results etc., versus later methods. I don’t have access to the paper (probably need to find a hard copy in a University library somewhere). The results of the paper and the methods can be found in most texts on heat transfer in the section on radiant heat transfer. Some texts still use Hottel’s curves (note that I spelt Hottel incorrectly previously). Others have switched to Leckner’s curves. I’ve reviewed Hottel’s curves and see that they DO NOT go to 0 F. I was definitely wrong about that. Need to be careful of statements without authoritative references handy!
John Eggert
That might explain why Ramanathan and others of the same era didn’t cite Hottel.
I’m still intrigued by the calculations of Hottel that match the later results solving the RTE. If you come across the paper please post a link.
The graph you borrowed:
“Radiation spectrum from the earth showing absorption from atmospheric gases”
appears to have some problems, and if I am right they are all too common with graphs of this type.
The vertical scale has the units W/m^2, which does not make any sense. If one judged by the horizontal scale (microns) one would expect W/m^2/micron or W/m^2/micron/steradian.
But then another problem is that the spectral peak is at or around 17microns which is I think correct for the radiance as a function of frequency (the spectral peak for radiance as a function of wavelength would I feel be at around 10microns).
This indicates to me that the values are derived from the frequency form of the function but a horizontal scale in the equivalent microns substituted when it came to making the graph. I am afraid that this does make a difference.
I have fiddled with the values as produced from the frequncy form of the radiance function to try and come up with the values indicated on the graph but I cannot. To be honest I do not know how they are scaled.
Compare this to your graph:
Radiation vs Wavelength – Sun and Earth
which does appear to have the right values and scales.
A small point that is just a “reader beware”: the flipping to a logarithmic scale can confuse as the integral of the radiance no longer corresponds to the “visual” area under the graph but that is a small point and is as I said just a case of “reader beware”.
Alex
Alexander Harvey:
You are right about the graph, thanks for pointing it out. I will replace it with a better one.
scienceofdoom:
OK, but when you do replace it, could you please annotate my entry to show that I am talking about something that has disappeared.
Alex
Hottel and Leckner data is intended to be used to calculate heat transfer in things like boilers and flue gas where the temperature is high and the concentrations of CO2 and H2O from fuel combustion are also high. Using that data to calculate atmospheric heat transfer is extending it far beyond its intended purpose. I’m not sure how much is empirical measurement and how much is an actual solution of the RTE either.
The principles are the same, DeWitt… Laws of thermodynamics works everywhere.
scienceofdoom:
I have to say that I think you be very brave to try to boil this kind of stuff down into bit sized chunks.
It ain’t easy. Some of the concepts are very slippery and promoting understanding vs bewilderment can turn on the interpretation of a single word; one example being equilibrium.
Anyway Good Luck, and Bon Voyage.
Alex
scienceofdoom:
Have been searching for absorption spectras regarding greenhouse gases and found a lot of graphs. One of them led to this site.
I have a question for you about the graph from Linacre and Geerts. The graph shows no absorbtion from H2O for longer waves than about 8 microns and that CO2 absorbs a lot from about 14 microns up to where the graph ends.
Several other graphs show that Co2 absorbs between 14-16 microns and nothing at longer waves. They also show H2o absorbing from 10 microns, increasing up to about 16 microns and then “full” absorption up in the microwave interval.
One example here: http://www.brneurosci.org/spectra.png
There are other graphs showing the same thing.
These frequencies are clearly important in the earth longwave radiaton spectra so this really has to matter.
Since I am far from an expert on the subject it would be helpful if you could explain the difference between the graphs.
Best regards
/G
Greg M:
Thanks for asking the question. I think the Linacre and Geerts graph isn’t so good and I’m glad you pointed it out. When I did this first post on CO2 I had some handy basic material that I used but just a few days ago Alexander Harvey (above) pointed out correctly that the Grant Bigg graph was flawed and now you have pointed out that the L&G graph is quite basic.
Both need replacing with better quality data otherwise they can cause confusion.
For water vapor the absorption across the longwave spectrum is very extensive. The subject gets “simplified” and perhaps that makes it harder to understand.
Here are two plots from http://spectralcalc.com/spectral_browser/db_intensity.php which uses the HITRANS database. You can play with it yourself.
The first graph is linear on the vertical axis, and you can see where water vapor is strongest. The second graph is logarithmic on the vertical axis and you can see that it has an effect across most wavelengths:
Of course, for people less used to seeing logarithmic graphs they can also confuse as each horizontal line represents a factor of 100.
Scinceofdoom,
thank you for your answer and the link to spectralgraphs.
I realize I´ll have to study this subject a bit more.
/G
I have been looking for a mathematical overview of global warming that develops the key equations & the physical underpinnings so I am delighted to find at this site exactly what I was looking for!
[…] is the long-promised eighth part of the seven-part series on CO2 basics. Part One introduced the idea of CO2 with some basic concepts. Part Three opened up the radiative transfer […]
[…] 30, 2010 by scienceofdoom In the CO2 series we looked at the effect of CO2 without climate feedbacks. The “answer” to the doubling […]
Science of Doom.
Thanks for an informative series. I am hoping you can help clear up some conceptual issues I have.
Where (at what altitude above the surface) in our atmosphere is downward ghg LW being radiated from?
My understanding is that the atmosphere cools by 7 deg C for each 1000m rise in altitude. If this is so, heat must be moving away from the earth, and not towards it.
Often one hears that ghg acts like a blanket trapping escaping heat and the re-radiating it in all directions. Is this a useful metaphor? If so, what proportion of LWR is directed downwards, and do your calculations take this into account?
cherry picked:
It varies depending on the wavelength – and in the case of water vapor, it depends on the concentration profile of water vapor. And it’s also important to realize that there will be a range of heights from which radiation will be coming. For some conceptual understanding on this, even though it is about the outgoing radiation, see The Earth’s Energy Budget – Part Three
The surface is hotter than the atmosphere. But the amount of heat received by the surface is the same as the amount of heat removed from the surface (otherwise it would be heating up or cooling down).
The same goes for the atmosphere.
The surface receives radiation from the sun (during the day) and from the atmosphere (24 hrs a day) and loses heat (to maintain balance) by radiation, by convection and conduction. Included in convection is latent heat (evaporation of water).
Note that strictly speaking this is considering a part of the surface in steady-state, but the concepts don’t really change for surfaces which are heating up or cooling down.
So the fact that the atmosphere is colder the higher you go doesn’t mean that no radiation is downward. Gases which absorb radiation will radiate in all directions.
For some more background take a look at Tropospheric Basics and Sensible Heat, Latent Heat and Radiation
I don’t like the metaphor myself because a blanket doesn’t really explain why solar radiation goes right through the atmosphere, but various trace gases absorb the terrestrial radiation.
But the second part is correct.
A simplistic way to think about it is that half is up and half is down. But in fact because the radiation is in all directions, the radiation from one layer in the atmosphere will radiate to many locations on the ground and less than half will be absorbed by the surface.
The full solution to the radiative-convective model (see CO2 – Part Five ) takes these factors into account.
Thanks for the considered response, SOD.
I will read the links you provided before asking folllow questions, if any.
Thanks
[…] attacking skeptics and defending questionable science like the famous hockey stick. The series of articles on CO2 as a greenhouse gas I would recommend to anyone and certainly have been useful to me better understand […]
[…] of 230K (-43°C). The same calculation for the earth gives 255K (-18°C) – see CO2 – An Insignificant Trace Gas? – Part One. So in terms of a simple energy balance with the sun, Venus should be colder than the […]
NASA has flown some interesting instruments that may help with understanding the radiation. These pictures were taken in the central US in 1996.
http://mas.arc.nasa.gov/gallery/comparison.html
The photos are from the visible (0.55 um) to far infrared (14.21 um). They appear to be calibrated but the details are not given. One easily notes the slight increase in land brightness as you go into the near infrared (0.7 to 1.75 um) as should be expected by anyone who has used IR photo film. Plants get very bright in this range. The clouds are white and reflecting in the range from 0.55 to 2.40 um. The land gets dark around 1.8 to 1.95 um and then brightens up again (perhaps ground vapor in the H2O band near 1.8). At 2.89 um the band goes gray, perhaps the CO2 absorption band. And surprise above 3.21 um, the clouds start to go dark and the land starts to become very bright. I would say we are starting to see the “blackbody” radiation from the land here.
Interesting how the clouds reflect the visible and near IR but go dark in the far IR. They appear to reflect most the incoming solar radiation but would absorb and re-emit the ground radiation.
The question I have is: why don’t we clearly see the CO2 absorption if it is such a problem? Perhaps the gray-out is CO2 at 2.89 and 14.21 um. We sure don’t see it obstructing the bands further out in the IR where the land is very bright but the clouds sure do. In fact, it is possible that the solar radiation is much smaller here and we are mostly seeing the outgoing land radiation and the clouds are simply dark because the sun doesn’t reflect much far IR off their tops. In this case I would expect the bottom of the clouds to be reflecting some of the far IR back down to the ground.
Perhaps SOD can comment on these sensor photos.
Here is a high resolution false color image of the IR images I previously posted (an area north of Denver):
We can now see that there are shadows to the lower left of clouds, jet contrails and some small water features (lakes or reservoirs). This helps in the analysis of the original spectral bands at lower resolution:
http://mas.arc.nasa.gov/gallery/comparison.html
I believe that the following analysis is correct, but as I am not an expert (only a BS in physics), some of the details may be wrong. Take it as an opinion.
It is noted that the spectral band images are calibrated for radiance and I will assume that equal brightness will mean similar radiation power flux (although radiance is not exactly equal to flux due to vector angle differences). These were shot by an E2 (modified U2) and could be from about 70,000 feet, but this is not mentioned in the data description. The fact that large jet contrails are in the images suggests it was much higher than the 40,000 feet that jets fly below. There is also very little atmosphere above this altitude so most of what we see will be similar to a view from space. I also assume that the grayed-out images at 2.89 and 14.21 um are caused by CO2.
In the incoming Sun shortwave flux the dark areas will indicate absorption while light areas indicate reflection back to space. For the outgoing “blackbody” longwave flux (above about 4 um where the cloud shadows disappear because the land is so bright) the bright areas indicate emission while dark areas indicate lower emission. The Sun has very low output in this region so the clouds will appear dark (reflecting very little IR from the Sun compared to the emissions from the land below).
While bands above 14.21 um were not captured by the sensor, they should look similar to 14.21 um. This is because CO2 has strong absorption in this region. There is still about 25% of the total longwave emission in this area so this is where CO2 has the most effect on warming.
Concerning the different features in the images:
Land: strongly absorbs in the visible and near IR, strongly emits in the far IR. Bare land heats the most and also emits the most. Urban features would be worse (pavement, etc.).
Vegetation: strongly absorbs in visible bands, some reflection in near IR, strongly emits in the far IR but would be cooler than bare land.
Surface Water: strongly absorbs in visible and near IR (more so than land), doesn’t emit much in far IR until you get above 5 um. Appears to reflect or emit around 3.21 to 3.52 um and absorb around 4 um.
Water Vapor: there appears to be strong absorption near 1.9 um which may be surface water vapor.
CO2: Appears to absorb at 2.89 and 14.21 um. The fact that it is a gray fog seems to suggest that it is re-emitting some radiation downward or transferring it to the surrounding air as thermal heat. It it were saturated (no longer able to absorb and emit) it would be as bright as the land and more transparent.
Clouds: Highly reflective for most incoming Sun near IR. Appears dark in longwave IR but may still be reflective. The Sun’s output in longwave IR may actually be reflecting off the top of the clouds but is much lower than the flux from the land below. Provided the clouds reflect longwave IR (and there are suggestions that cloud cover causes heat retention) this should cause warming due to partial downward (diffuse) reflecting of the outgoing earth flux.
The real question concerning AGW: is the CO2 really that important? It appears to be above 14 um and surface water appears to have some output above 5 um (it is a cooler blackbody than the hot land and should have more output at longer wavelengths). Clouds could be a negative feedback because they reflect so much of the incoming flux, but they also may reflect some of the outgoing flux back to the surface.
The spectral images have been contrast extended so no real conclusions can be drawn without access to the raw data and knowing the calibration factors. However, they do provide a layman’s view of what is actually happening by showing the CO2 fog bands, incoming flux absorption, cloud reflection and blackbody flux out.
There’s a sentence in the article where it says “If you measure radiation above 5μm, you know it’s generated by the terrestrial system.”, which is utter nonsense.
A hot object radiates more energy at _all_ wavelengths than a cold object. This can be seen from the figure “Energy intensity versus wavelength for different temperature objects”. The figure “Radiation vs Wavelength – Sun and Earth” conveniently scales the energy from the sun by 10e-6 to hide this.
The sun emits much more radiation at 5μm than the earth does.
Symon
Well, take a look at The Sun and Max Planck Agree and the followup in minute detail: The Sun and Max Planck Agree – Part Two
Seeing as the sun is a long way from the earth, the solar radiation is reduced according to the inverse square law.
You can take the total solar radiation and divide it by 2 billion to get the amount absorbed by the earth, or alternatively, the solar radiation per m^2 and divide by 215^2 to get the amount per m^2 reaching the earth.
Anyway, take a look at the graphs in The Sun and Max Planck Agree – Part Two.
Thanks for your reply. The link makes clear that the 5μm statement refers to measurements made on or near the earth, which this article doesn’t mention.
Thanks again.
No trouble. Your comment prompted me to the long overdue overhaul of this article in attempt to explain everything more clearly.
The article has now been significantly rewritten. Earlier comments might confuse current readers if they refer to specific diagrams or calculations.
Thanks to the many people who have made a contribution by asking questions, asking for clarification or explaining their alternative point of view.
[…] 6, 2010 by scienceofdoom Just a note that CO2 – An Insignificant Trace Gas? – Part One has been significantly […]
I can see that a lot of work went into this revision,
and that is much appreciated. I will give this a
careful reading.
I still have the feeling that you have not yet
read Miskolczi’s papers, both the theoretical
and the experimental. If I am right in having
this feeling, then, to honor the commitment to
objectivity you seem to have made, you should
do so. I do not assert that Miskolczi is “right”, I argue that
he deserves to be heard.
As well as I am able, I make it my practice to listen
more carefully when I disagree than when I agree.
The lucid, orthodox exposition notwithstanding, an alternative approach which concludes that CO2 is after all an insignificant trace gas is worthy of consideration:
From K&T(1997) we see that the atmosphere emits to space 195 Wm-2 of the total 235 Wm-2 required to balance the net solar flux. It emits the heat that it takes in from the solar flux, 67 Wm-2, and from the surface: through evapotranspiration, 78 Wm-2; through convection, 24 Wm-2.; and, imlicitly, 26 Wm-2 through absorption by GHGs of surface radiation. Of the absorption, CO2 accounts for 26%. That is, CO2, which accounts for 0.06% of atmospheric mass accounts for 0.03% of the climate system’s radiation to space. The possibility, canvassed by K&T, that the atmosphere absorbs 25 Wm-2 more of incoming radiation than they have allowed, reducing absorption of surface radiation by GHGs to 1 Wm-2, virtually relieves CO2 of any significance in the climate system.
Correction: for 0.03%, read 3%.
John Millett:
I don’t understand your calculation.
What do you think the surface would radiate (global annual average) if there were no “greenhouse” gases?
What do you think the surface radiates in our current climate?
The surface radiates at an intensity determined by its own temperature and the temperature of the sink to which it radiates. The smaller the difference between these temperatures, the lower the intensity of radiation. If, as I have seen stated seemingly with some authority, most of the absorption by greenhouse gases of surface radiation occurs relatively close to the surface in the turbulent boundary layer, it seems likely that the temperature difference would indeed be small. This would be in accord with the analysis above of the K&T energy budget showing that radiation plays a small role in the transport of heat from the surface to the atmosphere for ultimate radiation to space.
In that tantalising imaginary world without life-essential CO2 and H2O, molecules with the added property of wavelength-specific absorption of radiation, would the sink temperature be much different? Not really, since the atmosphere gains its energy from the sun, directly by absorption and indirectly by non-radiative means of transport from the solar-heated surface. This imaginary atmosphere, the absence of its IR-absorbent molecules notwithstanding, would continue to diffuse the heat thus gained by radiation to space. The imaginary earth would be uninhabitable for lack of food and water not lack of shelter from the cold of deep space.
I suspect that the difference between the AGW view and this one reflects the apparent conflict between Kirchoff’s Law equating absorption and emission and the more general thermodynamic theorem that all matter with temperature above absolute zero radiates. Something to get your teeth into, scienceofdoom?
Hi, thanks for the update, it’s very clear now.
BTW., the reason I came to read this article a few days ago was because of an article on WUWT entitled “CO2 heats the atmosphere…a counter view” in which the author, Tom Vonk, says “Internet sites that are said to have a good scientific level like “Science of doom” publish statements similar to those quoted above . These statements are all wrong”. Anyway, I thought you might be interested.
[…] is composed of gases that can’t radiate any significant heat – N2 and O2. As shown in CO2 – An Insignificant Trace Gas? the absorption and emission ability of these gases is more than a billion times less than water […]
how do you calculate respiration rate from a photosynthesis over PAR graph?
and how do you convert ul O2 g-1 min-1 to umol CO2 m-2 s-1 ?
should the respiration rate of a shade plant be greater than a sun plant in low light intensities? 20 – 60 PAR?
I’m unsure as to whether or not this is the proper place to ask this question, but…well, if not, my apologies.
First, am I correct in thinking that if Earth’s atmosphere had no greenhouse gases present, nothing that absorbed or emitted longwave IR at all, it would still show a typical blackbody spectrum for a gas at its temperate? And if so…well, why don’t gases like nitrogen absorb longwave IR? I believe I understand why they wouldn’t be able to absorb the energy to alter their vibrational energy levels, since the molecules are symmetrical, but why couldn’t the energy be absorbed to change their translational energy (I presume, in a normal blackbody, that’s where the emissions come from? Changes in translational energy as a result of sudden collisions between molecules, etc?) I would think that if the gas is capable of emitting light of a certain wavelength as a result of blackbody emissions, it should also be able to absorb it. That’s obviously not the case (if it were, nothing would be transparent to any wavelengths of light), but I don’t understand why not. If anyone can help me with this, many thanks.
Sam Yates:
If the atmosphere had no “greenhouse” gases present then the satellites orbiting the earth would see the blackbody spectrum for the surface of the earth – from the particular area the satellite was measuring.
The atmosphere would be completely transparent to the radiation from the surface of the earth.
If a gas can emit light of a particular wavelength it can absorb it. This is correct.
I think you are probably confused about “blackbody emissions”. What does this mean?
It means that a perfect emitter/absorber – called a “black body” – radiates according to Planck’s law.
No gas radiates like that. Gases have “discrete wavelengths” that they can emit or absorb at. Well, in practice these discrete wavelengths are broadened slightly for a number of reasons.
Take a look at Part Two for more about the characteristics of different gases.
Hm. I think that helps a bit, but I’m still a bit confused. I’m correct in thinking that, although Earth’s atmosphere is far from being a true black body, the light emitted by it roughly follows the expected curve for an object at its temperature, yes?
In Part two, you wrote that: “The layer itself will act as a blackbody and re-radiate infrared radiation. But it re-radiates in all directions, including back down to the earth’s surface. (If it only radiated up away from the earth there would be no “greenhouse” effect from this absorption).”
If I’m understanding this correctly, then, much of what’s doing the re-radiating is ordinary oxygen and nitrogen that have had the energy from excited CO2 (and other greenhouse gases) transferred to them by collisions. Is that so? Or does the energy from the greenhouse gases just get spread out only to other greenhouse gases (in which case, why do you get a curve at all, instead of just a bunch of somewhat-blurry peaks?)
And by the way, thank you for putting so much effort into this site; it’s clear, concise, and has helped clarify a lot of details of the greenhouse effect for me that realclimate.org (although a wonderful resource) hadn’t really made clear.
John Millett on August 15, 2010 at 1:10 am:
Not true unless you have overturned 100+ years of physics.
The surface radiates at an intensity determined by its own temperature and nothing else.
Here is the famous Planck equation for intensity of radiation:
The values of h, k and c0 are constants. T is absolute temperature and λ is wavelength.
You will notice that there is no variable in this equation for “temperature of where the radiation might end up“.
Thanks for that, sod; confusing intensity and heat transport wasn’t smart. What’s your take on the principal point of the post?
Sam Yates
Thanks for the kind comments.
I think I need to review some of Part Two. Probably some careless writing on my part.
On the other parts of your question, I have seen many other similar questions from other people so I will write a post on the topic.
John Millett:
I can’t work out the main point of your question. Maybe you can try and explain it another way?
But this is wrong. If the atmosphere can’t absorb or emit radiation how does it “diffuse” heat to space?
This, precisely, is the point. You say there can be no radiation to space from an atmosphere without the 1% of it capable of absorbing and emitting radiation. So, what does the other 99% do with the heat it gains from the sun-warmed surface by non-radiative means? Since it constitutes matter with a temperature above absolute zero wouldn’t it radiate it away to surrounding space ( the atmosphere, after all, is mostly space as your bycycle pump exercise on another page demonstrates)? If not, it would have to accumulate heat until an isothermal atmosphere is achieved. This world would be hotter than the real one, a direct contradiction of AGW hypothesis.
John Millett:
If the atmosphere can’t radiate and absorb heat it can’t radiate and absorb heat. The atmosphere will reach an equilbrium condition via conduction and convection with the surface (which is radiating).
Have a read of The Hoover Incident, it’s written to answer this exact question.
The Hoover Incident
You conclude, conventionally, that the post-hoovering equilibrium temperature equals the effective radiating temperature of the net solar flux as a consequence of the OLR exceeding the incoming net solar flux. The energy imbalance would be reduced by taking account of atmospheric reflection of surface OLR; and lower surface emissivity values ( I note from your piece on emissivity that the near unity value occurs over a limited spectral range; and various emissivity tables elsewhere show somewhat lower values); and using day-night hemispherical averages rather than global ones. But these adjustments wouldn’t change the result. What would change it is the accumulation of heat in the atmosphere gained by non-radiative means from the surface (which would be greater than before, as solar energy previously absorbed in the atmosphere is switched to the surface, compensating for the elimination of direct absorption of solar in the atmosphere). As the surface and atmosphere move towards a new thermal equilibrium by non-radiative means of heat transport, the rate of radiative heat transport from the surface decays, falling to zero when the surface-atmosphere system becomes isothermal. The post-hoover world heats up continuously, directly contradicting AGW hypothesis – unless we relax the imposed constraint that the non-absorbing 99% of air can’t radiate. What law says that heat gained by non-radiative means can only be lost by similar means? Don’t room heaters gain heat by conduction and lose it by radiation? Why is thermodynamics different for air?
John Millett:
I don’t think you have followed the argument.
No law says that heat gained by non-radiation means can only be lost by non-radiation means.
Re-read The Hoover Incident.
The point is that if a gas is unable to emit or absorb radiation – what will the world be like?
If the gas is still able to absorb and emit radiation then that is a completely different situation.
In the confused comment that you write I can’t extract enough to assist you in understanding the subject of heat transfer.
Perhaps if you would like to address specific aspects that you don’t understand/agree with of the chain of events explained in The Hoover Incident – at that article I might be able to explain a few things.
Extract from The Hoover Incident:
“And no matter what happens to convection, lapse rates, and rainfall this cooling will continue. That’s because these aspects of the climate only distribute the heat. Nothing can stop the radiation loss from the surface because the atmosphere is no longer absorbing radiation. They might enhance or reduce the cooling by changing the surface temperature in some way – because radiation emitted by the surface is a function of temperature (proportional to T4). But while energy out > energy in, the climate system would be cooling”.
Convection etc distributes the heat in ways that affect radiative heat transport from the surface. While the intensity of radiation is a function of temperature, radiative heat transport is a function of a temperature difference. No heat may be exchanged between regions with the same temperature. In an isothermal atmosphere there would be no temperature difference between it and the surface and therefore no heat loss from the surface. The accumulation of heat in a radiatively-constrained atmosphere by non-radiative means of heat transport from the surface would produce an isothermal atmosphere. Then, energy out = 0 and < energy in and the climate system would be heating.
John Millett:
In the light of your comment, I’ll shortly post a new article trying to explain the basics a bit better. Watch this space.
Now posted – Heat Transfer Basics and Non-Radiative Atmospheres
[…] one commenter said in response to this (but in another article): Convection etc distributes the heat in ways that […]
[…] the surface of the earth would radiate at an average of around 240 W/m² (see The Hoover Incident, CO2 – An Insignificant Trace Gas? and many other articles on this […]
[…] The higher up you go, the colder it gets. The explanation is somewhat involved, so check out the link and also the series: CO2 – An Insignificant Trace Gas? […]
scienceofdoom I am very impressed with your website, excellent work. As a “skeptic” (those who call me that are now dubbed AGW extremists lol), of course I doubt the impact of CO2 on the climate, as H2O is by far the dominant greenhouse gas. Though you agree with me on this point, I suspect you think that CO2 is amplified by H2O.
So…
1) I would be interested in your hypothetical mechanism for amplification.
2) Could you please answer James Gibbons questions posted on July 25? He made some tough points that are worth of addressing.
Thanks, great site, and I hope to convert you to our camp soon. 🙂
Marcus Ortiz:
1. There are two obvious mechanisms that might cause positive feedback.
a) One is easy to understand and uncontroversial – snow/ice albedo. If more ice melts, then less solar radiation will be reflected.
However, this feedback is not so strong.
b) The second potential mechanism for positive feedback is more difficult to understand – water vapor.
There is a series on this Clouds and Water Vapor, which is currently up to Part Three.
It’s not a simple subject.
2. I didn’t really understand James Gibbons’ point.
Take a look at Theory and Experiment – Atmospheric Radiation, recently posted.
You will see one graph of top of atmosphere radiation, and one graph of downward surface radiation.
Maybe that article will answer some of your questions. But if not feel free to ask again.
You make the claim that without greenhouse gases the earth would be considerably colder, this is contrary to what I was taught about the water cycle. Which is, that without the greenhouse gases the earth would be 20-30 degrees hotter, because water vapour/water in the atmosphere has the effect of cooling the planet as it absorbs the great heat generated by the sun.
As your idea, that the earth would be colder, creates in effect the concept that greenhouse gases warm the planet I think this an important point to clear up.
Myrrh:
If the atmosphere did not absorb any radiation then the surface of the earth would end up radiating around 240 W/m^2 to balance the radiative heating from the sun.
Simple application of the first law of thermodynamics.
At the moment, the surface of the earth radiates, on average (globally annually) 396 W/m^2.
If the atmosphere was transparent to radiation then 396 W/m^2 would be lost to space.
And so the earth would cool down.
Check out The Hoover Incident.
Re the Hoover page,
“But without an atmosphere that absorbs longwave radiation there is no way that radiation from the surface can be greater than the radiation from the top of the atmosphere.”
– the sun isn’t the only heat source for the earth, so that doesn’t apply here.
Anyway, I was taught re the water cycle that the earth would be 20-30 degrees hotter, that it is the water vapour/water cycle primarily that cools down the earth, and makes life possible. This can be seen locally re deserts versus wetter areas, but globally as an ‘average’, it is the water cycle that takes out the greater heat and cools the earth via evaporation and rain, for example, which cycle is by displacement of hot air near the surface by colder air as gases heat and rise.
Myrrh:
If the atmosphere did not absorb radiation, then apart from geothermal activity what else are you thinking of?
If you are talking about water vapor? Well, we just posed the question – if the atmosphere (which includes water vapor) does NOT absorb radiation what happens?
Geothermal is estimated at way below 1W/m^2.
“What you were taught” isn’t gospel.
– you need to explain this, not state it.
What heat source?
Re the sun isn’t the only heat source for the earth.
Geothermal is a huge heat source, surely? Volcanoes are continually erupting, there are thousands of earthquakes going on in the land and in the ocean, the ocean boundary plates release great heat which rises to the surface, and so on, these can’t be insignificant in accounting for the difference. And, living matter itself. Plants give off heat, in transpiration, that’s all the trees, grass and other plants and then all the fauna. I don’t know what that is in total, but the planet and the life on it generate heat.
But my interest is still in this difference between your premise that the earth without greenhouses gases would be colder and what I was taught, that it would be hotter.
By saying “what you were taught isn’t gospel”, is a good reminder, for both of us.
However, the explanation as I was taught makes sense to me. If I can be convinced that the wisdom gained in science by the 60’s and 70’s has actually been overturned by present day science fact, then of course I’m prepared to change my view of it.
I was taught that the earth’s temperature, difficult as it is to measure such a thing anyway, was around 18 degrees centigrade, (65 degrees F), and it would be 20-30 degrees centrigrade hotter if there wasn’t the water cycle in our immediate atmosphere which reflects back into space some of the heat in one form or another, clouds, ice, and by the evaporation of water, which has a very high capacity to hold heat, which then rises up into the cooler layers of our immediate atmosphere where it precipitates out as rain, snow. Not just water vapour, so all gases in the atmosphere, as they heat they rise and redistribute heat energy around the planet, our winds.
In other words, I was taught the earth’s greenhouse effect, contra planets such the moon which doesn’t have an atmosphere, was what gave us the possibility of life on earth. The moon has massive extremes of heat and cold.
The earth’s atmosphere, its greenhouse, regulated those kinds of extremes; by stopping all the heat escaping it brought up the lower temperature it would be without the greenhouse and by blocking and releasing heat it brought down the higher temperature it would otherwise get to, the model was convection. As in a real greenhouse, when it gets too hot inside we open a window and release the heat. It made sense to me then and still does.
Plants and animals, all get their energy from the sun. Through photosynthesis, locking it in chemical bonds, and it is released through animals etc metabolizing it, breaking those bonds releasing the energy. But it should average out to be the same as the measured solar radiance.
Matter is energy, but atomic decay is the only way it is really released on earth in the planets core(maybe, also uranium etc do a little bit, but inconsequential in the larger scheme o things), the sun however has the mass for fusion en mass.
I seem to have jumped into two different aspects of the discussion when I thought I was only coming in to discuss one! Here it’s my off the cuff thoughts about the difference in energy received from the sun v the energy radiated back from the earth, which above is attributed to greenhouse gases making the difference as there is an imbalance, from earth the greater.
I think ‘geothermal’ is likely underestimated. Very little has been known about the heat generated from underwater; before the discovery of tectonic plates, the formation of crust, the ring of fire, all really very recent knowledge. There was something a few weeks ago about the discovery of more underwater volcanic activity than previously thought, and we’re learning more about what we thought we knew. Hawaii for example, we now know is a massive hot spot creating volcanoes and has thousands of underwater earthquakes a year, continually releasing heat (and CO2).
Plants, and animal life, don’t have a direct balance to energy received from the sun. Plants generate growing energy from food, water and carbon dioxide, besides needing blue and red light, so there are three sources of energy in this. In respiration they need oxygen and give out carbon dioxide and water and in that heat. The sun isn’t the only source of our energy on earth for life because as you remind, matter is energy. To establish a balance has to take that into consideration and also include time, for what is energy from the sun only will also take time to balance out.
So, my thought on this difference being attributed to greenhouse gases only is that these things have to taken into consideration and could account for the discrepancy at any given time, perhaps even for the majority or all of it?
One thing I’ve noticed is that descriptions of plant life in AGW gives the impression of a less than dynamic system, perhaps it’s just the wording, but describing plants as ‘carbon sinks’ appears to have confused some. I’ve had a couple of discussions where on mentioning that carbon dioxide is heavier than air have got replies saying that’s impossible because then our atmosphere would separate out into layers and we’d be walking around in a layer of CO2. It seems, from my limited forays into such discussions, that the concept of us and life on earth as carbon life forms and intrinsic to this the Carbon Life Cycle, is no longer taught. I don’t know which of us was more surprised the first time I came across this, me for discovering that this wasn’t taught, or those who had never heard that carbon dioxide was plant food..
Myrrh:
No, overall, it is a tiny heat source. Less than 0.1 W/m^2 (averaged over the globe).
So if the sun is the only significant heat source – and the atmosphere (including water vapor) didn’t absorb or emit radiation – what do you think would happen?
Well above reply to Mike, I think geothermal in all its forms could be very much underestimated, some say that the present cause of arctic ice melting is geothermal in origin.
I’m not sure I understand your question. Certainly the sun is provider of massive amounts of direct energy in the form of heat, but I’ve already said that without an atmosphere we would be like the moon, extremes of temperature.
Ah, OK, what I was saying at the beginning of our discussion is that I was taught something different from what you presented. You said that without greenhouse gases the earth would be colder, (I was taught hotter), so this is about ‘greenhouse’ gases specifically rather than the atmosphere in general.
Water is the main ‘greenhouse’ gas, so taking that out of the picture gives us a hotter atmosphere not a colder, mainly because water’s capacity to store heat and take it up higher into our troposphere, which also brings in colder air below it. ‘Averaged’ out, is what keeps what would be the greater heat in check globally, because without it the sun would still be heating up the atmosphere and earth. Locally, deserts are an example of this and easily compared with the cooler coastal regions because of this cooling effect of evaporation created by the sun. The earth’s surface is some 70% water and compared with what’s left as land, this makes it a major player in heat exchange.
How all this, the role of water, could be calculated mathematically I don’t know, because such things as cloud cover acting as a ‘blanket’ to delay heat loss from the earth, which helps in regulating the colder extreme temperature possible on earth, also has to be considered, and that’s a two way process, because too much cloud cover also cools by blocking the sun’s energy.
Overall though, I think the balance would go to making the atmosphere hotter globally without it as I was taught, the desert condition would prevail as we see locally. The other gases heating and rising and so creating colder air beneath just isn’t enough to cool a desert in the day time, while the heated earth is able to radiate that back out at night so keeping the atmosphere from going to extreme minus degrees – it can certainly be cold in the desert at night, but a good blanket or small fire sufficient for comfort.
I am a bit confused about the data from some of your graphs. From your ‘Wisconsin, Ellingson & Wiscombe (1996)’ source, we get an average value of about 133mW for CO2 radiance at the 15um band, as CO2 emits photon energy gained from earthshine.
However, when we look at earthshine in the ‘Outgoing longwave radiation at TOA, Taylor (2005)’ source, we see that it has a distinct drop at the 15um band. This drop is roughly 60mW, assuming the drop started at 0.10W.
If am am reading these graphs correctly, when we observe earthshine from space, we see about 60mW of energy missing at the 15um band, and correctly attribute this to CO2 absorption. But when we observe the atmospheric spectrum from sealevel, we see 15um radiation at 133mW power.
Since energy cannot be created or destroyed, where is the extra 73mW of power coming from?
[…] […]
[…] […]
Looks as though you may need to update your article again…
“Planet is ‘more sensitive to carbon dioxide than we thought
If carbon dioxide emissions continue at their current rate through to the end of this century, atmospheric concentrations of the greenhouse gas will reach levels that existed about 30 million to 100 million years ago, according to Jeffrey Kiehl from the US National Centre for Atmospheric Research (NCAR).”
http://environmentalresearchweb.org/cws/article/news/44990
Sod,
Still working my way through this so please bear with me (decided to start again just to make sure I understand everything). I think I understand the concept of energy arriving at the Earth from the Sun and the Earth radiating that energy back into space. However, one aspect I have not seen discussed (might have just missed this and apologies if that is the case) is what about the fact that the center of the Earth is infact very hot? Based on this I would expect that heat energy from this source must also be a factor in the calculations? Of the top of my head I guess this has the following possibilities:-
A) No energy escapes from the center of the earth and hence the Earth’s surface or mantel is in effect a perfect insulator? I guess this is highly unlikely?
B) Some energy does enter the “system” from the center of the earth (hence the center is actually cooling) but the amounts involved are neglible with respect the energy balance of the surface?
C) Relatively speaking a lot of energy enters the system from the Earth. If so how does this affect the equations?
I can only assume from your analysis that the answer is A or B (most probably B). If that is the case how do we know this?
Lastly, this assumes that infact no energy is in fact being “generated” in the center of the Earth? I had always thought that to be the case but recently I am sure I saw a Science program on the Earth Moon system which stated that in fact heat is being continually generated inside the earth (can’t recall if this was “nuclear” or from the movement of the Earth and Moon)? Obviously additional heat energy would compund the matters I raised above? Any comments of this?
Once again many thanks
Neil:
The answer is B. I have been asked about this a few times, so hopefully will get around to writing an article about it (eventually).
From Impact of Geothermal Heating on the Global Ocean Circulation:
Check out The Heat Flow from the Continents, by Pollack, which talks about some of the measurements techniques and the first calculation by Kelvin in the 1880s for the UK where he determined it was 68 mW/m^2.
And by the same Henry Pollack: Heat flow from the Earth’s interior: Analysis of the global data set, REVIEWS OF GEOPHYSICS, VOL. 31, NO. 3, PP. 267-280, 1993:
Many thanks
[…] with vertical gray lines." Sure doesn't look like saturation to me. Got any ad homs for scienceofdoom's explanation of saturation: […]
I enjoy the content and tone of your blog. I’ve only recently discovered it.
I think of it this way. If earth were a perfect reflector, the incoming 239 watts would immediately leave with little effect. Instead gas solid and liquids at the surface of the earth do not perfectly reflect so that the time consumed as the non radiant energy of space translates into heat and temp for the surface of the earth. Time always strikes me as a critical component of the explanation. With time in mind, it seems sensible that any additional CO2 in the atmosphere should tend to slow the passage of that wattage back out to space.
One nitpick which doesn’t really interfere with the thrust of what you are saying, is the surface temp of the earth really 15C? Think about all the extreme cold in the depths of the ocean and compare that to temps at comparable depths under continents. I think of the depths of the ocean as being full of polar water. I may be wrong but I doubt that it is cold left in the oceans since the last ice age, and I don’t think it’s cold just because the ocean depths are dark.
[…] […]
[…] […]
Was hoping you could in more detail explain that interesting cross-over area around 4.3 microns in wavelength, where there is still some amount of incoming solar LW radiation and we also see some outgoing LW. As this is one of the major absorption bands for CO2, as it would seem logical that both the incoming and outgoing are then re-radiated in all directions, what would the net effect be related to CO2 at 4.3 microns?
R. Gates,
Someone has probably articulated the net effect at 4.3μm.
However, the net effect is encapsulated in solving the equations of radiative transfer using all of the spectroscopic data for CO2 (and other gases) at all wavelengths.
So the standard result includes 4.3μm but I don’t know whether if we removed the spectroscopic data at these wavelengths it would result in more or less heat into the climate system.
scienceofdoom,
Thanks for the reply. It would be interesing to see the some model results with 4.3 microns removed. Obviously the big effect is out at 15 microns, but we know all of that (or virtually all of it) is outbound LW. It seems 4.3 has about equal shares of solar incoming and outbound LW. The net effect of how CO2 responds to being “hit from both directions” so to speak, would be interesting to know.
From first glance it looks like downward directed radiation is very tiny. For instance, your Wisconsin, Ellingson & Wiscombe (1996) graph shows CO2 peaking at 135 mW/m^2.
Perhaps I am mistaken, but is such a tiny fraction of power really able to warm earths surface above its theoretical 255K blackbody temperature to its 288K observed temperature?
My guess is that, while CO2/H2O do indeed absorb energy, this in turn warms the surface primarily by conduction and convection (I would guess 95%?), with the remaining part being back-radiation.
Mark,
Your first glance is wrong.
You can see the actual measured values of total flux (W/m2) in The Amazing Case of “Back-Radiation”.
For example:
And in Part Two of that series I said:
and followed up with an explanation in the comments.
In essence the value you describe as peaking at 135 mW/m2 is actually peaking at 135 mW/m2 per steradian per cm-1. [mW/(m2. sr. cm-1)]
The measurement of cm-1 is wavenumber and you can see on the x-axis that there are around 1000 of them on that graph. So that means you would have to take the average value of the graph and multiply by the 1000 cm-1 to get W/m2 per steradian.
The measurement of steradian is solid angle, so you would have to multiply by the number of steradians in a hemisphere to get W/m2.
scienceofdoom, thank you for that prompt reply, it greatly helped to clear up my understanding.
Thinking about this CO2 forcing business, it seems to me that fairly simple calculations can show that the lower atmosphere becomes saturated with CO2 at around 10ppm, therefore adding more has minimal effect. This has in fact been recognised since 1905.
The notion was then proposed (around 1950) that stratospheric CO2 can provide additional warming. This was based on the premise that the colder gas (~-40C) in the stratosphere loses heat to space less easily than the troposphere, hence it is able to retain and re-radiate heat more effectively due to CO2 absorbtion. This, as I understand it, is the basis of the whole AGW campaign.
The fallacy that I see here is that while temperature affects black body radiation, it does not significantly affect spectral-line absorbtion. The upper-atmosphere does not in any case behave as a black body but a transparent one. Even if it were a black body though, its temperature would still be irrelevant to its scattering of photons through CO2 molecular resonance. Gas at -40C will perform this function just as effectively as gas at +20C. Or, even 1000C. Only the photon energy and quantum considerations determine whether this happens, or not.
This I see as the flaw in the argument of the AGW proponents, and the reason why anthropogenic upper-atmosphere CO2 does not in fact cause enhanced global warming.
Your thoughts on this?
Anteaus:
What “fairly simple calculations”?
And what do you mean by saturation?
In fact, solving the equations of radiative transfer for the real atmosphere it is very easy to demonstrate that doubling CO2 causes an increase in radiative forcing. See Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations for these equations and their derivation.
The subject of “saturation” was first covered in Part Eight of this series.
You can also see calculations of atmospheric transmittance across the whole CO2 band in Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Nine, for example:
There is no confusion or controversy about this subject in the science world. Radiative transfer is well understood and theory, derived from first principles, matches experiment (see Theory and Experiment – Atmospheric Radiation).
To demonstrate that a doubling of CO2 doesn’t cause the pre-feedback radiative forcing claimed you need to either:
a) show the equations of radiative transfer are flawed (well proven over 60 years)
b) demonstrate that the solution to these equations has been miscalculated by everyone for the last 40+ years
It is not a simple calculation that you can do on a pocket calculator. You need to integrate across all wavelengths using the spectroscopic data in the HITRAN database, and you need to integrate up through the atmosphere to take account of the various line width phenomena at different pressures.
The Atmospheric Radiation and the “Greenhouse” Effect series might be of interest.
The graph above seems to confirm that the lower atmosphere’s CO2 is saturated, there being zero difference between 280pppm and 560ppm in the centre of the absorbtion band.Whether the slight differences in the fringes of absorbtion affect tempertures significantly is a moot point, and would I imagine be very hard to calculate. If you do the math based on the mean free path of photons you find that saturation occurs at less than a tenth of the values in this graph at sea-level pressures, and still at less than the values for the stratosphere.
Though, I digress. The main point I am making here is that while the land is a black-body radiator, the atmosphere is not. Or, if it is, it is an exceedingly poor one. It is therefore correct that the Stefan-Boltzmann law will determine the amount and spectral distribution of infrared received by the atmoshere from the surface, but it is incorrect to apply black-body equations to the transmission of IR through the atmosphere.
AFAICS It is from this mixing of incompatible math that arises the statement that ‘the colder upper atmosphere will show an enhanced global warming effect.’ It will not, because molecular or atomic resonances are temperature-independent*.
You can’t extract more light from a neon indicator by applying a blowtorch to it, or change its color by freezing it. For that matter, the output wavelength of a CO2 laser is not determined by its temperature, but by the gas properties and, to a certain extent, the optics. In both cases it is not a thermal mechanism which produces the photons, but one of quantum physics. It is a spectral emission, similar to that of CO2 infrared scattering. Likewise, it would seem to me that you cannot change the photon-scattering effect of CO2, and hence the amount of IR leaving the atmosphere or being reflected back, by changing its temperature. Therefore the ‘cold upper atmosphere’ postulate is a red herring.
That is my take on the subject, anyway.
*Provided we ignore the doppler effect of molecule velocity, which is not significant here.
“ is a moot point, and would I imagine be very hard to calculate.” ? It is not a moot point and I already explained the calculation requires an integration across wavelengths and up through the atmosphere, so it is a numerically challenging calculation.
You haven’t done the calculation but you imagine it is irrelevant and also too difficult to calculate.
This is not a scientific approach. Do the calculation yourself (it will take a lot of time) or accept the results from people who have done the calculation.
If you decide you want to believe something different, up to you, but don’t call it science.
No one is applying these black-body equations to transmission of IR through the atmosphere.
At this point I realize you are not interested in learning anything. You haven’t even read the articles I just pointed you to that explain these fundamental points.
If you want to learn science, read some science.
Alternatively, if you want to randomly claim stuff and pretend those random claims are what climate science believes there is big community to join full of happy people, but it isn’t on this blog.
I don’t ‘believe’ anything, in that sense. Belief is a function of religion, not of science. I question, because only by picking holes in arguments can their validity or otherwise be determined. If the question can be answered satisfactorily, then that adds validity to the proof.
I don’t, for example, question special relativity because although I don’t fully understand Einstein’s proof of concept, I do understand enough of the math to know that his arguments make sense, and that real-world examples bear them out. Therefore I am prepared to accept that he was probably right. His work also has the hallmark of someone who checked his facts carefully, and always questioned his own assumptions. This gives me confidence in his findings.
WIth climate science we are faced with arguments which at a superficial level seem plausible (which is why it gains so many supporters among non-scientists) but which when examined more closely don’t make sense. The proofs offered are couched in math so impenetrable that it would be hard even for a World-class genius to say whether they are valid or not. If we question these proofs we are simply told that since we don’t have the IQ to understand them, we should just accept them.
We also see a situation, again and again, where one AGW postulate being disproved simply results in another postulate being produced which allegedly proves the same hypothesis but in a different way.
The objective, clearly, is not so much to discover the truth, but to prove the original hypothesis at any cost. Cost being the operative word, since large amounts of money are now staked-on its being vindicated.This is not the behaviour typical of scientists, but that of a bunch of panicking investment speculators.
[…] Incorrect. The science and math is well known to anyone who cares to learn. From ScienceofDoom: Part One of the series started with this statement: […]
[…] […]
pretty graphs – I can see you like pictures
-again co2 significant – NO certainly not
just more well written rubbish as far as most can tell
simon,
This is a science blog, so no one’s opinions are particularly interesting.
If you have a scientific point it will be welcome. A science point will be identified by experimental evidence demonstrating something relating to the topic of this article, or deriving a new or contrary result from established theory. Please stay on topic and please use science.
The physics of radiative transfer is well established and derived from fundamental physics.
And as you don’t like pictures – neither do I particularly, I prefer maths – perhaps as a start you can review Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations and EITHER explain which of the equations are wrong (and why of course) OR demonstrate the result you get from applying the radiative transfer equations to the atmosphere (and as a nice extra you could touch on why every other person solving these equations has got the wrong result).
If you prefer to voice opinions you will be more welcome at many other blogs which happily for you have much larger audiences.
The problem with all of this is that there is more solar than terrestrial IR radiation entering the atmosphere, even with the 46,000 fold reduction.
So as CO2 increases more of the solar IR will be absorbed and re-radiated back into space thus reducing surface warming. This is clearly demonstrated by the graph titled “solar radiation at the top of atmosphere, and at the surface:”
Yes, GH gasses will store the heat thus providing warmth during the night, but dont forget they insulate the surface from heat during the day aswell. If not earth would have a daytime high similar to the moons.
So increasing GH gasses does not so much cause warming as narrowing the extremes of temperature variation.
You’re making the same mistake Wood made in 1909. The IR energy in sunlight is in the range of 0.8-5 μm. The thermal IR emitted by the atmosphere and surface is in the range of 5-50μm. CO2 only has a few bands in the 0.8-5μm wavelength range and most of those are severely overlapped by water vapor. The emission of CO2 from those bands is infinitesimal because the temperature of the surface and the atmosphere is too low. The total absorption of incident solar radiation by CO2 is about 2 W/m². Doubling CO2 would increase that absorption by about 0.4 W/m². That’s not going to have much effect on surface warming compared the the 3.7 W/m² of outgoing radiation that is absorbed.
matt,
What is clearly demonstrated is that CO2 absorbs both terrestrial radiation (“longwave”) and solar radiation (“shortwave”).
But these are not absorbed equally. CO2 does not absorb X% across all wavelengths, it is very wavelength dependent.
So to determine the effect of more CO2 we need to calculate the effect on shortwave and the effect on longwave.
You write as if this calculation is a simple one that can be done in your head.
When you write “..as CO2 increases more of the solar IR will be absorbed and re-radiated back into space..” it indicates that perhaps you haven’t examined this subject at all.
The calculation is very complex. CO2 at atmospheric temperatures (220K-300K) absorbs solar radiation at specific wavelengths (e.g. around 1.4μm) and then emits at what wavelength? CO2 at these low temperatures cannot emit any significant radiation at 1.4μm. CO2 at atmospheric temperatures emits strongly in the 14 – 16μm region (as well as others).
In effect what has happened to solar radiation as a result of more CO2 is that the atmosphere has absorbed a little more radiation at the expense of the surface.
To solve the problem and find the net effect (what happens to the temperature of the surface and the temperature of the atmosphere) under specific conditions you need to solve the equations of radiative transfer.
These equations are slightly more complicated than “it sorts itself out without changing anything”. They calculate the transfer of energy between each layer in the atmosphere as a result of absorption and emission of radiation. Absorption depends on incident radiation and the absorptivity of the atmosphere at the wavelengths of the incident radiation. Emission depends on temperature and the emissivity of the atmosphere at these temperatures.
Apologies it this has been mentioned, but the conversation is focused on CO2 being .04% of the atmosphere. Which is correct.
But the man-made portion is only 4% of the total atmospheric CO2.
So the question is, how does or how can the man-made CO2, which is .0016% of the atmosphere, heat up the other 99.9986%.
I would label the man-made portion at .0016% insignificant. No?
Nope. The man-made portion of the CO2 in the atmosphere is whatever the concentration is today less the pre-industrial level of about 280 ppmv. That’s on the order of 100 ppmv or about 25% of the current concentration. The 4% number is relative to the annual fluxes between the biosphere and the atmosphere which are on the order of 100Gt/year and is dated to boot. It’s more like 8% now. But the flux into the biosphere and the flux out of the biosphere are essentially equal over the course of a year causing no net change in the atmospheric concentration. The flux into the atmosphere from burning fossil fuels, producing cement and changes in land use/land cover stays in the system for on the order of 100,000 years.
Next you’ll probably trot out the residence time of an individual molecule of CO2 in the atmosphere of only about 5 years, not 100,000 years. While that’s true, it doesn’t change the rate of accumulation in the atmosphere, ocean and biosphere system. It’s the residence time of CO2 in the system as a whole that counts, not how long it resides in one part of the system. We’re currently adding about 40 times as much to the system each year as is removed in the geologic carbon cycle every year. And the addition rate is increasing.
You can put whatever label you like on the proportion of anthropogenic CO2 out of the total atmosphere. I don’t think your 4% is correct, but that’s not an interesting question for me.
Physics questions are not solved by your approach of “it’s so small how can it have any effect” but instead by understanding the fundamental physics, and solving the relevant equations for whatever changes have occurred or are expected.
In this case, the solution comes from the Radiative transfer equations which have been well-known and proven for more than 60 years.
So what you need to do is prove the equations wrong, or prove that they can be solved to give a different answer from what the climate science community has calculated.
Amazingly you can’t do the calculation with a pocket calculator or in your head. Take a look at the link provided.
More insignificant than you think – or astoundingly significant! CO2 concentration of 0.04% of atmospheric matter, which accounts for 90 ppm of the volume of the spherical shell we call the atmosphere (the rest comprising radiation), therefore represents spatial occupancy of 36 parts per billion. Prior to a couple of centuries of life-enhancing industrialisation, occupancy was 11 ppb lower and, according to mainstream climate science, rendered life possible by raising surface temperature 33 degrees C. However, the mainstream continues, that increase in spatial occupancy has raised surface temperature a further 0.8 degrees C and models warn of a business-as-usual two degree increase this century, when CO2 occupancy doubles from pre-industrial levels (to 50 parts per billion), and a consequential great and dangerous regress in the human story. Given the sparseness of CO2’s presence in the climate system, its capacity to raise surface temperature (by absorbing ougoing LW radiation from the surface) must rely on extremely efficient unit absorptivity about which, strangely, little is said.
John Millet ends his comment “.. efficient unit absorptivity about which, strangely, little is said”. That’s a rather strange comment in this thread where many comments have discussed how the well known quantitative values of that absorptivity are used in detailed calculations whose results have also been in many ways been verified by empirical data.
Every comment where MODTRAN is mentionned is on that and so are many others.
Has no one here ever heard of the SUN? There is direct correlation between the activity of the Sun and temps on the Earth based on historical data. And the other planets. What little warming has taken place on Earth has also taken place throughout the Solar Syetem.
Has no one here ever heard of the Milankovitch Cycles. The Earth is headed for an Ice Age over the next 1- to 40,000 years. Celestial mechanics cannot be denied. Google Milankovitch Cycles and Procession of the Axis.
Recent research has shown that the variation in solar radiation is less than had been thought.
The Milankovitch cycle isn’t headed for a big drop for ~50,000 years. We’re currently in a period where all the variations cancel each other out.
Of course there are those like Ruddiman that think that if we hadn’t started major land use changes by the invention of agriculture 8,000 years ago, we’d already be well on our way to an ice age. CO2 is just icing on the cake.
http://en.wikipedia.org/wiki/William_Ruddiman
Oh, Dewitt you assuming that ALL of the change in CO2 concentration is from man-made sources. There is no proof of that. CO2 in the atmosphere can increase from natural sources as temps increase. Coincidence is not causality. And yes, I am familiar with the C12/C13 ratio argument.
Anthropogenic CO2 emissions are nearly twice as large as is necessary for the measured increase in atmospheric CO2. The 13C/12C ratio argument is simply icing on the cake. There is no proof of anything in science. There is only disproof. The people who claim that the CO2 increase is not anthropogenic have never outlined a consistent explanation of how that could happen in the face a massive human emissions. They just wave their hands. The anthropogenic mechanism, however, is self consistent.
So if as you say we can measure and distinguish between
(a) the energy arriving from the sun, and
(b) the energy leaving the earth
that means we can straightaway tell if the earth is warming up or not.
And since I’m sure we can also measure atmospheric CO2 concentration, we are thus in a position to easily see if the planet warms in line with CO2 increases or not.
Case closed or what?
Handel,
It would be if we could measure with sufficient precision. We can’t yet.
Dewitt
Yes, the inability to accurately measure the incoming and outgoing energy from the earth system, does seem to be what is standing in the way of settling the AGW hypothesis one way or the other.
Seems like such a basic and obvious issue – so why don’t they prioritize it? Why not divert some of the $millions/billions wasted on models that can’t seem to predict anything, into the empirical side? Don’t the people controlling the funds want the issue convincingly resolved?
One of the problems is that we don’t have a flyable, high precision IR detector that goes to long enough wavelength. By flyable, I mean something that can be launched into orbit and stay active for years. This isn’t a problem that can be solved by throwing money at it. It requires some research breakthroughs.
The Glory satellite would have helped a lot with the problem of aerosols, but the launch failed. Now that is something that could have been fixed with money by building more than one satellite. When I put my tinfoil hat on, I sometimes wonder if they didn’t really want to know because aerosols are a major tuning kludge used in climate models.
hi out there
(First excuse me – i am not english speaking // im not a scientist)
What we not know despite of all the scientific facts is:
How long do the atmospherical phaenomena last.
For example:
before i was born, my mass was close to zero
after i die my mass will be zero
so you might guess – from a scientific viewpoint – that i never existed
second example: water in a storage reservoir
Flowing out = Flowing in minus evaporation minus what s.o. may have drunk
(note the easy maths)
as a result there is no water in it
see where this may lead us to (Handel)
“(a) the energy arriving from the sun, and
(b) the energy leaving the earth
that means we can straightaway tell if the earth is warming up or not.”
But no we can´t! Cause we do only look at the system from outer space ignoring that there are lots of processes working between incoming and outgoing radiation.
Look at the second example and you will see, that the amount of water in the reservoir depends on its dimensions, not on the flow.
The main questions are: How long is energy kept in the biospherical system and what makes it stay? What are the main characteristics of the thermal capacity of the whole system?
I think we are far away from any answer yet. The incidental relationship between co2 and temperature in any vostok icecube can only be a hint. Stop linear thinking in polydimensional systems.
[…] might be a factor but it is not Here is an explanation of why saturation does not apply https://scienceofdoom.com/2009/11/28/…-gas-part-one/ Beyond that – argue it with Mannie or PB they have a lot better understanding than I of the […]
The use of green bags will immediately be the solution to
this problem that we are looking for. It is predicted that “global warming” will increase water shortages in the near future.
We cannot use technology to refreeze disappearing glaciers or
the ice caps, refill rivers, remake their entire ecology or stop rising oceans due to
global warming.
[…] calm way he approaches his material is admirable, and astonishing. He has, for example, a multi-part treatment of the various interpretations assigned to carbon dioxide being a trace gas in …. His very first page, limited in scope, is assailed by some claiming he has an agenda. As he […]
Very nice addition to the web. Could you put up (or link to) your define_atmos_0_3.m and define_atmos_0_2.m file? I am in urgent need!
thank you very much.
[…] series of blogs begins here, at the “Science of Doom” site, called Visualizing Atmospheric Radiation. […]
“Nope. The man-made portion of the CO2 in the atmosphere is whatever the concentration is today less the pre-industrial level of about 280 ppmv.”
Wasn’t there a time in the distant past when the CO2 was in excess of 4000ppm but the Earth’s temperature was cooler?
https://encrypted-tbn2.gstatic.com/images?q=tbn:ANd9GcQbJQFl9pDl5XprngsaNgP-u2QFLBx7_OLTs-fxgjTUYTZYPxtt
Hi! I could have sworn I’ve visited this blog
before but after looking at a few of the articles I realized it’s new to me.
Anyhow, I’m certainly pleased I came across it and I’ll be bookmarking it and checking back
often!
Abierto un correo sin embargo mi, en el que un nos lo bloquea antivirus no
lo detecta.
Excellent blog you have! Very informative! Thanks! Now that I’m done buttering you up, I have a couple of concerns:
I was surprised that N2 and O2 has so little impact on the atmospheric temperature. According to dQ=mc(dT) from my thermodynamics text, N2 and O2 and air warm up faster than CO2 and H20. This was confirmed by experiments and has led to the specific heat coefficients for these molecules.
I also wonder why you assume that only greenhouse gases are responsible for the increase in temperature from (-18C to 15C). The earth is a source of energy apart from the sun. Beneath the crust there is massive amounts of geothermal energy that can transfer to the atmosphere through conduction and convection. Let’s not forget the oceans’ ability to store heat and transfer it through convection. It seems the only thermal transfer you are willing to consider is radiative transfer.
mikejacksonauthor,
They (N2, O2) definitely have an impact on the atmospheric temperature. But they don’t absorb any significant radiation.
The way they have an impact is by constant collision with molecules that do emit and absorb radiation.
The result is that a “local” portion of the atmosphere is all at the same temperature. So the energy absorbed by water vapor, CO2 and other GHGs increases the local temperature of the atmosphere – of all molecules, including O2 and N2. The energy emitted by water vapor, CO2, etc decreases the local temperature of the atmosphere – of all molecules, including O2 and N2.
The estimated global average geothermal energy transfer is less than 0.1 W/m2. So it is insignificant compared with the energy transfer to and from space.
This is just one article. Radiation, and the absorption of radiation by GHGs in the atmosphere, is necessary to explain the difference between the surface radiation (390 W/m2) and the radiation to space (240 W/m2).
Convection makes the surface temperature cooler than it would be without convection. It effectively “short-circuits” the temperature gradient that would be there from radiation. That is, if we had no convection the surface temperature would be a lot higher.
Another article to read is The “Greenhouse” Effect Explained in Simple Terms.
Thanks for your reply. Let’s see if I understand you correctly. N2 and O2 would not warm up significantly if there was no CO2 or H2O in the atmosphere? I would love to see that experiment!
After doing a little math, it looks as though the geothermal energy of .1W/m^2 would add 36.4K to your calculation of -18C. That would bring the temperature up to 17.86C. Too high! Anyway, there’s more than enough heat to jiggle those O2 and N2 molecules. I’m assuming of course that radiative thermal transfer isn’t the only thermal transfer available in the system. And I haven’t even factored in the oceans yet.
I’m also wondering how much water vapor is in the atmosphere at -18C, since water freezes at 0C. Probably not much. I would imagine there isn’t much CO2 either, since CO2 gets absorbed by the oceans at colder atmospheric temperatures.
I may have screwed up on the math, so .1W/m^2 wouldn’t add much to the overall temperature. But it seems to me that water vapor at -18C would be insignificant compared to the oceans’ ability to store heat and transfer it by means of conduction and convection.
“Convection makes the surface temperature cooler than it would be without convection.”
That’s true but it should also make the atmospheric temperature warmer. The oceans can store energy that would otherwise be lost. The question is how much of the energy is transferred by radiation, conduction and convection? If I heat some water using sunlight and then seal it in a glass jar so no IR radiation can escape, will the jar feel hot? Will it heat up the surrounding air?
mikejacksonauthor
The ocean has 100 times the heat capacity of the atmosphere. Therefore the atmosphere has 1% of the effect?
As a starting point to understanding heat transfer basics – heat capacity affects the lag. It does not affect the equilibrium temperature.
If you created an artificial earth, E2, like the current ones and put the oceans in at 60’C they would gradually cool down. If you created another artifical earth, E3 with oceans only 350m deep and started them at 60’C it would take 1/10 of the time as for E2 for the ocean to reach its equilibrium temperature.
Both E2 and E3 would end up with ocean temperatures like today’s (note 1).
The reason the atmosphere affects the temperature of the ocean is the climate system emits radiation mostly from the atmosphere. The atmosphere is the “interface”.
If you heat water above the ambient using sunlight and then seal it in a jar the water in the jar (and the jar) will cool down a little while the ambient air will heat up a little. What are you expecting to demonstrate here?
The reason for explaining that convection short-circuits the “radiative transfer” in the lower atmosphere is a little complex. I hesitate to use an analogy because it won’t be a perfect analogy and then discussions seem to go in the wrong direction.
Instead I will point you towards:
– Radiative Atmospheres with no Convection
– Temperature Profile in the Atmosphere – The Lapse Rate – why the temperature change with height is like it is
The key to understanding why this makes a difference comes with appreciating that the emission of radiation by the climate system is mostly from somewhere in the atmosphere:
The Earth’s Energy Budget – Part Two
The Earth’s Energy Budget – Part Three
Note 1 – Of course there is a complexity in a non-linear dynamic system that I am avoiding in this basic discussion.
“The reason the atmosphere affects the temperature of the ocean is the climate system emits radiation mostly from the atmosphere. The atmosphere is the “interface.”
From what I gather, the ocean, being the huge black body it is, emits a heck of a lot of radiation, a small portion of that gets absorbed by the occasional water vapor molecule (which probably also came from the ocean) or CO2 molecule (which also may have come from the ocean). Now, this process can only take place if the ocean is warmer than the atmosphere. In which case, the atmosphere can’t warm the ocean. P = eoA(To^4 – Ta^4). To = ocean temperature. Ta = atmospheric temperature.
mikejacksonauthor,
“Warm up significantly”? What exactly do you mean? Do you think I am saying they will be at absolute zero or something??
Where did you reach the conclusion that N2 and O2 absorb significantly terrestrial radiation? It wasn’t from reading a textbook on atmospheric physics. It wasn’t from reading a paper.
I provided the absorption data (graphs) on different molecules in this article from spectralcalc. It’s an online resource. In another series of articles – Visualizing Atmospheric Radiation – Part One – I took the data from the HITRAN database and reproduced a lot of “basic” atmospheric physics.
This database was developed over a few decades and from the input of thousands of papers by spectroscopy professionals over many decades. They publish in journals like Journal of Quantitative Spectroscopy & Radiative Transfer.
Here are two papers on the database, the older one (2004) is probably a more interesting read.
The HITRAN 2008 molecular spectroscopic database, by L.S. Rothman et al, Journal of Quantitative Spectroscopy & Radiative Transfer (2009)
The HITRAN 2004 molecular spectroscopic database, by L.S. Rothman et al., Journal of Quantitative Spectroscopy & Radiative Transfer (2005)
mikejacksonauthor – please clarify why you think you know anything about the subject of atmospheric absorption and emission – what is your source for believing that N2 and O2 don’t absorb terrestrial radiation?
Are you interested in learning anything? If you want to tell myself and readers here that everything written on this subject on this blog is wrong you should start with your research – textbooks, papers.. or your original research and why all the spectroscopy professionals are wrong.
Mike: Proof that N2 and O2 aren’t “warmed” by thermal infrared can be seen in the spectra in Figure 5-2 of this post. If all the radiant energy is transmitted, none is absorbed.
More CO2 is found in the oceans than in the atmosphere, but MEASUREMENTS show 400 ppm (and rising from year to year) in the atmosphere. We know how much water vapor (measured twice daily at about 100 locations up to 20 km (down to 220 degK) with weather balloons.
A blackbody at 36 K emits about 0.1 W/m2. The earth at about 288 K emits 390 W/m2. The temperature would be only slightly higher, not 36 K higher, if we accounted for the additional 0.1 W/m2 from geothermal (390.1 W /m2).
Frank, thanks for your reply. Yes, N2 and O2 aren’t warmed by IR, but I suspect they are warmed by shorter frequencies that are more intense energy. Why is the sky blue? There is a lot of activity going up there. Activity should create heat.
Also, I think there is another way to account for earth’s 15C temperature: check out the moon. It has no atmosphere; thus, no atmospheric pressure. It’s average temperature, coincidentally, is around -20C. According to Doom’s calculations, earth’s temperature comes out to be -18C. Add an atmosphere (increase the pressure) and the temperature will also increase. So greenhouse gases may not be significant at all.
Pump up a tire and the temperature increases.
That is because you have put work in.
Leave a tire and it cools down to the ambient temperature.
The pressure of the tire has absolutely no effect on its final temperature.
Convection, Venus, Thought Experiments and Tall Rooms Full of Gas – A Discussion – three of us put forward explanations and ideas about the high temperature of Venus.
I’ll leave it there. If you believe changing the pressure of the atmosphere changes its equilibrium temperature then you don’t understand conservation of energy.
Mike: You are correct that N2 and O2 absorb shortwave UV. Only a very small fraction of the sun’s energy is emitted at these short wavelengths and they are absorbed well above the troposphere. So these absorptions have little impact on our climate.
The sky appears blue because blue light is scattered by the molecules in the air more than other visible wavelengths. Scattering redirects EM without absorption or emission.
I prefer to quantify the greenhouse effect using W/m2, not degK. Every model for an earth without GHGs – including the moon – makes dubious assumptions (albedo, surface heat capacity, emissivity, rate of rotation) when calculating expected surface temperature. The surface of our planet averages about 288 K and emits an average of about 390 W/m2. Only about 240 W/m2 escapes to space, equal to post-albedo incoming SWR. The difference between 390 and 240 W/m2 is the greenhouse effect. If you think an earth without GHGs should be 255 K, then the GHE is 33 K. If you prefer a different model for earth without GHGs, you will calculate a different GHE in terms of degK, but it will still be 150 W/m2.
Some of that 150 W/m2 is due to CO2. It will increase to 154 W/m2 when CO2 doubles, based on laboratory measurements of the spectrum of CO2 and our knowledge of the absorption and emission of electromagnetic radiation by gases – until the earth warms enough to emit 4 W/m2 to compensate and restore steady state.
When you have an object initially at constant temperature, the RATE of energy (power) entering and leaving must be equal. 1LoT. You can make that object warmer by increasing the incoming power (radiation, for example) OR by decreasing the outgoing power. We often call decreasing outgoing power “insulation” and say we are “conserving” power (not creating energy by violating the 1LoT). Usually we think insulation prevents heat/energy loss by slowing convection or conduction, but we also can insulate by slowing NET loss by radiation. GHGs in the atmosphere “insulate” by slowing outward radiation from 390 W/m2 at the surface to 240 W/m2 at the top of the atmosphere.
The ocean has had thousands of years to equilibrate with the surface land and atmosphere and reach a steady state where there is negligible heat flux into or out of the ocean. Both will absorb the energy increasing CO2 is keeping from reaching space.
Climate physics can be tricky, but no one explains it more accurately or clearly than SOD’s early posts. Some readers here are skeptical of the IPCC consensus on climate change and some are supporters, but most of those who seem technically competent (IMO) find the info here highly reliable. Good luck in your search for the truth about this subject – it will be hard to find if you waste most of your time making challenges that are trivial to refute. Questions (“I don’t see why …”) teach one more than statements (“You’re wrong about …”). By the time one has formulated a sensible question, one often can answer it.
“by slowing outward radiation from 390 W/m2 at the surface to 240 W/m2 at the top of the atmosphere.”
Frank, it seems to me you are moving the goalpost. After reading the above post, I was under the impression that the maximum power reaching the planet surface is 239W/m^2, so the outward radiation couldn’t be more than that. In fact that would be the initial maximum power, not the mean power leaving the surface. To get the mean power, you have to take into account that as the black body loses energy, it cools off. When it cools off, the power drops. When it cools off enough so that it’s temperature is the same as the surrounding air, the power drops to zero. P = eAo(Ta^4 – Tb^4).
Thank you for your comment, scienceofdoom. I’m pretty sure that if the pressure of the atmosphere increased, so would the temperature: P1/T1 = P2/T2.
I’m also highly certain that the earth can’t end up being warmer then its energy source: -18C. If the system is 100% efficient and no heat escapes, you will have -18C, but never higher than that. If your system is less than 100% efficient and heat can escape, then your temperature should be less than -18C. I refer you to the first law of thermodynamics.
All GHGs can do is prevent IR radiation from escaping. They don’t add to your power source. If they could, why not build an engine with more than 100% efficiency? You start with X amount of power, then add some GHGs and voila! You get X+ amount of power. You could make billions!
So if you want to end up with a mean temperature of 15C, you need more sun energy, preferably more than 15C worth (388W/m^2). You also need to factor into your analysis the exponential drop in the earth’s emissivity when it radiates energy. As it gives up energy, its temperature drops and that drop in temperature (according your equation above) causes the power to drop. Thus, the earth, by default, saves some of the energy it receives from the sun. You don’t even need GHGs. An ocean works nicely! This process can cause a temperature increase when the sun revisits the next day. However, no amount of temperature increase will exceed the temperature of your power source: X W/m^2.
mikejacksonauthor,
Take a thin metal gas chamber with the outer surface held at 25’C and the gas temperature, Tg, in equilibrium with the outer surface – i.e., Tg = 25’C. Increase the gas pressure inside from 1bar to 2bar.
You are claiming that the new temperature, Tg(t=1minute) = let’s say 30’C. I’m putting a number into your claim to help other readers get a practical sense of what you are claiming and thereby helping them to realize what a colossally ridiculous idea it is. At this point – 1 minute after – it is not a colossally ridiculous idea, it is a correct idea – because the act of pumping extra gas into the chamber is doing work, which adds energy. So we expect, immediately after the increase in gas pressure for the gas to be at a higher temperature.
However – you claim – one day later, Tg(t=1 day) = 30’C.
One year later – you claim – Tg(t=1 year) = 30’C.
All this time heat is being conducted from the gas through the metal walls of the chamber to the outer surface.
Why doesn’t the gas cool down to 25’C? Has the fact of the gas being at a higher pressure prevented the law of heat transfer working? Do you know there is a law governing conduction of heat?
I say, that the gas will cool down over a period of time (governed by the fact of conduction of heat from a higher temperature to a lower temperature) until Tg(future) = 25’C.
Therefore, higher pressure cannot create an equilibrium higher temperature.
I look forward to your explanation.
The climate system absorbs (globally annually averaged) 240 W/m2.
The climate system emits (globally annually averaged) 240 W/m2.
There is no “more than 100% efficiency” here. Because there is nothing preventing the surface of the earth being at a higher temperature than the atmosphere.
It’s as simple (although a different mechanism) as lagging a pipe. Why do people do it? Idiots! They think they can create energy.
Still, you clearly have the basics understood. So I challenge you to the same challenge given to “physics degree” Bryan:
A Challenge for Bryan
Please state your answers there. As predicted (“we will wait a decade..”) Bryan did not attempt to answer the question but later decided, when I provided the worked answer that my answer was correct.
It is solved using conservation of energy (as you find in heat transfer textbook problems that need to find equilibrium solutions) and yet the mystery of it..
I hope we can get an answer on this problem within a few days from you.
There is one other challenge for you. What is the total annual average emission of thermal radiation from the surface of the planet? Is it 240 W/m2.
SOD: I hate these “shell” models for the GHE. A shell, no matter how thin, can not conduct any heat outward when the temperature of the interior and exterior surfaces is exactly the same. Even when you postulate infinite thermal conductivity and/or negligible thickness, calculating heat flow involves multiplying infinity times zero. The problem could be restated in terms of a limit as the thickness of the shell and the temperature difference approaches zero.
The reason I object to these shell models is that the same assumptions and math are next applied to models for our atmosphere with isothermal optically thick layers. The problem of transferring heat through an isothermal layer can’t be overcome by making it thinner – an infinitely thin layer is not optically thick. Sensible models for the atmosphere are optically thin.
(Sorry to complicate your job of explaining this physics.)
Frank,
Well, then you must hate all numerical radiative transfer programs. They all, every single one, approximate the atmosphere as a series of isothermal layers. Once you get enough layers, about 30 seems to be a good number, increasing the number of layers does not significantly affect the results. It also matters little whether the layers are optically thin or thick. In fact, they are both at different wavelengths.
You’re over thinking the problem. Sure you could include a temperature gradient in the shell, but again, as the shell becomes thinner, it makes less and less difference, except in the calculation time and program complexity.
DeWitt: There is nothing wrong with subdividing the atmosphere into a series of optically thin layers and doing radiative transfer calculations between layers. Of course, you should check when you are done to be sure that the approximations made during this process – each layer has a single density and temperature – don’t distort your answer and use more layers if necessary. This is true for anytime you use numerical integration to solve a problem.
My objection is to an atmosphere composed of isothermal, optically-thick layers. The mathematics of optically thick layers is the same as the shells in SODs question. As I noted, the problem with conducting heat through an isothermal solid can be addressed by considering the limiting case of a thin, highly conductive material. However, that solution doesn’t work for an optically thick layer that emits like a black body. When you make an optically thick layer thinner, it no longer emits like a black body.
Upon further thought, you can’t make a solid shell infinitely thin and expect it to still emit like a black body either. Nothing emits blackbody radiation without enough molecules in the path of the radiation to bring absorption and emission into equilibrium. Too many people don’t understand when it is and is not appropriate to assume that an object emits blackbody radiation.
Perhaps I am overthinking the problem. There is nothing wrong with a problem with physically unreasonable assumptions, unless it leads to misunderstanding.
Frank,
It’s just a teaching tool.
It’s like basic mechanics where “assume a perfect frictionless surface” is a staple, or “the pendulum rod has zero mass, all the mass is concentrated in a point at the end”.
It just helps to simplify the problem to provide some conceptual understanding of a principle.
Imagine if you had to start explaining the problem of radiative transfer through the atmosphere with the numerical integral across wavelengths and up through a number of layers.
The benefit of the “multiple blackbody layers” is it is the simplest mechanism to show that the surface temperature of a planet can increase due to radiation transfer.
If that was where the lessons ended it wouldn’t be particularly helpful.
All that said, if the model doesn’t work for you as a teaching tool, throw it out. Once you’ve grasped the real equations (as you have) the teaching tool isn’t of interest anyway.
Final answers to challenge: Ta1 = 193K; Tb = 192K; Ta2 = 231.43K
P = 1000W; Aa = 12.56m^2; Ab = 12.81m^2;
Ta = (P/Aao)^25 ; Tb = (P/Abo)^25; Ta2 = (.1576P/o)^.25
I’m copying your answer to a new comment at the end (the replies appear in something of a random order).
My response is there.
“Therefore, higher pressure cannot create an equilibrium higher temperature.”
Try this experiment: continuously add energy from a power source like the sun. Increase the pressure of the system. The temperature should increase. Reduce the pressure, the temperature should decrease. In both cases there is an equilibrium if your energy in matches the energy loss to the outside. If your experiment was really analogous to the earth and moon, they would both eventually end up with a temperature close to 0 Kelvin because you did not mention any new energy being added to the system.
“There is one other challenge for you. What is the total annual average emission of thermal radiation from the surface of the planet? Is it 240 W/m2.”
(Esolar = 1367 x (1 – 0.3) / 4 = 239 W/m²}
No. The figure you came up with is the power that hits the surface. The power leaving the surface would be substantially less if you account for the fact that the power drops at night when there is no added energy from the sun. During the day, there is no emissivity at all if the temperature of the atmosphere meets or exceeds the temperature of the surface.
“1. What is the equation for the equilibrium surface temperature of the sphere, Ta?”
If the energy source is from within the sphere, there would be no temperature equilibrium initially. As the interior gives up energy, none is being replaced, so the temperature would drop until it matches the temperature outside of the sphere. T1 = P/eAo + T2 T1 is the surface temperature; T2 is the outer temperature. Assuming T1 > T2 initially. At the end of time t, T1 = T2 and P = 0. So the equation for the equilibrium surface temperature is T1=T2.
Forgot to add the exponents. P = eAo(T1^4 – T2^4). When T1 = T2, P = 0.
What I love about this equation is it can be used to check if a 15C or 288K temperature is possible with a P of 239W/m^2. Let’s say T1 is 255K and T2 is 0.00K. P = 239W. Let’s suppose the T1 source warms up the T2 sink (GHGs). Once T2 warms up to 255K, P = 0. There is no power to increase T2 to 288K. Nor does T2 have power to warm up T1 beyond 255K.
Assume the energy source is radioactive decay with a half life orders of magnitude longer than the equilibration time, not stored heat.
Whatever the energy source, you can find the surface temperature with Ts = (P/eAo + To^4)^.25. If To is 0 Kelvin and P is constant, then Ts = (P/eAo)^.25. If To is the temperature of something surrounding the disk other than space, Ts can be found the same way.
<<>>
Same answer as above, only one T is the shell instead of space.
2b. What is the equation for the equilibrium temperature, Tb, of shell B?
Same answer as above, only one T is shell B and the other T is space.
If no new energy is added to the system then eventually T1 = T2 = T3. T1 is the surface; T2 is the shell; T3 is the outer space.
Can’t edit comments. I should have put your questions in parenthesis, not brackets.
2a. What is the equation for the new equilibrium surface temperature, Ta’?
Same answer as above, only one T is the shell instead of space.
Hey, guys, I like your blog so much, I gave it it a shout out on facebook!
mikejacksonauthor,
The problem stated at the outset:
So your initial statement:
– is incorrect. If the first part of your statement “As the interior gives up energy, none is being replaced, so the temperature would drop..” was correct then the equilibrium temperature of the sphere would be 0K. We are not very interested in that case.
We have a “Case A” and a “Case B” in the problem.
I think (from your answers above) you are going with:
Answer 1 (case A), Ta = (P/Ao)0.25 ?
The correct answer for case 1, Ta = [P / (Aaσ)]0.25
where I put in the area of sphere A = Aa = 4π.ra², where ra is the radius.
If your value “o” in your equation is the constant σ = 5.67×10-8 then your answer is correct.
I defined ε = 1 in the problem.
Do you agree with this?
If so, what are your answers for case B (questions 2a and 2b)?
You can find the surface temperature with Ts = (P/eAo + To^4)^.25. If To is 0 Kelvin and P is constant, then Ts = (P/eAo)^.25. If To is the temperature of something surrounding the disk other than space, Ts can be found the same way.
I can find the formula – correct I can.
But you need to state the formula and then we will either point out the flaw in your answer or you will have made the opposite case to what you earlier claimed.
What is the formula in case B for Ta and Tb?
Please don’t go the same way as Bryan on this one.
Scienceofdoom, let’s go over your proof line by line to see where you went wrong, OK?
“Tb = (P/Aσ)0.25 ….[eqn mk1]”
OK, let’s assume the above is true.
“and now if we substitute eqn mk1 into your “general” formula we get:
Ta = (P/Aσ + Tb4)0.25”
OK, here is where you screwed up. The Tb in the above equation does not equal the Tb in your first equation. Above Tb = (Ta^4 – P/Ao)^.25. In your first equation, Tb = (P/Ao)^.25. You can’t make the substitution. You have two different Tbs!
“therefore Ta = (P/Aσ + P/Aσ)0.25”
Therefore this is wrong and so is everything that follows. Ta does not = 1.2 Tb based on your wrongful substitution.
mikejacksonauthor,
In my last comment – May 7, 2015 at 3:10 am – I said:
In the comment above yours here (May 6, 2015 at 1:22 am & earlier than my last comment) I said:
I’ve already disproved one assertion (that the solution of the equations gave Ta = 0). Doesn’t it bother you that you were wrong on such a simple point? Why should I disprove more mistaken assertions that you make?
Go ahead and write out your formulas for Ta and Tb under case B.
I will help you:
…i) You have two boundary conditions
…ii) One for the temperature of shell A, and one for the temperature of shell B
…iii) Both involve the conservation of energy and both involve the Stefan-Boltzmann law.
Two boundary conditions gives two equations. Energy in = Energy out in both case. You can calculation energy out and energy in for both boundaries using the Stefan-Boltzmann law
It should be so easy for you. Why haven’t you written them down?
Ok, I know the answer to that last question..
I thought you were looking for the surface equilibrium temperature for various scenarios? If so, you can use Ta = (P/eAo + Tb^4)^.25. If P is constant.
mikejacksonauthor,
What you have written here almost answers the problem.
I will substitute values into the formula you wrote if you like.
First of all Tb has no outer shell, so your “general” formula you wrote as Ta, when applied to Tb reads:
Tb = (P/Aσ)0.25 ….[eqn mk1]
note: I have removed emissivity, e=1 for simplicity
and now if we substitute eqn mk1 into your “general” formula we get:
Ta = (P/Aσ + Tb4)0.25
therefore Ta = (P/Aσ + P/Aσ)0.25
therefore Ta = (2P/Aσ)0.25
therefore Ta = 20.25.Tb = 1.2 Tb ….[eqn mk2]
would you agree with this?
No. If Ta = (P/Ao + Tb^4)^.25, then Tb = (Ta^4 – P/Ao)^.25, not (P/Ao)^.25.
Therefore Ta = (P/Ao – P/Ao + Ta^4)^.25
Ta = Ta when simplified.
If you assume that Ta = 0, then Tb = (-P/Ao)^.25. |Tb| = (P/Ao)^.25.
But then Ta != 1.2Tb unless Tb = 0. So what you end up with is P = 0; Ta = 0; Tb = 0.
mikejacksonauthor,
This is why I asked you to write down the equations for 2a and 2b and not make a general assertion with “you can calculate using..”
You claimed in case A (May 5, 2015 at 8:56 pm) that the temperature of the shell, Ta = (P/Aσ)0.25
Your statement was:
Are you saying that in case B the temperature of the outer shell, Tb is not this value?
Tb = (P/Aσ)0.25
If not, then why has the Stefan Boltzmann law changed? We have a constant internal power source of P. And a surface of area, A, emits power at the rate AσT4.
In steady state, power in = power out. Power input = P. Power output = AσT4.
What is the formula for Tb in case B? Please explain.
mikejacksonauthor,
In your statement of May 6, 2015 at 5:00 pm you said:
You have taken one equation and by re-arranging it have proven what? You could re-arrange it and prove P=P or 0=0 or 1=1. You can do this with any equation.
E = mc2, so m = E/c2, therefore – amazing step, substitute m into the original equation and we get E = Ec2/c2, which when simplified gives E = E.
Hilarious! And, because E=mc2, no other equation for E or m can ever be derived by knowledge of physics principles?
In a system with 2 unknowns we need 2 independent equations to solve for both values. The fact that an equation exists for a value doesn’t mean it is impossible to derive a second equation from physics principles.
In fact the second equation is necessary.
Luckily, we have a second boundary condition, which creates a second equation. I just showed that it comes from your earlier claim – at 9:59 pm.
I look forward to you explaining why your earlier equation for a shell with an internal power source no longer applies.
Scienceofdoom, you asked me if I agree whether Ta = 20.25.Tb = 1.2 Tb. My answer was no, because, as you demonstrated yourself, it is the wrong answer. If you do the algebra properly, you end up with nonsense like Ta = Ta. To get Ta = 1.2Tb, or Tb = (P/Ao)^.25, Ta must be set to zero. As a result your answer is 0 = 1.2Tb, so Tb must be zero also. You have 0 = 0. More nonsense.
“I look forward to you explaining why your earlier equation for a shell with an internal power source no longer applies.”
Why should it no longer apply. I’m assuming the shell has a temperature and the surface has a temperature. The goal is to find the temperature of the surface. So Ta = (P/Ao + Tb^4)^.25. Put in values for Tb and P and A, calculate Ta and all will be well.
mikejacksonauthor,
You agree that in case B, Tb = (P/Aσ)0.25 ….[eqn mk1] ?
Or not?
You say:
I have not demonstrated it is the wrong answer. It is the right answer.
Do you know how to solve 1 equation with 2 unknowns where you would like to find the actual numerical values?
It is not possible.
You need 2 equations. 2 unknowns requires 2 equations.
If you have one equation you can’t find the values.
To get Ta = 1.2 Tb, Ta must be set to zero?
No. It is the solution to the two equations. If you plug Ta = 1.2 Tb, and Tb = (P/Aσ)0.25 you get an answer for Ta and Tb which are not zero.
For any readers who don’t like maths but do have a calculator and are wondering if mikejacksonauthor is onto something, just try:
P = 1000
A = 12
σ = 5.67×10-8
Tb = [ 1000/(12 x 5.67×10-8) ]0.25 = 196 K
Ta = 1.2 Tb = 235K
mikejacksonauthor says Ta has to be zero but we can see that Ta is not zero and the equations are easily satisfied. This hopefully demonstrates to all readers that mikejacksonauthor is incorrect in very simple algebra.
I asked:
“I look forward to you explaining why your earlier equation for a shell with an internal power source no longer applies.”
You respond:
You say “why should it no longer apply?” yet your other statements I cited here claim the equation is wrong. At this point I realize you have no idea how to do simple maths or even confirm or deny a statement. Go back to my question at the start of this comment.
The only way you will learn anything is actually write out the 2 equations for yourself.
If at some stage you want to post up your 2 equations for Ta and Tb under case B along with the working then I will respond. If not, I will ignore following comments.
Anyway, scienceofdoom, I think I’ve solved your missing power problem. The sun provides 239W/m^2, but this is not enough. The highest temperature you can achieve is -18C or 255K. I pointed out in an earlier comment that the moon has a mean temperature of -20C. It gets as much sunlight, but it does not have an atmosphere.
So what happens when you add an atmosphere? You add mass and energy to the system. How much is the question and how much does it contribute to the mean temperature?
Using the formula PV = RnT, I was able to calculate that air pressure at around 6atm causes your temperature figure of 255K. This is equivalent to the earth with no atmosphere. Add another atmosphere and we get 7atm. Since the volume of earth’s atmosphere is fairly constant, we can calculate the new temperature with P1/T1 = P2/T2.
6atm/255K = 7atm/T
6T = 1785
T = 297.5K = 24.5C.
Now I’m 9.5 degrees too high, but you can see just how much earth’s atmosphere contributes. Now the question is what brings the temperature down to 15C?
Mike: You may want to distinguish more carefully between energy (P*V work) and power (energy/time). When we discuss an object with a constant temperature, that object radiates eoT^4 W/m2 (power) indefinitely. No one-time source of energy, such as PV work, lasts indefinitely. So the trick to solving any problem involving an object at constant temperature is to start with the principle that power in equals power out. When power in doesn’t equal power out, the object’s temperature rises or falls until it does.
“No one-time source of energy, such as PV work, lasts indefinitely.”
Frank, there is nothing one-time about earth’s atmosphere. The atmospheric pressure is constant thanks to the mass of the molecules and gravity.
“So the trick to solving any problem involving an object at constant temperature is to start with the principle that power in equals power out. When power in doesn’t equal power out, the object’s temperature rises or falls until it does.”
Well let’s look at the equation: T = PV/Rn. There is no power going in and no power going out. 0 Power in = 0 Power out. Problem solved. Temperature can rise and fall or stay constant on the basis of pressure and volume as well as power.
Mike wrote: “Well let’s look at the equation: T = PV/Rn. There is no power going in and no power going out. 0 Power in = 0 Power out. Problem solved. Temperature can rise and fall or stay constant on the basis of pressure and volume as well as power.”
You are correct about the ideal gas law, but incorrect about no power going in or out. Collisions between gas molecules produce excited rotational, vibrational and electronic states that spontaneously emit photons. If EM is passing through the gas molecules, they may absorb photons. In theory, power out never equals zero unless the gas is at 0 degK and power in never equals zero unless the surroundings are at 0 degK. In practice, mono-atomic and symmetrical diatomic gas molecules have negligible emission/absorption at most wavelengths including thermal infrared. The gases of most interest – GHGs – strongly emit/absorb some of the thermal infrared wavelengths that transfer heat from the earth to space.
Let’s try a thought experiment with a can of compressed air (like those used to blow dust off computer equipment). V, n, and R are fixed. P changes linearly with T and/or T varies linearly with P. When the manufacturer filled this can with compressed air, the air inside the can became hot because PV work was done on the gas. Sitting in my house, however, P is determined by the temperature of the room, because the can has reached thermal equilibrium with its environment.
To make things clearer, let’s imagine the same can of compressed air in space where the only way heat can enter or leave is by radiation. (Let’s also imagine it is spinning rapidly so that all surfaces receive equal irradiation.) If the can is orbiting the sun at 1 AU (the distance from sun to earth), we can calculate how much power it continuously absorbs from the sun and therefore how much power it can emit as blackbody radiation without its temperature changing. Knowing how much power it emits AT STEADY STATE, we can calculate its temperature and therefore its internal pressure. If we add gas to the can, the gas will heat up because PV work has been done. Due to its higher temperature, it will radiate more power than it receives from the sun and gradually cool until it reaches the same steady state temperature it had before gas was added.
Now let’s instantly move the can to an orbit 9 AU from the sun where it receives 1/81 as much energy, but is initially radiating just as much power as it did at 1 AU. The absolute temperature AND pressure inside the can will gradually drop until it is 1/3 what it was at 1 AU.
In all cases, the final steady state temperature the can reaches depends only on the power of the incoming radiation (and the emissivity of the can). The steady state internal pressure depends on that steady temperature (and n and V).
A planetary atmosphere behaves somewhat differently because it has: a pressure determined by the weight of the gas above every square meter at a given altitude, a undefined “volume”, and a density that changes with altitude/pressure and temperature (n/V = P/RT). Photons are absorbed and emitted by the gas molecules, not the surface of a can confining the gas. However, the steady state temperature adopted by the atmosphere depends on the incoming power it absorbs and the atmosphere temperature needed to emit the same amount of power to space.
Mike also wrote: “So the thing that is putting the work into the system on an ongoing basis is gravity. Gravity and the sun warm the earth.”
Work is force times distance (or, more accurately, the dot product of the force and displacement vectors). Gravity is the force, but it currently doesn’t produce any net vertical movement of the atmosphere and therefore does no work on the atmosphere.
When the earth, sun and other planets were coalescing out of a rotating cloud of gases and solids, gravitational potential energy was converted to kinetic energy. That provided enough heat to melt the coalesced solids and produce spherical planets. PV work was done on the atmosphere as the gas molecules “fell” closer to the surface and occupied a smaller volume, which eventually initiated fusion in the sun. However, this happened 4.5 billion years ago. The heat from gravitational collapse was lost by the surface and atmosphere long ago by radiative cooling to space. Any heat from coalescence or radioactive decay that remains is buried deep inside and produces outward fluxes too small to be relevant to surface climate.
Frank, thanks for you comment. Your can in space has weak gravity. According to the equations I listed, the more gravity you have the higher the pressure and the higher the temperature. Technically gravity does no work in the sense of W=mcd. Gravity is like you pushing against a brick wall. No work is done (technically), but you will work up a sweat and your body temperature will rise. Go figure!
To further demonstrate how atmospheric pressure is a major factor in the warming of a planet, here are some comparisons using the formula T = PV/Rn. P = Mg/m^2. M is the mass of earth’s atmosphere, g is the acceleration due to gravity. The moon has a g of 1.6m/s^2. Earth has a g of 9.78 m/s^2. Jupiter has a g of 24.7m/s^2.
Notice that when you increase g, the pressure increases, so does the temperature T. The converse is also true.
So the thing that is putting the work into the system on an ongoing basis is gravity. Gravity and the sun warm the earth.
Mike wrote: “Technically gravity does no work in the sense of W=mcd [mgh?]. Gravity is like you pushing against a brick wall. No work is done (technically), but you will work up a sweat and your body temperature will rise.”
Frank replies: Work is work – a form of energy usually measured in Joules. Some “technical” legal laws may be broken, but not the laws of physics. If gravity doesn’t cause net vertical movement of the atmosphere, additional kinetic energy will not be available to be converted to heat. In the case of pushing against a brick wall, force doesn’t do any work or produce any heat (unless the wall moves). The energy that warms your body is chemical energy from hydrolysis of ATP. That energy comes from eating food. P*dV is also work. It is F*ds work in disguise, (F/A)*(ds*A). Pressure alone is not work or energy. The pressure at the bottom of the ocean is many orders of magnitude larger than atmospheric pressure, but the bottom of the ocean is colder than the surface.
Your mistaken idea that gravity – and the atmospheric pressure it creates – warms the atmosphere without net vertical motion is a violation of the principle of conservation of energy.
You correctly note that planets with thicker atmospheres and higher surface pressure usually have higher surface temperature, but this happens because atmospheres usually contain GHGs. Such atmospheres slow down rate at which radiation escapes from the surface to space: from 390 W/m2 to 240 W/m2 on Earth, and from 16,700 W/m2 to 65 W/m2 on Venus. The optical properties of these gases – not their weight – are critical to slowing down this radiative cooling. (Pressure does broaden the absorption bands of GHGs.)
In an ideal science experiment, we would replace Venus’s CO2 with N2 and see what happens to its surface temperature. Unfortunately that isn’t practical. We are currently conducting an experiment here on Earth by adding CO2 to our atmosphere, but that experiment is complicated by changes in water vapor, clouds, lapse rate, surface albedo, solar output, volcanic and anthropogenic aerosols, and unforced variability arising from chaotic changes in ocean currents. Little dynamic range and numerous poorly controlled variables make this a terrible science experiment. It is difficult to draw conclusions from the 0.5 K of warming in the last quarter of the 20th century or the hiatus in warming since. Instead, we have carefully studied the interaction between GHGs and radiation in the laboratory for more than a half-century – well before fears of CAGW politicized climate science. Those experiments explain why GHG’s reduce outward radiation. We can’t accurately quantify this reduction, because temperature-dependent changes in water vapor, clouds, lapse rate and surface albedo also influence outgoing radiation.
mikejacksonauthor said on May 8, 2015 at 2:07 am – copied and pasted from his comment of above:
First of all, I take my hat off to you for proving me wrong.
Earlier, May 7, 2015 at 10:03 pm, you said:
Here you have Ta = 1.2Tb. So, a big hats off.
1. You have a formula for Tb that has variables and a physics constant. It’s clear where they come from.
You have a formula for Ta that contains a number, 0.1576.
Can you give us the formula that creates this value?
2. In case B, the emission of thermal radiation from shell A, from the Stefan-Boltzmann law:
Ra = Aa.σTa4 = 12.6 x 5.67×10-8 x 2314 = 2,034 W
Correct?
If so, how is it possible for the surface of shell A to radiate at over 2,000 W when it is only heated internally by a source of 1,000 W?
Of course the answer to Ta2 is based on your assumption that Tb = (P/Ao)^.25. And that Ta = Tb. We could start with the assumption that Tb = (Ta^4 – P/Ao)^.25 and that Ta > Tb, which is always true. So the fact that Ta > Tb doesn’t necessarily mean Ta2 > Ta.
If we assume Ta and Tb are the same temperature, they could together become Ta2 and the zero Kelvin surroundings could become Tb2. Since Tb2 = 0.00K, the new equation would be Ta2 = (P/Ao)^25. In that case, Ta2 = Ta.
If Ta2 > Ta, then the mean temperature of a combined Ta2 and Tb would be greater than Ta and P would increase as you have shown. You can’t have more P then you started with. Here’s the equations:
Tab = (Ta2 + Tb)/2
Ts = surrounding environment at 0.00K.
P = oAe(Tab^4 – Ts^4) > oAe(Ta^4 – Ts^4)
If you really could create more power than you started with, think how rich you would be selling your new unlimited power machine!
“You have a formula for Ta that contains a number, 0.1576.
Can you give us the formula that creates this value?”
Cross multiply the fractions then add them and convert to decimal.
One form of this “unlimited power machine” – i.e. creating a higher internal temperature by making it more difficult for outgoing heat to leave – is called pipe lagging and people make a bit of money off it but there is a lot of competition, which reduces the profit margins.
If you read the Solution article and tried to learn something you would find out that the “conservation of energy” law has not been violated.
It’s pretty simple. Energy gets stored, manifests itself in higher temperatures.. anyway why am I repeating myself? It’s there for you to read.
Instead you will meander around with pointless comments for a few days, like everyone else who can’t write down a conservation of energy equation.
And finally leave without writing down two equations. And not realizing why.
My hat is firmly back on. My hat was off for 2.5 hours and that was obviously too good to be true.
mikejacksonauthor comments in another page:
And now it turns to rubbish so quickly. My hat is temporarily back on.
Don’t base on anything on my assumptions.
Please produce 2 equations with your working which are based on physics.
“Please produce 2 equations with your working which are based on physics.”
Why do you believe you can have more power than you started with? There’s an old saying in physics: “You can prove anything if you start with the assumption that fits.” That’s why scientists do experiments and carefully check their assumptions.
Equations are only a feeble attempt to model reality. They are beautiful even when they spout utter nonsense.
Did you read the article?
Conservation of energy produces the solution. That is – no energy has been created.
The inner surface (A) emits more radiation because it is hotter. It isn’t “producing power”. You can’t tap into this power. It is a store of energy. Counted in Joules.
Joules in – Joules out = change in energy.
The countless hordes of well-meaning citizens who think they know something about heat transfer and conservation of energy and yet we wait for a single one to produce two equations to be verified.
Instead they write profound statements.
Strictly speaking you produced two equations for 2.5 hours and then apparently recanted them. How did you produce them?
An insoluble problem?
Impossible to work out the answer?
Hopefully the silent readers are learning something..
At the heart of mikejacksonauthor’s replies is a conceptual problem, captured in:
What is important to understand is that the equations that calculate the temperature and emission of radiation in the example are from the conservation of energy.
They are explained in the article that mikejacksonauthor is responding to here.
So if you use conservation of energy to get “the result” and “the result” says that an inner surface is radiating 2000W when the internal power source is only 1000W then either you have made a basic maths error that should be easy to spot – OR – the result doesn’t violate the conservation of energy.
Which is it? How can shell A radiate 2000W when it is only powered by 1000W?
The answer is simple, and explained in many other articles on this subject.
Shell B, outside shell A, radiates back 1000W. So every second, shell A absorbs 2000 Joules and emits Joules.
No violation of the conservation of energy.
Shell A is hotter than shell B. When there was no shell B in place, shell A was cooler. It radiated out 1000W. That is because it was on the boundary with deep space and received no energy back from deep space. So it was cooler.
Then along came shell B, placed around shell A – like lagging a pipe.
Heat could not get out so easily, so shell A heated up. And heated up. And heated up. The more shell A heated up, the more it radiated. Until eventually it reached a steady state condition where it radiated 2000W – 1000W to balance the internal source and 1000W to balance the outer shell radiating back.
Shell B, just to complete the happy picture, is receiving 2000W and radiating 1000W to outer space and 1000W back into the interior. So it is in a steady state.
Everyone is happy because all Joules have been conserved.
Everyone is happy, that is, except those people with conceptual problems.
In this case, you have the patience of a saint. I tend to not bother reading posters who routinely make several consecutive posts. RW is a classic example of the breed. It indicates to me that their thinking isn’t particularly well organized. The lack of an edit function is no excuse.
Scienceofdoom, If you can get 2000W out of 1000W, again, what is stopping you from building a machine that produces unlimited energy? You also have a second problem: a violation of thermodynamics. Heat always flows from hot to cold, never from cold to hot. So shell B can’t heat up shell A beyond what 1000W will give.
You you start with a temperature for shell A that corresponds with your maximum power 1000W, then you add to that! As if you could! You start out with a system that is 100% efficient, then add more! You can start with less and add more.
For example, if the shell A was less than 193K and your heat source was suffering heat loss, and then you added shell B to insulate, so you have less heat loss, that could bring your temperature up to a maximum of 193K. 100% efficiency! You don’t get to have more than that! In reality, both shells lose heat to space, since hot always goes to cold, not vice versa.
I’ve worked it all out for you:
Your problem really has four variables: the mysterious power source P, Ta, Tb and space Ts.
Ta = 193.4K; Tb = 192.4K; Ts = 0.00K;
Aa =12.56m^2; Ab = 12.81m^2
P=1000W; P = Pab + Pbs (law of conservation)
This is the total power between Ta and Tb:
Pab = o((12.56m^2)193.3^4 – (12.81m^2)192.4^4) = 0.00W.
This is the total power between Tb and Ts:
Pbs = oAb(192.4^4 – 0) = 1000W.
Notice you have 1000W going in and out and no violations of heat transfer rules. So what has happened when shell B was added? It caused the area that was formerly occupied by space to warm up to 192.4K. When there was only shell A, the mean temperature was 193.4/2 = 96.7K. When shell B is added, it increased to (193.3 + 192.4)/2 = 192.85.
What happens in a greenhouse? Same thing. The ground warms the air when there is a glass shell around it, but the air does not warm the ground that is warmer than the air. Understand?
mikejacksonauthor,
Shell B radiates how much energy into space? Pbs (shell b to space)
Shell B radiates how much energy inwards? Pba (shell b to a)
I think you are claiming Pbs = 1000W.
Pba = ?
Total energy radiated by B, Pb = Pbs + Pba ?
I expect you will now invent a new law for emission of thermal radiation.
I wait. If you do respond and your answer is not Pba = σTb4 as found in all heat transfer textbooks you will need to provide evidence of your new law of radiation that doesn’t work from the argument from your personal incredulity.
If the answer is something like this:
“The two surfaces of a shell would be expected by crazy people (like Science of Doom) who don’t understand conservation of energy to have the same formula for emission of radiation. But, magically one shell knows it is facing inside and that another shell is radiating towards it, and then the easter bunny appears and then.. ” – it will make my day.
This is actually most people’s answer when they share your convictions. They just miss out the bit about the bunny and add some technical words. Same result.
Scienceofdoom, I didn’t make up the first and second law of thermodynamics, nor did make up energy and power conservation. I took those out of College Physics 10th Edition. What I don’t find in any books is your new law of physics that says Tb gets to transfer energy to Ta when Tb <= Ta. Nor do I find your other law that says you can start with 1000W and end up with 2000W.
Now you are obviously headstrong, so I think it's time for an experiment, a real one, not a fantasy thought experiment with a magical power source . I answered your challenge, now here is mine:
Heat some water to 373K. That should give you a nice hot power source (14kW). Put the water in a thermos bottle. The water is your Ta and the thermos is your Tb. Now, if your theory is correct, after time t you should be able to open the thermos and measure the temperature and … eureka! The temperature will be over 373K!
Now if I'm right, or I should say, if my college textbook is right, the water temperature will remain at 373K or be lower.
Now 373K is an arbitrary number. You can do this experiment with what ever temperature suits your fancy. Now go to it and let us know how you make out. Personally, I hope you are right and my physics book is wrong. It's always been my dream to create energy out of nothing. Good luck and God speed!
Well, not really. You wrote down two equations (after much pressing).
You then recanted one equation and wrote down a different equation once I pointed out that it contradicted your earlier assertions.
As requested on May 8, 2015 at 11:03 pm I want you to write down your equations for emission of radiation from shell B.
You need to defend your answer. Then I will be able to demonstrate that you have invented a heat transfer equation to get the answer you believe in.
If you understand heat transfer you will be able to write down these equations and they will match textbook answers.
If you don’t understand heat transfer you will “do a Bryan”.
In other news, I hope you will enjoy Geese, gold, bunnies and the First Law of Thermodynamics.
Scienceofdoom, Brian is right. You have a problem with reading comprehension. Scroll up. I put the actual solution (not your imaginary one) to the problem. As you know, I also came up with your solution when I used your imaginary assumptions. But I prefer a solution that matches reality. If you are too lazy to scroll up, here is the solution again:
Ta = 193.4K; Tb = 192.4K; Ts = 0.00K;
Aa =12.56m^2; Ab = 12.81m^2
P=1000W; P = Pab + Pbs (law of conservation)
This is the total power between Ta and Tb:
Pab = o((12.56m^2)193.3^4 – (12.81m^2)192.4^4) = 0.00W.
This is the total power between Tb and Ts:
Pbs = oAb(192.4^4 – 0) = 1000W.
0.00W + 1000W = 1000W. All power drops in the system must add up to your total power. If they don’t, you have made an error.
Here is your version of reality:
Ta1 = 193K; Tb = 192K; Ta2 = 231.43K
P = 1000W; Aa = 12.56m^2; Ab = 12.81m^2;
Ta = (P/Aao)^25 ; Tb = (P/Abo)^25; Ta2 = (.1576P/o)^.25
In your universe Ta2 = 1.2Tb. But you didn’t check your work. Do the power drops add to 1000W? Let’s see:
Pab = 1000W, Pbs = 1000W
1000W + 1000W = 2000W.
Nope! You’ve made an error. Do the experiment I suggested above and you will see. Or you could do this experiment:
Let the sun heat some water. Measure the temperature. When you reach a desired temperature, put the pot of water in the shade and surround it with a glass container. The shade will prevent the sun from heating the water further. The point of the experiment is to see how much the IR from the water heats the water. The glass container will reflect the IR back to the water. After time t, check the temperature again. Is it higher or lower than your starting temperature? If your theory is right, the water should be hotter than when you first put it in the shade in the glass container. Good luck!
mikejacksonauthor,
I appreciate you giving me the solution again but I did ask for a value that you didn’t provide.
That value was the power radiated by shell B to shell A, Pba.
You did give a value Pab which I think is the power radiated by shell A to shell B.
You say Pab = 0
Is this what you claim is the steady state solution?
There is a problem (more than one) with the above graphic which interested readers will spot right away.
Of course you might have different values for Pab and for Pba, so I will wait for your confirmation before pointing out the problem.
“I appreciate you giving me the solution again but I did ask for a value that you didn’t provide. That value was the power radiated by shell B to shell A, Pba.”
The value you are seeking is zero. Why? Basic thermodynamics. This the last time I’m going to explain it to you. If Tb is the same temperature as Ta or less than Ta, Tb radiates nothing to Ta (area and emissivity are also factors, but for simplicity, let’s assume they are equal in both A and B).
It might help to think of P as the amount of power a black body radiates when its temperature is Ta and its environment or another object is Tb. That’s what it was originally designed for. So you have A and B. What is the power between them? P = oeA(Ta^4 – Tb^4).
Notice what happens when Ta and Tb are the same temperature as per your challenge. P = 0. If you want Tb to radiate to Ta, Tb must be a higher temperature than Ta, not the same temperature.
Think of power transfer as water and gravity. Water (power) always flows from the highest point to the lowest, never the other way around when there is no added work or energy.
That’s actually the first time you have asserted it.
I wish you had explained your ideas about radiation at the start. It would have saved me the effort of responding at all.
It is not what is found in heat transfer textbooks. It is something you have invented.
Here is an extract from an undergraduate heat transfer textbook, Incropera & DeWitt (2007):
I posted the extracts from six heat transfer textbooks from the university library in Amazing Things we Find in Textbooks – The Real Second Law of Thermodynamics.
This blog accepts standard textbook physics:
You can produce the textbooks that support your point of view if you like. I know for sure you don’t have any. There are quite a few blogs where invented physics is very popular and well-received, so you will get a good reception for your ideas there.
“You can produce the textbooks that support your point of view if you like. I know for sure you don’t have any.”
LOL! I can’t believe I’m reading this! There is nothing in your textbook that contradicts anything I’ve said! Read it carefully. If the surface temperature is warmer than the temperature of the surroundings, the net transfer will be away from the surface. That means the surroundings will not warm the surface. Tb will not warm Ta.
There’s even an equation that allows you to tell what will happen:
P = eAo(Ta^4 – Tb^4).
I’m flattered that you think I made this up, but alas, somebody beat me to it.
I can’t take credit for the second law of thermodynamics either:
“In this sense, energy or heat cannot flow form a colder body to a hotter body.” Source: http://www.physicsplanet.com/articles/three-laws-of-thermodynamics.
What I’ve told you is so basic and well-understood it is hard to believe you think I’m making it up! LOL!
By the way, I have another experiment you can try:
1. Let’s suppose your body temperature is 310K. (About 1000W assuming an area of about 2m^2 and an emissivity of 1.)
2. Wrap yourself in a blanket that is also 310K.
3. After time t, measure your body temperature again.
4. Pursuant to your equation, Ta = 1.2Tb, your body temperature should be 1.2 times higher. Or 372K! That’s 99C! 210F! LOL!
5. Now put on a second blanket, and a third, put on an infinite number of blankets and your temperature should be infinite, right? LOL!
See the problem yet?
“Tb radiates nothing to Ta”
This is true on the macro scale. Your net answer is still zero. If you get into quantum mechanics you could say that the expected value of all probable outcomes = zero.
– You said the colder surface doesn’t radiate. (May 9, 2015 at 11:04 pm) And tell me this is the “last time I’m going to explain it you”.
– I show one textbook (and give a link to many others) that says both the hotter and the colder surface both radiate. (May 9, 2015 at 11:22 pm).
– You say there is nothing in the textbook that contradicts anything you have said and this time make a different statement. (May 10, 2015 at 4:46 am). In fact you can’t even believe that I have claimed you said what you said just 5 hours earlier.
Who can know what you believe?
You have your mind made up and it is questionable whether it is even the same mind from one hour to the next.
No more from me.
SoD, maybe you’re right. Maybe Tb radiates to Ta. It’s hard to say in your imaginary universe where we can’t really observe what is happening. It’s a trivial point since it does not effect the bottom line, so it’s OK to treat Tb as not passing anything on to Ta.
Anyway, just to satisfy myself I worked out the real-world equilibrium temperatures of Ta and Tb. To do this, however, I had to give your magic power source a surface area of 12.31M^2.
When it’s the power source alone, 1000W (194.3K) is lost to space Ts (0.00K).
When you add shell A, it’s equilibrium temperature is 162.6K. Only 500W is lost to space and 500W is used to maintain Ta. 500W + 500W = 1000W.
When you add shell B, Ta increases to 174.7K. Tb = 146.14K. 333.3W is used to maintain Ta; 333.3W is used to maintain Tb; 333.4W is lost to Ts.
333.3 + 333.3 +333.4 = 1000W.
Note that Ta does increase like you said, but also note that its temperature never exceeds its power source, nor does the power ever exceed 1000W.
To solve this problem I simply divided the total power by the number of shells and Ts (space). That provides the value of each power drop. The sum of the power drops should equal 1000W. The power drops should be equal to each other so each shell receives the same power in as power out to maintain equilibrium.
Mike wrote: “Equations are only a feeble attempt to model reality. They are beautiful even when they spout utter nonsense.”
Equations appear to be beautiful when they make predictions that agree with our expectations. Equations are useful when they make predictions that always agree with experimental observations. When I walk on a concrete sidewalk, I don’t expect or perceive that the concrete bends under my weight until a force of mg prevents me from sinking further into the concrete. However, Newton’s laws of motion apply to this situation whether I like it or not.
The development of quantum mechanics (and chaos theory) has forced scientists to recognize that the physical world is probabilistic, not deterministic. Physicists don’t believe in QM because it is a beautiful theory – it is a very strange theory. See Schroedinger’s cat. Physicists believe in the theory because it makes extremely accurate predictions – albeit for reasons they don’t understand. Perhaps these 6 minutes from Feynman will help:
http://www.youtube.com/watch?v=_sAfUpGmnm4
The clip is from a longer lecture:
http://www.youtube.com/watch?v=xdZMXWmlp9g
When the IPCC tells us that the 70% confidence interval for ECS is 1.5-4.5 K, it reminds me of 18:45-20:00.
Frank, thanks for the update. I had no idea that physics had so much to it … well … actually I did. I’m also aware that the sky is blue. Are you aware that the sky is blue, Frank?
Anyway, to paraphrase Feynman, it doesn’t matter how elegant or beautiful your theory is, it doesn’t matter how smart you are or how clever you are, it doesn’t matter how many Nobel prizes you’ve won, if an experiment disproves your theory, it is wrong.
I have asked scienceofdoom to do an experiment to confirm or falsify his theory re: the magic power source inside the magic shells. Personally, I hope he is right–it would enable mankind to have an unlimited supply of energy. But what I hope has nothing to do with the scientific method, but then you know that like you know the wonders of physics.
Mike: Since you are challenging accepted physics, perhaps you should be the one to do the experiments. There have been numerous experiments reported in the blogosphere attempting to prove or disprove that radiation from the atmosphere is absorbed by the earth. It turns out to be very difficult to stop heat transfer by conduction and convection with amateur equipment and still permit heat transfer by radiation. Roy Spenser, a prominent skeptical climate scientist, has published several efforts on his blog.
http://www.drroyspencer.com/2013/08/revisiting-woods-1909-greenhouse-box-experiment-part-ii-first-results/
http://www.drroyspencer.com/2010/08/help-back-radiation-has-invaded-my-backyard/
IMO, the simplest experiment is to point an infrared thermometer at night at the sky and at the ground and interpret those measurements as the two-way flux of radiation from cold to hot and hot to cold. Roy discusses such experiments. As SOD has undoubtably told you, the 2LoT applies only to the net flux. (However, some skeptics say IR thermometers don’t work as scientists believe they do. No experiment will convince someone whose mind is closed.)
Those who normally deal only with bulk materials think the Second Law of Thermodynamics (2LoT) applies to all situations. As a chemist, I realize that the 2LoT does not control the behavior of individual molecules and photons – only the net behavior of large groups of molecules. (This caveat isn’t mentioned in most science courses.) Individual molecules have kinetic energy, but are not hot or cold. Only large groups of colliding molecules have a temperature – which is proportional to their mean kinetic energy. So individual photons can travel both directions between SOD’s shells (oTa^4 in one direction and oTb^4 in the other) or between the colder atmosphere and the warmer ground without violating the 2LoT. Collisions between individual molecules also produce some molecules with more kinetic energy than others – the Boltzmann distribution. (Statistical mechanics is branch of physics/chemistry that explains how the behavior of individual molecules produces bulk behavior that obeys the laws of thermodynamics.)
When you wear a jacket outside on a cold day, you feel warmer because you skin IS modestly warmer. This is insulation – making something with an internal source of heat warmer by slowing down the rate at which that heat escapes. Insulation certainly doesn’t violate conservation of energy. When we wear a jacket, however, we usually think we are reducing heat lost by conduction (which is very slow through air) or convection (which isn’t important on a windless day). A jacket also reduces heat loss by radiation using the same principles as SOD’s shells.
Your intuition, however, prevents you from accepting how much warmer things can get with really good insulation. SOD’s shells permits heat loss only by radiation from a source surrounded by two totally opaque shells. We don’t encounter insulation that effective in everyday life. As the video from the Feynman lecture points out, real scientists don’t let their intuitions about how things should behave interfere will discovering and correctly applying theories that accurately explain the way our world behaves. As Feynman says: If you don’t like it, go to another universe where the rules make more sense! Or as SOD has occasionally said, go to another blog – this one is based on accepted physics.
Very effective insulation does make the temperature of the center of the earth is roughly the same as the temperature of the surface of the sun. The only energy source below the surface of the earth is the decay of few rare radioactive elements. Heat transfer through solid earth (conduction) is so slow that the earth warms until the rock partially melts and heat transfer by convection becomes possible. When this molten rock reaches the surface as lava, it is still about 1000 degK and radiates 56,700 W/m2. Inside the earth, that molten rock radiates roughly a million W/m2 – to itself. Does this violate the law of conservation of energy?
“Mike: Since you are challenging accepted physics, perhaps you should be the one to do the experiments.”
Well I tried taking an ice bath but my body temperature didn’t go up. It went down. I don’t challenge accepted physics, only false claims posing as accepted physics. SoD claims you can have a power source of 1000W and end up with 2000W because he believes that Tb can warm Ta to a temperature that corresponds with 2000W, and, when Tb and Ta are the same temperature.
“There have been numerous experiments reported in the blogosphere attempting to prove or disprove that radiation from the atmosphere is absorbed by the earth.”
Well of course radiation is absorbed by the earth–especially when the earth is colder than the atmosphere.
“However, some skeptics say IR thermometers don’t work as scientists believe they do. No experiment will convince someone whose mind is closed.”
Well there’s another possibility, Frank. The skeptics could be right. And your mind is closed to that possibility.
“Those who normally deal only with bulk materials think the Second Law of Thermodynamics (2LoT) applies to all situations. As a chemist, I realize that the 2LoT does not control the behavior of individual molecules and photons – only the net behavior of large groups of molecules.”
Entropy controls nothing. It is not a force of nature, it is a measurement of disorder. And it isn’t limited to “bulk materials.” When 2H2 molecules and an O2 molecule combine to form H2O, the reaction is exothermic. Energy is lost. Entropy increases. Entropy also increases when a photon loses energy or when a high frequency EM wave loses energy. And I’m surprised you missed the increase in entropy of a CO2 molecule when it emits radiation. In a closed system, entropy can decrease if energy or work is added, but this is at the cost of an entropy increase outside the system that is greater.
“(This caveat isn’t mentioned in most science courses.)”
Gee, I wonder why? LOL!
“Individual molecules have kinetic energy, but are not hot or cold.”
But when they lose their kinetic energy, does entropy increase?
“Only large groups of colliding molecules have a temperature – which is proportional to their mean kinetic energy. So individual photons can travel both directions between SOD’s shells (oTa^4 in one direction and oTb^4 in the other) or between the colder atmosphere and the warmer ground without violating the 2LoT.”
But the shells do have a temperature and that’s what is measured, not individual photons. As long as the wayward photons don’t change the expected value of zero between Ta and Tb, I don’t see why they would violate the 2Lot. However, SoD claims they do and can! Tb warms up Ta!
“When you wear a jacket outside on a cold day, you feel warmer because you skin IS modestly warmer. This is insulation – making something with an internal source of heat warmer by slowing down the rate at which that heat escapes. Insulation certainly doesn’t violate conservation of energy. When we wear a jacket, however, we usually think we are reducing heat lost by conduction (which is very slow through air) or convection (which isn’t important on a windless day). A jacket also reduces heat loss by radiation using the same principles as SOD’s shells.”
You really should read SoD’s comments and mine. No one is disputing the fact that a jacket can prevent heat loss. SoD’s claim is not the prevention of heat loss. If it were, I wouldn’t bat an eye. He is claiming that the jacket increases your body temperature 1.2 times! And, if you put on another jacket (or shell), your body temperature would go up another 1.2 times! If you put on an infinite number of jackets (shells), your body temperature would be infinite! He treats each jacket or shell as an additional power source! Not as an insulator. Maybe this was not his intention, but that is what his math shows.
“Your intuition, however, prevents you from accepting how much warmer things can get with really good insulation.”
Straw man! Reading comprehension problem!
“SOD’s shells permits heat loss only by radiation from a source surrounded by two totally opaque shells. We don’t encounter insulation that effective in everyday life. As the video from the Feynman lecture points out, real scientists don’t let their intuitions about how things should behave interfere will discovering and correctly applying theories that accurately explain the way our world behaves.”
I agree. That’s why I mathematically disproved SoD’s solution and proffered a solution that was more in line with reality. Scroll up, read it, and don’t let your intuition make you weep.
“As Feynman says: If you don’t like it, go to another universe where the rules make more sense! Or as SOD has occasionally said, go to another blog – this one is based on accepted physics.”
I never said I don’t like it. I love it! If SoD is right, then he has found a source of unlimited energy! But like Feynman, I’m skeptical. Maybe you don’t like the fact that I’m skeptical. Feynman also said, “Science is a culture of doubt.”
“Very effective insulation does make the temperature of the center of the earth is roughly the same as the temperature of the surface of the sun. The only energy source below the surface of the earth is the decay of few rare radioactive elements. Heat transfer through solid earth (conduction) is so slow that the earth warms until the rock partially melts and heat transfer by convection becomes possible. When this molten rock reaches the surface as lava, it is still about 1000 degK and radiates 56,700 W/m2. Inside the earth, that molten rock radiates roughly a million W/m2 – to itself. Does this violate the law of conservation of energy?”
The earth? No. Your explanation? Yes! Here’s where you went wrong:
“Heat transfer through solid earth (conduction) is so slow that the earth warms …”
The earth does not warm. No new energy is added by insulation. You said yourself that insulation slows heat loss. When you put hot coffee in a thermos you do not warm the coffee, i.e., add energy; you slow down entropy.
Amendment to my last comment. Insulation does cause a body to warm by preventing heat loss, but no new power is added as SoD’s shell game suggests.
Mike:
Oh, where to begin in such a target-rich environment?
Your most basic error is that you consider the 1st LoT to be “conservation of power”. It is not! “Conservation of energy” is fundamentally a different thing, but you completely confuse the two concepts.
Just as “conservation of mass” does not constrain the magnitude of mass flow rates, “conservation of energy” does not constrain the magnitude of energy flow (a.k.a. power) rates.
Or to build on SoD’s financial analogy, let’s look at a comparable example employing “conservation of money”, the basis of all accounting.
Let’s say you have a $1000 weekly paycheck deposited to your savings account P. Each week you spend $1000 out of your checking account A. Your total account balance remains constant from week to week. Let’s say it’s $20,000.
To cover your spending from your checking account, each week you transfer $2000 from your savings account to your checking account (to prepare for any additional spending). But then each week you transfer $1000 back, because you did not spend it. Both accounts are in steady state balance from week to week.
By your logic, this should not be possible, because your monetary input is only $1000 per week, and you are transferring out $2000! But this kind of thing is done every day!
But this is exactly analogous to SoD’s shell thermal example. Energy must be conserved, but power rates are not constrained.
I am dumbfounded that you believe that the P+A system could have 1000 Joules/sec input and 500 Joules/sec output, and believe that the system is in steady-state condition (constant internal energy). If you deposited $1000/week into savings, transferred $500/week into checking, and spent $500/week out of checking, and your bank told you your total account balances did not change week to week, would you be satisfied? (If so, I would happily be your banker!)
Amendment to previous comment. Again using SoD assumptions and parameters:
Pba = P – oAaTa^4
Pba = 1000W – 2000W
Pba = -1000W
Or if you prefer to make Pba positive: Pba = 1000W.
[…] and recent comments in CO2- An Insignificant Trace Gas? – Part One […]
Mike: If you assign SOD’s power source a physical size, then it absorbs radiation from the inner shell and cast a shadow, complicating the solution to the problem.
The problem isn’t complicated at all if you keep the 2LoT in mind. Focus on the “net” direction of the flow. The Boltzman equation does the work for you. If shell if P puts out 1000W and shell A puts out 500W, what is the net power from P to shell A? It’s 500W. Shell A inturn puts out 500W into space. So 500W is lost. Now lets say we are not happy losing 500W to space. What can we do? I know! Let’s add shell B! When we add shell B, shell A’s power increases to 667W, so does it’s temperature. P continues to output 1000W. The net power from P to A is now 333W. The net power from A to B is also 333W and shell B loses only 333W to space.
As we add more shells we lose less power to space and we increase the power of each shell and its corresponding temperature. But no shell’s power will ever exceed P.
By contrast SoD’s solution has 1000W being lost to space. So either is insulation isn’t working or he has increased his power source. He chose the latter. For his system to output 1000W from shell B, P would have to equal 3000W! So he has created energy!
Mike:
The 1st LoT says that the change in internal energy of ANY system is the difference between the energy input and the energy output. Expressed as a rate, the rate of change of internal energy of ANY system is the difference between the power input and the power output.
Let’s use this for the combined P+A system. It has a 1000W input. If it has a 500W output, the internal energy of the system will increase at a rate of 500W. In this simple system, this internal energy increase will create a temperature increase. The temperature increase will cause increased radiative power increase.
The system will not reach steady state until the power output from the outer surface of Shell A to space is equal to 1000W. To do this, it will have the same temperature as Shell P alone in space had (or slightly less to account for the greater radius).
In this condition, Shell A will also be radiating 1000W inwardly toward Shell P. So now let’s apply the 1st LoT to Shell P alone. It has a power input of 1000W from the separate source, and now 1000W of radiative input from Shell A. Its only method of power output is radiative, so it must reach a steady-state temperature high enough to output 2000W. This temperature is significantly higher than if it were alone in space, radiating directly to space.
If you are more comfortable thinking about net radiative transfer, this net from P to A must be 1000W for the system and its components to be in steady state. Still, Shell P must have a significantly higher temperature to transfer this power to Shell A than it would have to transfer this same amount of power to space.
Undergraduates get problems of this level of difficulty in the third or fourth week of an introductory thermodynamics class. Those who cannot handle them by the end of the class are typically encouraged to find a less demanding field of study.
Curt, you wrote:
“Let’s use this for the combined P+A system. It has a 1000W input. If it has a 500W output, the internal energy of the system will increase at a rate of 500W. In this simple system, this internal energy increase will create a temperature increase. The temperature increase will cause increased radiative power increase.”
Curt, your assumption is wrong. You need a 1000W power source to have a 500W input to shell A. The net input between P and A is 500W, not 1000W. You can check this with the Boltzman equation. Let N = the net power input to shell A. Let Tp be the temperature of the power source; let Ta be the temperature at A. N = eAb( Tp^4 – Ta^4) = (1000W – 500W) = 500W. The corresponding temperature at A is in equilibrium, since A emits 500W to space.
“The system will not reach steady state until the power output from the outer surface of Shell A to space is equal to 1000W. To do this, it will have the same temperature as Shell P alone in space had (or slightly less to account for the greater radius).”
According to Boltzman, this would be physically impossible. If A exceeds 500W, then the power input will be less than 500W and the power output will be greater than 500W. A cools off and goes back to an equilibrium of 500W. If A is 1000W then P must increase to 2000W to keep 1000W of input going. Here’s where the 1LoT slaps your hand!
“In this condition, Shell A will also be radiating 1000W inwardly toward Shell P. So now let’s apply the 1st LoT to Shell P alone. It has a power input of 1000W from the separate source, and now 1000W of radiative input from Shell A. Its only method of power output is radiative, so it must reach a steady-state temperature high enough to output 2000W. This temperature is significantly higher than if it were alone in space, radiating directly to space.”
LOL! No, you don’t get to have more than 1000W in your budget! Again, you can check your assumption with Boltzman. We will now switch P and A around, since, according to you, A is now kicking back to P: N = eAb(Ta^4 – Tp^4) = (1000W – 1000W) = 0.00W. Gee, what happened to your 2000W?
“Undergraduates get problems of this level of difficulty in the third or fourth week of an introductory thermodynamics class. Those who cannot handle them by the end of the class are typically encouraged to find a less demanding field of study.”
After you, sir! The nearest exit is to your right. LOL!
Curt, as an illustration, add another shell to the system and see what happens if we follow your assumptions. Add shell B and P = 3000W. Add shell C and P = 4000W. Keep going … and P will explode to infinity! Isn’t that amazing? You started with a measly 1000W and you end up with unlimited power! With a 1000W battery you could power the world! LOL!
Curt, here is another problem with your analysis: If A has to warm up to 1000W and P warms up to 2000W, then A should then warm up to 2000W and in turn warm up P to 4000W and so on. This is far better than perpetual motion!
My May 12,2015, 1:52am replay ended up a little bit above.
Mike: You say, ” If A has to warm up to 1000W and P warms up to 2000W, then A should then warm up to 2000W and in turn warm up P to 4000W and so on. This is far better than perpetual motion!”
You obviously are completely unfamiliar with this type of problem. In a thermodynamics course, you would have solved dozens of these.
With a constant 1000W input, the P+A system MUST output 1000W from the outer surface of A to be in steady-state condition. So A must be at a temperature that provides this. If it started out at a higher temperature, it would cool to this temperature in the steady state; if it started out at a lower temperature, it would warm to this temperature.
With this same constant 1000W input, the P shell by itself must output a net 1000W to the rest of the universe. Since it is completely surrounded by shell A in a vacuum, this means that it must transfer a net 1000W to shell A.
Whether you consider this transfer 2000W out and 1000W back, or simply a net 1000W, you get the same answer for the (higher) temperature of the P shell surface.
These are STEADY-STATE conditions, so there is no “runaway”, no creation of energy, no “perpetual motion”. I repeat, if you had actually taken (and understood) an introductory thermodynamics course, these concepts would be second nature to you.
“You obviously are completely unfamiliar with this type of problem. In a thermodynamics course, you would have solved dozens of these.”
Well I took a thermodynamics course and I must have blinked because I missed your solution to this problem.
“With a constant 1000W input, the P+A system MUST output 1000W from the outer surface of A to be in steady-state condition.”
So how do you achieve a steady 1000W in and out? Your previous assumptions fell flat when put to the Boltzman test.
“So A must be at a temperature that provides this.”
And that temperature is?
“If it started out at a higher temperature, it would cool to this temperature in the steady state; if it started out at a lower temperature, it would warm to this temperature.”
Right. I put it at 500W for one shell and 667W, 333W for two shells. What’s your estimate?
“With this same constant 1000W input, the P shell by itself must output a net 1000W to the rest of the universe. Since it is completely surrounded by shell A in a vacuum, this means that it must transfer a net 1000W to shell A.”
Well here’s the rub: to get a net 1000W to A, you need 2000W. You then end up with 2000W going in and 1000W going out. Now we’re worse off because the power is 1000W.
“Whether you consider this transfer 2000W out and 1000W back, or simply a net 1000W, you get the same answer for the (higher) temperature of the P shell surface.”
Or maybe P counts as +1000W and the back radiation from A counts as -500W and the forward radiation from A counts as -500W. Now if you add that all up, it comes out to zero! Energy conserved!
“These are STEADY-STATE conditions, so there is no “runaway”, no creation of energy, no “perpetual motion”.”
Well my solution doesn’t have any of these problems. Yours has all of them.
“I repeat, if you had actually taken (and understood) an introductory thermodynamics course, these concepts would be second nature to you.”
Were you looking in the mirror when you said this? LOL! If you really want to convince me that I’m the dummy and your smart, put down your solution to this problem or fade away.
Curt,
I have encountered this mistaken idea many times on this blog.
Some variant of:
“..if A being surrounded by B causes A’s temperature to rise, then that will cause B’s temperature to rise and that in turn will cause ‘another’ rise.. so an infinite supply of energy”
[Obviously, general reader, this is a completely unfounded idea.. as Curt says the steady state solution is.. the steady state solution]
I believe the principal cause – apart from no training in solving steady state heat transfer equations – is the radiation value.
Most people are familiar with lagging pipes to increase the temperature of the water, but are not familiar with radiative exchange.
Mike himself first solved the equations to get the right answer, then back-tracked after I pointed out the emission of radiation this implied.
-Then suggested no radiation from B back to A and said A and B were at the same temperature.
-Then asked how I could possibly imply he had said that there was no radiation from B back to A after I produced a textbook.
-Then asserted it didn’t matter.
-Then confirmed that the temperature of A was higher than B but the overall radiation from B was lower than 1000W (at this point I gave up – as you point out this doesn’t satisfy conservation of energy).
In Do Trenberth and Kiehl understand the First Law of Thermodynamics? I gave a different thought experiment with a hollow PVC sphere. I demonstrated that the inner surface of the sphere had to be hotter than the outer surface.
I’m sure, if I had left it there, no one would have disagreed.
But I pointed out the radiation value from the inside of the sphere. You can read the comments (and in the follow on articles) to see how many different ideas are proposed to solve the conundrum. They are quite bizarre and all contradictory.
Later another blog wrote an article with their own calculations. I covered it in Interesting Refutation of Some Basics. In their article, to solve the problem, radiation was mysteriously transmitted through solid PVC. Then later, radiation cancelled itself out. Others on this blog suggested that no temperature differential was necessary to conduct heat through the PVC. And so on.
Many in the scientific quarters of the blog world impute nefarious motives to people with such beliefs. I just see people with great self-confidence but who would fail their physics degrees. And that’s fine.
Not everyone is cut to out to write down 2 equations and solve them.
I didn’t start this blog to convince the “physics-degree” Bryan’s and the mikejacksonauthor’s of the world. Of course, at the outset I don’t assume a particular commenter can’t be convinced of physics basics.
But I do believe many (often-silent) readers have genuine questions and so there’s value in highlighting their confusion.
“as you point out this doesn’t satisfy conservation of energy).”
Well Curt and ScienceofDoom, you have both failed to show how 1000W can push through two shells and come out 1000W. Using the Boltzmann equation, it would take 2000W to push out 1000W. By your logic, energy is not conserved in your solution and you created an extra 1000W!
Now I start with a different assumption: I assume it is NOT your power source’s power going in. It is the NET power going in. The net power going in in my solution is 500W for one shell. The net power going out is 500W. In your solution it’s 1000W net power going in and 1000W going out.
I’ve staring at this problem for a few days and it just dawned on me that the earth is very hot in the center, but the outer layers are cooler. Just like a Boltzmann! In the center of the sphere it is hotter (1000W), but at the surface it is cooler (500W). Add another shell and it is cooler still: 333W.
The sun works the same way. It’s millions of degrees at the center. Lot’s of power going in. But on the surface, only around 50K degrees.
So here’s a question: Why isn’t the sun and earth putting out the same power into space as you find at their centers? If you guys are right, they should. You might as how their energy is conserved. According to my calculations, energy is conserved, but let’s see if you two can figure out the puzzle.
mikejacksonauthor,
I said no more from me.
No more from you either.
The reason I said no more from me was because I couldn’t get you to provide a simple value. The value – radiation from shell B to shell A.
I would have used the value you provided to prove – using 1+1=2 style proof – that your claims are all flawed.
You chose to claim that it was zero. I provided textbook proof it wasn’t. You expressed surprise LOL that I would claim you had said it was zero. Later, you said it didn’t matter.
I can’t discuss physics with someone who won’t provide a simple value, or can provide an answer one day or a different answer another day and not even apologize for providing misleading answers. Physics is not like politics. The same answer that was true yesterday will be true tomorrow.
Equations for basic 100-year proven physics are written down and defensible. They are in textbooks.
You can – with one last opportunity – write down the value, and the equation that provides that value, Pba (radiation from shell B to shell A under case B) and I will respond.
You will need to be prepared to defend your answer with textbooks if people provide standard textbooks that show your equation is wrong.
Otherwise, there is no point in you clogging up this blog. You have 40 comments on this article in the last few days and a few in related comments on other articles. Everyone can see your LOL point of view. It is hilarious but we have other subjects to discuss.
Comments that fail to provide the value and the equation requested will be deleted. You can join an elite small, but proud, group of banned commenters.
One last comment from me. Mike’s fundamental error (which I reported in a comment that ended up too high in the thread) is that he believes that the 1st LoT requires “conservation of power”, which it most certainly does not! And then to keep conservation of power, he’s even willing to sacrifice conservation of energy.
Conservation of a quantity does not constrain the rate of transfer of that quantity. Conservation of mass does not constrain mass flow rates. Conservation of charge does not constrain electrical current rates. “Conservation of money” in accounting does not constrain money transfer rates.
And similarly, conservation of energy does not constrain power (energy transfer) rates. So a shell with a power input of 1000W can radiate 2000W steady state if it is also receiving 1000W from an outer shell. Energy is conserved. “Power conservation” is not an issue.
I pointed out above that this is exactly analogous to depositing $1000/week into Account 1, transferring $2000/week to Account 2, spending $1000/week from Account 2, and transferring back $1000/week back from Account 2 to Account 1.Both accounts are steady state week to week. By Mike’s logic, this should not be possible.
Curt, I had no idea there is no conservation of power. That’s definitely a new concept for me. LOL!
Anyway, I’m glad you’re not my accountant because you spend money you don’t have. The problem with your money analogy is that you are $1000 (1000W) short. You have to borrow $1000 (1000W) from the magic power machine to make it work. You start off with depositing $1000 into account one (1000W). So far so good, but then you transfer $2000 into account 2? Gee, where’d you get the extra $1000? The power bank? OK, we’ll let that slide and voila! You end up with an analogy that perfectly demonstrates the conservation of power that you say doesn’t exist? LOL!
So why did you complain about my so-called lack of conservation of power? I must say your logic and motives are confounding. As I recall, you were upset that only 500W was coming out of my shell rather than the expected 1000W. After all, according to you, energy in must equal energy out to maintain equilibrium. But work is also a factor. dU = Ein – W – Q.
To push 500W out of the system requires work of 500W. That adds up to 1000W, by the way. Here’s my analogy: You have a river flowing and a second river flowing against it. To maintain the same rate of flow the first river has to have a stronger rate of flow. It must work to overcome the opposing river.
Thus, if we start with 1000W and are opposed by 500W, then we output 500W. dU = Ein – W -Q becomes: 0 = 1000W – 500W – 500W.
Just for fun, let’s see what happens when we try to balance SoD’s assumption that shell B adds power to shell A. Both A and B start out equal at 1000W each. According to SoD, the 1000W at B is a positive feedback, so we give it a positive value in the equation: Ein + W – Q = dU. 1000W + 1000W – 1000W = 1000W.
Oh no! We have 1000W unaccounted for! dU should equal zero! Gee, I’m sure glad there’s no such thing as conservation of power. LOL! The good news is now we can build that power plant that provides unlimited energy on just a 1000W input. All we need do is add shells.
“You can – with one last opportunity – write down the value, and the equation that provides that value, Pba (radiation from shell B to shell A under case B) and I will respond.”
Using your assumptions and parameters:
Pba = oAbTb^4 – oAaTa^4
Pba = 1000W – 2000W
Pba = -1000W
Amendment to last comment.
Pba = P – oAaTa^4
Pba = 1000W – 2000W
Pba = -1000W
“I can’t discuss physics with someone who won’t provide a simple value, or can provide an answer one day or a different answer another day and not even apologize for providing misleading answers.”
SoD, I’m sorry if I upset you and I do apologize. You were right and I was wrong. Tb can send radiation to Ta. My zero answer should have been stated as the “net” transfer to avoid misleading the public.
I know I can be very critical and skeptical and I know I’m not always right. The good news is I wouldn’t be here if I thought I was always right. Unlike some commenters, I didn’t demean and dismiss your blog with a troll comment and click to another site. I consider myself an open-minded skeptic and devil’s advocate. I’m sorry I left so many comments. I will definitely cut back. I suppose I got hooked on the conversations. Your blog is the best in my opinion.
mikejacksonauthor said on May 12, 2015 at 9:24 pm
For reference, here is the graphic I drew on May 9, 2015 at 8:47 pm to show these variables:
You are claiming that the emission of radiation from P towards A is negative?
Please provide a textbook with your equation for the emission of radiation.
Textbooks universally provide this equation (for emissivity = 1, i.e. black bodies):
Pba = σAbTb4
e.g., from Fundamentals of Heat and Mass Transfer, Incropera and DeWitt (2007):
[this value is per unit area]
From A Heat Transfer Textbook, Lienhard & Lienhard (2008):
SoD,
The normal convention is to subtract the lower temperature from the higher. Mike appears to have done the opposite so the sign is also opposite, the flow is still from a to b, but as the equation is written to calculate the flow from b to a, the flow has a negative value, as expected.
“You are claiming that the emission of radiation from P towards A is negative?”
Pba in the context of your query is the radiation from B to A. I calculate it to be -1000W based on your numbers. Based on my numbers it would be -333.33W. I also should point out that your solution Ta = 1.2Tb is true in my solution as well. The point of contention is just how hot is Ta and Tb? And how hot can they get if more shells are added. My solution does not allow any figure equal or higher than the power source. Yours explodes to infinity. I have no doubt more shells will make A and B hotter, but I think there is an upper limit.
“Please provide a textbook with your equation for the emission of radiation.”
Your textbooks agree with mine for the most part and I’ve been using the Boltzmann equation, which agrees with your textbook. I also use an energy conservation equation to check my work. dU = Ein – W – Q. Change in internal energy = energy in – work – heat loss. By dividing both sides by time t, it is converted to a power conservation equation. This is not in my textbook. It is something I derived by converting energy (joules) into power (joules per second).
mikejacksonauthor
“Your textbooks agree with mine for the most part” ??????????????
In your last attempt you wrote down a different equation. There is no such thing as a negative value of emission of thermal radiation.
I’m asking you to:
a) justify your equation with reference to a textbook, Or
b) confirm the equation I have provided and give your answer
There is no fuzzy “inbetween” here. This isn’t a some subject where no one can be sure what the right equation is. If I ask for the current flow across a known resistor with a known voltage, everyone will give the answer I=V/R.
While you are at it, please also confirm the equation and value for Pbs (emission of radiation from shell B to space).
DeWitt,
I’m not asking for the overall net transfer of Pba – Pab.
I’m asking for Pba. And also Pab.
Once these values are provided I will demonstrate useful points that everyone can understand.
Mike:
You are just digging yourself deeper. You really don’t understand the difference between energy and power. Power is the rate of energy transfer. There is no reason for it to be conserved. Energy — the time integral of power — is conserved. If you cannot comprehend the difference, you cannot even start to deal with problems like this.
With regard to my bank account analogy, why do you assume the starting balance is $0? In our thermal problems we are not dealing with absolute zero temperature or zero internal energy. I almost stated a starting balance, but decided it wasn’t necessary to the problem. But because you could not understand the issue, I will state it explicitly now.
So the starting balance in Account 1 is $20,000, and Account 2 is $500. Each week, a $1000 paycheck is deposited in Account 1. Each week, $2000 is transferred from Account 1 to Account 2. Each week, $1000 is spent from Account 2, and $1000 transferred back from Account 2 to Account 1.
Both accounts are steady-state from week to week. But twice the amount of the money input rate to Account 1 is transferred to Account 2. According to your logic, this should not be possible!
Next, you claim that thermodynamic work is done in the power transfer between shells. No! You have no idea whatsoever of the concept of thermodynamic work. This is the simplest possible example of heat transfer with no work being done at all.
Besides, power transfers within a system, whether heat or work, do not change the amount of internal energy of the system, any more than transferring money between two of your own accounts changes your wealth. So for the system encompassing both shells, power transfers between shells do not affect the internal energy. In steady state conditions, for the internal energy to remain constant, all that matters is that the power transfer from the exterior of the outer shell to space match the power input of 1000W.
You get the most basic concepts of thermodynamics completely wrong. Until you can comprehend these points, you have NOTHING to contribute to the discussion.
“I’m asking for Pba. And also Pab.”
Pab = P + Pba
Pba = Pab – P
Translating to Boltzmann:
1000W = 0 + oATb^4
1000W = oATa^4 – 0
Pba = oATb^4 = 1000W
Pab = oATa^4 = 1000W
“So how can the temperature of Shell A (Ta) influence what Shell B emits.”
I don’t know that it does. All the Boltzman equation tells me is the net thermal transfer between the two shells and each shell’s rate of transfer to each other and away from each other.
“And as long as you properly record the power being radiated away from Shell B and absorbed by Shell A, energy will be conserved.”
You missed a couple of steps. You must also properly record the power being radiated away from Shell A and absorbed by Shell B. If Shell B is exposed to open space, then you must record what Shell B emits and receives from open space. Then energy will be conserved.
mikejacksonauthor,
Previously you confirmed Pbs = 1000W.
So shell B is radiating 2000W, 1000W outwards and 1000W inwards.
Every second it is losing 2000 Joules.
How many Joules is it absorbing every second?
“You are just digging yourself deeper. You really don’t understand the difference between energy and power. Power is the rate of energy transfer.”
You are correct. It is measured in joules per second or Watts. Sometimes horsepower.
“There is no reason for it to be conserved. Energy — the time integral of power — is conserved. If you cannot comprehend the difference, you cannot even start to deal with problems like this.”
You should go back and read your original comment. You complained that my solution didn’t conserve power. Now you are telling me power does not need to be conserved? Which story do you want to go with? Your first comment was at least on the right track: power conservation is the derivative of energy conservation. If you want to know how much energy goes in per second and how much goes out per second, you need power conservation. You dig?
“With regard to my bank account analogy, why do you assume the starting balance is $0?”
Because your real bank account is probably empty. lol.
“In our thermal problems we are not dealing with absolute zero temperature or zero internal energy. I almost stated a starting balance, but decided it wasn’t necessary to the problem. But because you could not understand the issue, I will state it explicitly now.”
Oh good. I’m such a dummy.
“So the starting balance in Account 1 is $20,000, and Account 2 is $500. Each week, a $1000 paycheck is deposited in Account 1. Each week, $2000 is transferred from Account 1 to Account 2. Each week, $1000 is spent from Account 2, and $1000 transferred back from Account 2 to Account 1.”
OK, it looks like you didn’t have to borrow any money this time. Good job. But what is your point? That power is conserved? No that can’t be it! You said power doesn’t need to be conserved.
“Both accounts are steady-state from week to week. But twice the amount of the money input rate to Account 1 is transferred to Account 2. According to your logic, this should not be possible!”
Actually, my logic has nothing to do with it. I just simply test your assumptions with the Boltzmann equation. And your assumptions don’t work. Your bank account analogy falls flat when applied to the actual physics. If you want to convince me you are right, put up your solution–and I don’t mean another cock-n-bull analogy or story. Show me your equations.
“Next, you claim that thermodynamic work is done in the power transfer between shells. No! You have no idea whatsoever of the concept of thermodynamic work. This is the simplest possible example of heat transfer with no work being done at all.”
I agree it’s not work as you understand it. The Boltzmann equation gives me the net transfer of power. It may not be like two rivers opposing each other. I just threw that out there since you like cute little analogies instead of math and physics. The bottom line is Pi = oAe(Ta^4 – Tp^4) = the net transfer in (Ein). Po = oAe(Tb^4 – Ts^4) = the net transfer out (Eout). Ein – Eout = 0. You’re telling me this is wrong–that the great Boltzmann is wrong! Incredible!
“Besides, power transfers within a system, whether heat or work, do not change the amount of internal energy of the system, any more than transferring money between two of your own accounts changes your wealth.”
I agree. But it can slow the rate of energy from 1000W to 500W; thus reducing the amount of energy going in per second. if you can get around the Bolzmann equation in mathematical terms instead of cute analogies that don’t fit, I’ll be impressed: Pi= 1000W – 500W = 500W going in. Po = 500W – 0W = 500W going out. 500W-500W = 0.
“So for the system encompassing both shells, power transfers between shells do not affect the internal energy.”
Agreed. They effect the rate of transfer in and out of the system.
“In steady state conditions, for the internal energy to remain constant, all that matters is that the power transfer from the exterior of the outer shell to space match the power input of 1000W.”
But it’s the net power input that goes in. 1000W only goes in if you have no shells. Add shell A and your net power in is 500W. Add shell B and your net power in is 333W. And the powers out match. Do the math and get back to me.
“You get the most basic concepts of thermodynamics completely wrong. Until you can comprehend these points, you have NOTHING to contribute to the discussion.”
Curt, with all due respect, if your so smart, show your math. Do the exercise. Show us how its done. So far you haven’t convinced me that you know what you are talking about. You can’t make up your mind if conservation of power is important or not. Now that’s sad.
Mike: Suppose I have a 2x2x2 m block of solid with emissivity 1 and temperature 288 degK. Total emission is 6 sides times 4 m2 times 390 W/m2. Now divide the block into eight 1x1x1 m blocks: 48 sides toms 1 m2 times 390w/m2. Twice as much. Now hollow out each block, which doubles the radiating surface area again. Are we violating conservation of energy?
Hell yes! To conserve, the temperature of the blocks should drop to 204K.
Mike: The block emits W/m2 depending on the behavior of the individual molecules in the block. How does any group of molecules in the block “know” how many photons to emits in each of these situations? Collisions with neighboring molecules excite a certain fraction of the molecules in any group into an excited state (usually vibration and rotation) and that fraction does depend on temperature. So any group of molecules “knows” the local temperature (but not the size of the blocks).
If a group of molecules emit some photons, how is that a violation of the law of conservation of energy? The block is a little colder and the photons are on their way to somewhere currently unspecified.
Is there some rule a small group of molecules in the block can follow without knowing what is happening elsewhere?
“Mike: The block emits W/m2 depending on the behavior of the individual molecules in the block. How does any group of molecules in the block “know” how many photons to emits in each of these situations?”
I doubt the molecules know anything.
“Collisions with neighboring molecules excite a certain fraction of the molecules in any group into an excited state (usually vibration and rotation) and that fraction does depend on temperature. So any group of molecules “knows” the local temperature (but not the size of the blocks).”
You talk about molecules as though they have a mind of their own. I’m not sure what you are driving at. It would help to know the temperature of the environment.
“If a group of molecules emit some photons, how is that a violation of the law of conservation of energy? The block is a little colder and the photons are on their way to somewhere currently unspecified.”
If the blocks are 204K, then energy is conserved. But I’m assuming the environment is 0 Kelvin and a vacuum.
“Is there some rule a small group of molecules in the block can follow without knowing what is happening elsewhere?”
I’m sure there are many rules, and I’m sure that the molecules know nothing about what is happening elsewhere.
Mike wrote: “I’m sure there are many rules, and I’m sure that the molecules know nothing about what is happening elsewhere.”
We are getting a little closer to the essence of the situation. Each group of molecules knows its own temperature, but nothing about its surroundings. However, I suggest there are not many sets of rules governing their behavior; just ONE set of rules that tell a group of molecules how fast to emit photons. If not, then the molecules would need some system for deciding which set of rules to follow at any given time and place. It is absurd to talk about what a molecule “knows”.
If so, what rule – preferably an equation – determines how fast a group of molecules emits photons. (You didn’t object when I claimed that emission of photons didn’t violate conservation of energy, so I’m hoping you will suggest something different.)
If the molecules are part of a solid object (and can only radiate outward) or a two-sided or hollow object (and can radiate either inward or outward depending on which face of the object they are found), is the rule/equation different?
(I’m referring to a group of molecules because a single molecule doesn’t have a temperature and because QM says the behavior of single molecules is probabilistic. A group of colliding molecules has a temperature that determines how many are in an excited state on the average.)
Mike: You may find an answer to SOD’s question (power flux from the outside of Shell B to the inside of Shell A in this series of questions. You have admitted knowing that a group of molecules on the outside of Shell B knows nothing about what is happening on Shell A when they are deciding how many photons (i.e. power) to emit towards Shell A. So how can the temperature of Shell A (Ta) influence what Shell B emits.
And as long as you properly record the power being radiated away from Shell B and absorbed by Shell A, energy will be conserved.
Frank wrote: “So how can the temperature of Shell A (Ta) influence what Shell B emits.”
Mike replied: “I don’t know that it does. All the Boltzman equation tells me is the net thermal transfer between the two shells and each shell’s rate of transfer to each other and away from each other.”
Frank responds: The Stefan-Boltzmann equation does NOT tell you about the net power transferred, it tells you only how much Shell A emits at any given temperature. Shell A may received radiation from Shell B or other somewhere else; it may receive heat from other source by conduction or convection, or the Shell A may be heated by radioactive decay, electrical resistance, chemical reaction, etc. No group of molecules in Shell A knows anything about the surroundings or these other processes, they simply emit eoTa^4. (In special circumstances, you can use the S-B eqn to prove that heat transfer between two objects is eoTa^4-eoTb^4, but this is not true in general.)
Frank also wrote: “And as long as you properly record the power being radiated away from Shell B and absorbed by Shell A, energy will be conserved.”
Mike replied: “You missed a couple of steps. You must also properly record the power being radiated away from Shell A and absorbed by Shell B. If Shell B is exposed to open space, then you must record what Shell B emits and receives from open space. Then energy will be conserved.”
Frank responds: One step at a time. As long as you account for the loss of energy from Shell A when emitting eoTa^4 and the gain of eoTa^4 by the surface that absorbs this radiation, energy will be conserved. It doesn’t make any difference whether Ta causes eoTa^4 to be 1000 W/m2, 2000 W/m2 or 1,000,000 W/m2; you don’t need to worry about conservation of energy. The same is true for Shell B (and any other component that may be present).
Shell A will cool if it doesn’t receive as much total power from all sources as it loses and will warm if it receives more. That doesn’t violate the law of conservation of energy. It does, however, contradict the premise of SOD’s problem – that the system is at steady state. You are being asked to solve for Ta and Tb such that: a) the net flux into and out of each Shell is zero AND b) each Shell emits eoT^4. There are many possible solutions if you only worry about constraint a).
I pointed out above that the amount of power radiated away from a 2x2x2 m block changes when you cut it up (exposing more surface). Energy is not being created. If the blocks are in dark space, they will simply cool off faster after being cut than before. If the blocks are in a room that is warmer than they are, they will warm up faster by absorbing more radiation through their larger surface area. And if the room is the same temperature as the block, nothing will change. If you hollow out a block, you create a new surface that can radiate away energy (inward), but the block will absorb all the inward radiation and be unchanged by hollowing – unless you put something inside. No group of molecules knows what is happening elsewhere, they simply emit eoT^4 – and absorb (or reflect or scatter) whatever radiation is incoming. For any wavelength, absorptivity equals emissivity.
If you do your calculations correctly, you will also find at the net flux of heat is always from hot to cold. If it isn’t, you made a mistake somewhere.
“S-B eqn to prove that heat transfer between two objects is eoTa^4-eoTb^4, but this is not true in general.) ”
So why are we using S-B eqn and not the right eqn (assuming it exists)?
Here’s my final answer to the puzzle: The center of the sphere must be the hottest place just like the sun’s core is the hottest place for the sun. Shell B must be the coolest place and shell A must me mediocre. The same is true for different layers of the sun. The surface is the coolest spot. 1000W is the power source. Since it is in the center, it must be the hottest thing in the sphere. Shell A is 667W. Shell B is 333W. These values put SOD’s sun in equilibrium. The net power drop (a term I stole from electronics) between P and A is 333W. The net power drop between between A and B is 333W. The net power drop between B and space is 334W. These power drops add up to 1000W in the same manner voltage drops at every point in a circuit must add up to the total voltage. 333W is also the net thermal transfer going in and the net thermal transfer going out. So overall energy and power is conserved. For more details, I put my solution above–way above! LOL! Scroll up.
I am 99% confident that my solution is correct. The challenge for you and everyone else to come up with a better one. Or, at the very least, demonstrate in mathematical terms why my solution is wrong and why shell A must be hotter than P and why shell B must be the same temperature as P when its facing 0.00K open space.
“So shell B is radiating 2000W, 1000W outwards and 1000W inwards.
Every second it is losing 2000 Joules.
How many Joules is it absorbing every second?”
2000 joules per second.
I updated the diagram with your last information:
Shell B receives zero energy from outside – in this example, deep space is at 0K.
It only receives energy from shell A. According to your last information, shell A is radiating 1000W to shell B.
I don’t see how you can claim shell B is absorbing 2000 Joules per second.
Do you want to revise your value for Pab?
“I don’t see how you can claim shell B is absorbing 2000 Joules per second.
Do you want to revise your value for Pab?”
Sure. By the way, how is my favorite blogger today?
I started out with your assumption that Ta = Tb. Ta warms up to 1.2Tb making it 2000W and Tb = 1000W. Your power source is 1000W. So the warmest place is at shell A and not the power source. When I look at the sun, the hottest part is the center. As you go closer to the surface it gets cooler. So naturally I’m wondering why your power source isn’t hotter than shell A.
So this is your equilibrium solution?
Do you want to confirm the temperature of shell A and B, Ta & Tb?
According to the crazy solution you have given, shell A has a power source of only 1000W but is radiating 2000W.
Where is this extra energy coming from?
That looks like your solution. My solution models the sun. The hottest part of the sun is the center, not an outer shell, and the surface of the sun is not the same temperature as its core. Your sphere (sun) is just as hot at the center as it is on the surface and shell A is the hottest spot. I don’t see how your sun can exist in the natural world. That’s why my sphere (sun) has P = 1000W; A = 667W and B = 333W.
Now some complain that my sun fails to output 1000W, but the real sun also fails to output the same power at its surface as it does in the center.
The way I see it, the only difference between the sphere and the sun should be that the sphere’s energy never runs out. It has a 1000W battery that never goes dead. This fact should not change the equilibrium temperatures; it only prevents entropy.
The whole time I have been asking you for your solution so I can demonstrate the flaws in your solution.
I have pressed you many many times for specific answers.
On May 12 at 7:08 I said:
May 13th 8:38 pm you said shell B emitted 2000W.
And now 3 hours later, you tell me that was “my solution” not “your solution”. In “your solution” shell B emits 333W.
This discussion is over.
This isn’t the blog for you.
Mike: https://en.wikipedia.org/wiki/Stefan–Boltzmann_law
Frank,
mikejacksonauthor’s further comments will not be making an appearance on the blog.
I can reveal his final thoughtful insights which didn’t make it out of the moderation queue.
I am a retard and this is an idiot blog.
So there we have it.
SOD: I limited my last comment to a reference, because it was clear that your patience had run out. I sometimes wonder why your patience survived my early comments (often immoderate from a mistaken sense that I was hearing a distorted version of science). My past frustrations make me think that I understand the source of some misconceptions. Experience proves that I usually don’t.
I wish more people would phrase their comments as questions, so they don’t get personally invested in trying to prove themselves correct (and others wrong). “Why isn’t the emission of X W/m2 of radiation a violation of conservation energy?” would start me on the path towards answering my own question – or at least framing my objection more clearly. “Why doesn’t DLR violate the 2LoT?” Science is about asking questions and seeking an answer to them. Religion (and usually politics) is about reciting a system of beliefs and values.
Frank,
Mike Jackson, like Bryan is a tr0ll. There’s probably even a name for the particular variety they represent. I’m not even convinced that they actually believe what they’re posting. I’m reminded of the Monty Python skit, The Argument Clinic.
[comment deleted]
Yet more tr0llish behavior, changing screen names to get past banning. And your solution is still wrong.
I have just found your blog and am trying to absorb the information contained in the pages that I have read through so far. Sorry if this has already been addressed by you but I can not help wondering why all the calculation on the emissions of the sun. This information is readily available and your calculated values don’t seem to add up. The same thing can be said about the earths emissions. NASA has a very nice graphic of energy emitted by the earth. Oddly enough all of the oceans are grey seeming that they do not emit energy but readily absorb long wave radiation.
crankyspanker,
Why calculate the solar radiation arriving at the earth? There are lots of different ways to approach a conceptual explanation like this. If it is not helpful, no need to consider it.
In a different series I go through the earth’s energy balance – start with The Earth’s Energy Budget – Part One. You can see the measured values in this article.
You say “..your calculated values don’t seem to add up..” – not a lot to go on there. If you provide specifics I will address them.
See the big dip centered at 667 cm-1? That’s CO2. That missing radiation must be compensated by increased emission at other frequencies for energy in to approximately equal energy out. That can only happen if the surface is warmer than it would have been without CO2.
When I read the I was depending on my memory and I should have just supplied references instead. I still contend that the energy that Carbon Dioxide is trapping thermal energy emitted by the surface of the earth, and if energy is being emitted the surface it would be cooler not warmer.
see link http://acmg.seas.harvard.edu/people/faculty/djj/book/bookchap7.html
Figure 7-8 Terrestrial radiation spectrum measured from a satellite over northern Africa (Niger valley) at noon. Blackbody curves for different temperatures are included for comparison. The plot shows radiances as a function of wavenumber (n = 1/l). The radiance is the radiation energy measured by the satellite through a viewing cone normalized to unit solid angle (steradian, abbreviated sr). Radiance and fn are related by a geometric factor. Major atmospheric absorbers are identified. Adapted from Hanel, R.A., et al., J. Geophys. Res., 77, 2629-2641, 1972.
By contrast, in the strong CO2 absorption band at 15 mm, radiation emitted by the Earth’s surface is absorbed by atmospheric CO2, and the radiation re-emitted by CO2 is absorbed again by CO2 in the atmospheric column. Because the atmosphere is opaque to radiation in this wavelength range, the radiation flux measured from space corresponds to emission from the altitude at which the CO2 concentration becomes relatively thin, roughly in the upper troposphere or lower stratosphere. The 15 mm blackbody temperature in Figure 7-8 is about 215 K, which we recognize as a typical tropopause temperature.
There is that pesky absorption at 1500 cm recorded by a satellite at noon and depending on which season the suns rays would be practically normal to the top of the atmosphere. I am not sure exactly how much energy this slice of IR radiation represents but since CO2 is well distributed so would that amount of thermal energy across a column of air 7 km high. One other thing when this curve hits zero for more and more of the world how exactly would doubling CO2 still cause temps to rise.
EL – I’m afraid you are getting it backwards. Let’s look at the Figure 7-8 in the e-book you cite. The total area under the solid curve (integrated over area) must basically match the incoming solar power. Any difference would result in warming or cooling of the earths’ thermal system.
The low spots in the curve on the left, marked H2O and CO2, show emission from cold high regions of the atmosphere, not from the warmer surface. Because absorptivity equals emissivity at any wavelength, we know that H2O and CO2 are absorbing the surface emissions at these wavelengths. You do see the higher direct emissions from the warm surface in the 12-10um and 9-8 um regions ( the atmospheric window).
Let’s say you could remove the H2O and CO2 from the atmosphere. Now the surface could emit straight to space in the 12+ um range, and the curve would pop up to the top line there. Now the total area under the curve, representing the power output, would be significantly greater than the solar input power, and the surface would cool. It would continue cooling until the area under the curve once again matched the solar input power.
That, in a nutshell, demonstrates why these absorptive/emissive gases lead to surface temperatures higher than would be the case with just transparent gases.
I think that is among the such a lot vital info for me.
And i’m happy reading your article. But wanna observation on few basic things, The web site
taste is perfect, the articles is truly great
: D. Just right job, cheers
[…] space. I tried to find a decent website and I found that this one was explaining it rather well: https://scienceofdoom.com/2009/11/28/co2-an-insignificant-trace-gas-part-one/ bigfooted, Nov 19, 2016 at 5:51 […]
“Is CO2 an insignificant trace gas?”
“No, CO2 AND water vapor are….”
Ask question “Is A significant?”
Answer with “Yes! A+B is significant!”
You see the sleight of hand?
Also note the Author buries CO2 saturation under the “Boring Numbers”. Aha… I think it’s interesting that pre-industrial CO2 downward radiation is already 95% of potential maximum. But I guess, “nothing to see here, move along”.
However much useful basic science was presented so plus one for that.
Arnim,
It’s A and B, not A plus B. That means both are significant. The sleight of hand is on your part by claiming that and is identical to plus. It isn’t.
There is no potential maximum downward radiation except under very well specified conditions. Downward radiation increases with temperature. There is no potential maximum, temperature that I know of.
DeWitt wrote: There is no potential maximum, temperature that I know of.”
The maximum that can be achieved by concentrating sunlight is somewhere between 5000 and 6000 K. It is less than the temperature of the sun’s surface, and is determined by considering the entropy of sunlight. The Second Law would seem to require that the same limit must apply at the bottom of an atmosphere, no matter the optical thickness. I think.
Picky, picky.
Of course you are correct, but that still makes Arnim’s 95% comment very wrong.
Arnim,
Good point. I intentionally misled everyone by writing an article to try and explain the simple stuff.
You can rest now that you have pointed this out.
A common criticism of articles that try to give an intuitive non-technical explanation to non-technical readers is that they are over-simplified.
A less common criticism of lengthy technical explanations is that people can’t understand them. The criticisms are less common because non-technical visitors can’t understand them and leave.
You can read Water Vapor vs CO2 as a “Greenhouse” Gas.
Likewise you can read the series Visualizing Atmospheric Radiation which explains lots of details and calculates the different effects of CO2 and water vapor for different concentrations.
There are many other series to read on this blog, you can find them in the Roadmap at the top.
“The energy radiated by the atmosphere which is received at the earth’s surface increases the temperature at the surface”
It depends. Anorganic or dead matter surfaces gets warmer as the air above from a certain solar irradiation influx on. Energy flows the other way around above energetical equilibrium.
It’s exactly this stoneoven heating effect, which spoils terrestrial temp observations: Most wether stations are situated in increasingly sub/-urban shaped environments or agriculturally devastated landscapes who expose bar dead soil for a couple of month (depending on latitude). But the bigger the air temp difference to surrounding air masses, the faster does convection transport the charged air up into the stratosphere with a huge leverage of “Self Loading water vapor Fright” (There is a steep Temp/saturation curve!)
Most models seem to ignore that a free floating humid atmosphere (especially in a H2O-optimized temp&density range) more or less the OPPOSITE represents
to a sealed greenhouse.
At least half ways: The body of Earth’s atmosphere equaly keeps as “Icehouse(!)” the lighted h a l f of Earth from heating up like moons surface (253F).
question regarding figure 2.9 from Taylor (2005) showing “carbon dioxide bite”.
The graph of flux versus wavenumber showing infrared radiation at the top of the atmosphere reveals the “CO2 bite”. As atmospheric CO2 concentrations increase, it might be expected that the “bite” would become larger or broader. Do you know of studies recording infrared radiance at the top of the atmosphere over time and if a change in the CO2 effect has been detected?
Thanks,
Bruce Krawisz
Bruce: You might see the reference below:
1) http://www.grandkidzfuture.com/the-climate-problem/ewExternalFiles/Harries%202001%20GHG%20forcing%20change.pdf
Increases in greenhouse forcing inferred from the outgoing longwave radiation spectra of the Earth in 1970 and 1997. NATURE | VOL 410 | 15 MARCH 2001 | http://www.nature.com
Abstract: “The evolution of the Earth’s climate has been extensively studied, and a strong link between increases in surface temperatures and greenhouse gases has been established. But this relationship is complicated by several feedback processes – most importantly the hydrological cycle – that are not well understood. Changes in the Earth’s greenhouse effect can be detected from variations in the spectrum of outgoing longwave radiation, which is a measure of how the Earth cools to space and carries the imprint of the gases that are responsible for the greenhouse effect. Here we analyse the difference between the spectra of the outgoing longwave radiation of the Earth as measured by orbiting spacecraft in 1970 and 1997. We find differences in the spectra that point to long-term changes in atmospheric CH4, CO2 and O3 as well as CFC-11 and CFC-12. Our results provide direct experimental evidence for a significant increase in the Earth’s greenhouse effect that is consistent with concerns over radiative forcing of climate.”
If you are a skeptic and look really closely at the data, you might find it raises as many questions as it answers. Why analyze only emission from clear skies in a small part of the Equatorial Pacific? Why didn’t they show the full CO2 band? 1997 was an El Nino year. Why if the CH4 change the clearest. We have another two decades of observations from space. Has the difference become better defined? The papers below show that the detection problem is more challenging than the above Nature paper implies.
2a) https://journals.ametsoc.org/doi/pdf/10.1175/JCLI4204.1
2b) https://journals.ametsoc.org/doi/pdf/10.1175/2009JCLI2874.1
HOWEVER, there has been a 1-2 W/m2 change in forcing from rising GHGs since instruments have observed the Earth from space. Some of the resulting radiative imbalance has been offset by increased OLR from warming. According to ARGO, the current imbalance is about 0.7 W/m2. Total OLR is about 240 W/m2. So the difference being sought is well below 1% of the total signal – measured with different instruments several decades apart. Looking for ambiguous evidence of an enhanced GHE from space is a challenging proposition.
Frank,
Thanks very much for your helpful comments.
Bruce Krawisz
Bruce: Others here have helped me, and occasionally rush to point out any mistakes I may make. That way we both profit.
Reblogged this on Climate- Science.
Excellent material!
I’m going to add the link to a posting that I’m preparing for my blog relating to global warming. I’ve been working on explaining the greenhouse effect in terms simple enough that a youngster could explain it to their parent(s). My latest will be:
Why CO2 Acts As A Thermal Blanket
As sunlight strikes an object, if it is white (like snow) then the photons mostly reflect off as light back into space. If the color of the surface is darker, then more of the photons are absorbed and converted into heat (infrared radiation). This heat is gradually radiated away from the surface of the planet, with the only thing ‘slowing it down’ being the larger air molecules like carbon dioxide, methane, water vapor, etcetera. Molecules like nitrogen and oxygen have no ability to absorb and reemit infrared radiation.
https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/a-greenhouse-effect-analogy.html
SamIAm. In an attempt to make everyone feel that they understand the greenhouse effect (and the enhanced GHE from rising GHGs), climate scientists have promoted flawed analogies to blankets and greenhouses. You have linked an ACS article that uses this strategy. Unfortunately, most people think blankets keep them warm because they block convection of heat. Although convection transfers heat within our climate system, our planet does NOT lose heat to space by convection. Radiation, convection and conduction are all operating when greenhouses, blankets and other insulators make things warmer. With three mechanism of heat transfer involved, a proper explanation for how blankets and greenhouse make things warmer is challenging. Unfortunately, skeptics point out the weaknesses in these analogies and create doubt in the mind of readers. The resulting confusion makes it difficult for readers to learn the correct explanation for the greenhouse effect.
Rising greenhouse gases are much simpler to properly explain than a blanket, because radiation is the only mechanism of heat transfer involved.
1) Greenhouse gases both absorb and emit thermal infrared radiation. (When you stand near a fireplace with glass doors that block convection, thermal infrared is what makes your skin facing the fireplace feel hot. With an ordinary fire, most heat is convected upward and the warmth you feel is also from thermal infrared.)
2) Emission of thermal infrared radiation (per molecule of GHG) increases with temperature, while absorption of thermal infrared (per molecule of GHG) changes little with temperature.
3) The higher the GHG concentration, the shorter the distance the individual photons comprising radiation travel between emission and absorption. Therefore, those photons that do escape to space have been emitted from a higher altitude when more GHGs are present.
4) Most photons escaping to space are emitted from the upper and mid troposphere, where temperature decreases with altitude. Therefore the initial response to an increase in GHGs is a reduction in radiative cooling to space – because the emitting molecules are colder.
5) Incoming and outgoing radiation were in balance before GHGs began rising. When radiative cooling to space is slowed by rising GHGs, the law of conservation of energy demands that the temperature rise until balance is restored. (It doesn’t tell us how much warming will occur, how fast warming will occur, where warming will occur, or whether warming can be detected against the background on natural climate variability.)
Our host provided a similar explanation at the link below:
“climate scientists have promoted flawed analogies to blankets and greenhouses.”
No one with any knowledge of spectral physics does that. Stop with the misinformation please.
Frank,
The thermal IR you feel from a fireplace with glass doors is coming mostly from the hot doors. Regular glass is nearly opaque in the thermal IR. The people who think that blankets work mainly by blocking convection are wrong. Even with fans, convection isn’t all that effective at moving energy in the absence of latent heat transfer. In a convection oven, for example, you only reduce the temperature setting by 25 degrees F compared to no convection. When baking, most heat is transferred by radiation even with convection. A sheet of glass is, in fact, a good approximation of the classic simple radiative transfer model of the atmosphere, the single layer non-reflecting model with high transmission in the solar energy range and low transmission in the thermal IR. It’s not difficult to demonstrate this, as Horace de Saussure demonstrated in 1767 (although Wood in 1909 somehow managed to fail to replicate this, leading to much misinformation being spread by people who should have known better).
Geoenergymath: Above, SamIAm linked part of the official explanation from the American Chemical Society on the greenhouse effect that used analogies to blankets and greenhouses. It is part of the ACS’s Climate Science Toolkit to be used by teachers and professional chemists. Dr. Susan Solomon, Mario Molina and a few other atmospheric chemists were on the committee responsible for the material that used these analogies. Professor Solomon was the co-chair of AR4 WG1. Are you saying that she and the others don’t have enough knowledge of spectral physics to provide an accurate explanation for the GHE?
https://www.acs.org/content/acs/en/climatescience/about/working-group.html
However, the truth is that I don’t know WHY these scientists are using these misleading analogies, nor am I supposed to speculate at this website about the motivations of those who have done so. My apologies. I simply know from personal experience that misleading analogies about blankets and trapping heat made it more difficult to learn the correct explanation.
Does “spectral physics” really provide a simple explanation for the GHE? Technically, Planck’s Law only applies to radiation in equilibrium with the medium through which is traveling. Black cavities produce such an equilibrium, but our atmosphere does not. When you integrate over all wavelengths to get the S-B equation, a mysterious emissivity fudge factor is added, which sometimes an extrinsic property (independent of quantity) and sometimes an intrinsic property (proportional to quantity). Why is this emissivity equal absorptivity? Planck’s Law was formulated without knowing anything about the fundamental physics of interactions between radiation and matter – Einstein coefficients – and their macroscopic counterparts, absorption and emission cross-sections. These parameters vary with wavelength, adding “color” to the blackbody spectrum. Finally, I’ve never been happy with models that contain an isothermal layer or layers of atmosphere. Without a temperature gradient, how does vertical transfer of heat occur by convection or conduction.
DeWitt: Thank you for the correction. The glass doors of a fireplace emit the thermal IR that makes one skin feel hot on one side, not the fire behind the doors. I wanted the doors to make it clear that radiation, not convection, was responsible and I failed to be accurate. Perhaps a Franklin stove would have been a better choice, but they aren’t very common anymore. They are basically a fireplace with an iron door.
Your comments about the relative importance of convection and radiation were thought-provoking. Several heavy blankets certainly keep you warmer than a thin sheet, but both appear equally effective in blocking convection. Wind chill is a measure of the importance of convection to our perception of cold. Convection appears complicated by the fact that air and other fluids adhere to surfaces, requiring conduction of heat across a small gap before bulk flow can convect heat.
Though the terms ‘greenhouse effect ‘ and ‘blanketing effect’ are analogies to help describe the result of the interplay between Newtonian physics and Quantum Mechanics as carbon dioxide and several other large types of molecules cause infrared energy to be delayed in its escape to space from the surface, it does not change the reality of the situation whereby things are getting warmer and will get warmer yet as the level of these so called “greenhouse gases”.
I offer this as a blanket response to replies to my original posting. I do find the courtesy offered between those posting conversational replies herein to be refreshing.
Carl Sagan Quote:
“In science it often happens that scientists say, ‘You know that’s a really good argument; my position is mistaken,’ and then they would actually change their minds and you never hear that old view from them again. They really do it. It doesn’t happen as often as it should, because scientists are human and change is sometimes painful. But it happens every day. I cannot recall the last time something like that happened in politics or religion.”
P.S.— Rather than post a separate reply regarding the big punch that trace ‘greenhouse gases’ have, I’ll add it here:
Some people are of the opinion that the percentage of greenhouse gases in the atmosphere is so small that their contribution to the greenhouse effect would be minuscule. I see their reasoning; however, the even smaller amount of carbon dioxide of the past has been sufficient to keep the planet from being a giant ball of ice. And with no carbon dioxide at all, the temperature on Earth would be below zero degrees Fahrenheit.
For perspective, if you could bring all the clouds and water vapor in the atmosphere to the surface, it would form a ‘liquid’ layer less than an inch deep, and clouds alone would create a layer no deeper than a coat of paint. If just this past year’s carbon dioxide emissions alone could be confined to an undiluted layer of pure CO2 at the surface of the Earth, the layer would be about 1.5 inches thick.
“Nitrogen, oxygen and argon together comprise more than 99 percent of the atmosphere. None of these three gases absorb either visible or infrared light. It is as though, when it comes to the absorption and emission of light, the atmosphere’s three main players don’t exist.”
So, the only thing left to consider is the actual total amount of the greenhouse ‘trace gases’.
SamIAm: Though the terms ‘greenhouse effect ‘ and ‘blanketing effect’ are analogies to help describe the result of the interplay between Newtonian physics and Quantum Mechanics as carbon dioxide and several other large types of molecules cause infrared energy to be delayed in its escape to space from the surface, it does not change the reality of the situation whereby things are getting warmer and will get warmer yet as the level of these so called “greenhouse gases”.
Blankets and some aspects of greenhouses are examples of insulation. You don’t need QM to understand the basics of insulation. Everyone knows that in thermodynamics heat flows from hot to cold, but many people fail to realize that energy flows in both directions and that thermodynamic heat is the net flux from all incoming and outgoing energy flows. So an object’s internal energy/temperature can be increased by slowing down the rate at which it loses heat (insulation) as well as by increasing inflow. Blankets are a simple way to illustrate this principle of heat transfer, but confusing because multiple mechanisms of heat transfer are involved. Many reject the idea that blankets make you warmer because they radiative thermal infrared towards your warmer body. These skeptic focus the fact that a blanket also warms by blocks convection. This website has many useful posts that try to properly explain heat transfer:
Increasing GHGs slow down (rather than delay) the rate at which thermal infrared escapes to space and that causes the internal energy/temperature of our climate system to increase. That increase continues until the increased emission caused by higher temperature restores a balance between incoming and outgoing energy.
SamIAm: “For perspective, if you could bring all the clouds and water vapor in the atmosphere to the surface, it would form a ‘liquid’ layer less than an inch deep, and clouds alone would create a layer no deeper than a coat of paint. If just this past year’s carbon dioxide emissions alone could be confined to an undiluted layer of pure CO2 at the surface of the Earth, the layer would be about 1.5 inches thick.”
Since I have personal experience with absorption of radiation by solids and liquids, but no personal experience with absorption by radiation, I imagine condensing the whole atmosphere into a layer of liquid with the same density as water. Such a “liquid atmosphere” would be 10 m thick. 400 ppm would be 4 mm or 1/6 of an inch, a little thinner than a pane of glass that lets visible light in, but doesn’t allow thermal infrared out. 1 ppm (methane) would be only 10 uM thick and some of the minor GHGs are even lower. Can quantities that small interfere with radiation? How thick is the layer of active ingredient in sunscreen that absorbs UV and prevents sunburn? Obviously, the absolute amount of an absorbing molecule isn’t what is important – both amount and potency are critical. The dose of typical medicine is a few mg/kg of body weight: a few ppm.
Frank said:
” Professor Solomon was the co-chair of AR4 WG1. Are you saying that she and the others don’t have enough knowledge of spectral physics to provide an accurate explanation for the GHE?”
Don’t try to bullrush this topic. It’s YOU that never mentioned the spectral properties of the medium, and now you’re trying to make up for your lack of awareness.
He isn’t bullrushing. It’s you that can’t admit that you could possibly be wrong. But that’s been obvious in all your comments here.
geoenergymath: Let’s review my alleged “bullrushing”. After reading and praising the above detailed article by SOD, SamIAm cited a webpage from the ACS Toolkit on Climate Change with problematic explanations for the GHE using blankets and greenhouses. I provided a more accurate explanation and a link to SOD’s equivalent explanation.
Then I warned SamIAm that: “… climate scientists have promoted flawed analogies to blankets and greenhouses. You have linked an ACS article that uses this strategy.”
geoenergymath accused me of misinformation: “No one with any knowledge of spectral physics does that [uses analogies to blankets and greenhouses]. Stop with the misinformation please.”
Frank replied by noting that Susan Solomon and Nobel Laureate Mario Molina were listed as members of the ACS Presidential Climate Science Working Group responsible ACS Climate Science Toolkit. This toolkit was intended to:
“deal relatively briefly with the basic science of climate change and can used by every ACS member. The purpose of this content is self-education. It is designed to equip you with the information and other resources necessary to develop a robust intellectual structure that can be the basis for your discussions with others.”
https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/a-greenhouse-effect-analogy.html
https://www.acs.org/content/acs/en/climatescience/about/working-group.html
https://www.acs.org/content/acs/en/climatescience/about.html
I personally resent all the time that I wasted with these flawed and misleading “robust intellectual frameworks” for the GHE. This website is loaded with comments from other technically competent scientists and engineers who are confused by what appears to me to be an oversimplified discussion is a complicated subject.
The rising characteristic emission level argument I presented to SamIAm is a decent “hand-waving” rational for why increasing GHGs initially reduce radiative cooling to space. I struggled trying to reconcile that explanation with my own alternative hand-waving refutation: Doubling CO2 doubles emission of photons carrying heat to space and halves the average distance these photons travel before being absorbed. Therefore radiative cooling to space should be unchanged by doubling. With 20/20 hindsight, that was a remarkably good first approximation. The radiative forcing from doubling CO2 is only about 3.5 W/m2 out of an escaping flux 100X bigger, a flux which is the net result of far less than 10% of emitted photons escaping to space.
Even worse, I’ve learned that Planck’s Law is only valid for radiation in equilibrium with matter, an equilibrium that doesn’t exist in our atmosphere. The derived S-B equation is problematic for the same reason and contains an emissivity fudge factor without a clear physical explanation. That fudge factor is sometimes treated as being independent of GHG concentration and sometime proportional to GHG concentration. The correct explanation lies in the equations of radiation transfer, which are sometimes mentioned in passing by climate scientists, but which encompass the phenomena of scattering and the possibility that a local thermodynamic equilibrium may not exist (a non-Boltzmann distribution of excited states). When LTE exists, the equations of radiation transfer simplify to an equation almost no one mentions. When you speak of “spectral physics”, are you referring to this equation?
Frank, I really don’t care. I learned spectral physics long ago, when I studied CO2 infrared lasers in preparation for our groundbreaking work on fabricating semiconductor infrared lasing material
https://www.frontiersin.org/articles/10.3389/fmats.2015.00052/full
CO2 has this amazing property of converting energy into infrared radiation, which is essentially radiative heat. This is all spectral physics.
If you want to discuss ENSO dynamics or how to make a solid-state infrared laser, those are actually topics that are interesting, in contrast to this CO2 stuff which is all old hat, ever since the first infrared CO2 laser was invented and fabricated in the early 1960’s.
“The question asked at the start was “Is CO2 an insignificant trace gas?” and the answer is no.
CO2 and water vapor are very significant in the earth’s climate”
Why is water vapor included in a defense of CO₂?
Desert’s day/night temperature differential show the effect of water vapor (lack thereof)
What demonstrates the effect of CO₂?
The infrared emission spectra of the atmosphere clearly demonstrates the effect of CO2. There are several examples in this post.
“Desert’s day/night temperature differential show the effect of water vapor (lack thereof)”
I don’t think so..
https://greenhousedefect.com/2/deception-with-emission-spectra-part-2
Livescience disagrees.
https://www.livescience.com/why-do-deserts-get-cold-at-night.html
NASA disagrees.
https://www.earthobservatory.nasa.gov/biome/biodesert.php
AskABiologist disagrees.
https://askabiologist.asu.edu/explore/desert
And weather records contradict NASA. You may want to browse both sites to find any desert climate with a day/night spread of over 20°C. You will fail.
http://worldweather.wmo.int/en/city.html?cityId=134
https://www.worldweatheronline.com/timbuktu-weather-history/tombouctou/ml.aspx
It is just the sand, not the vapor. Of course NASA measures surface temperature, opposed to air temperatures taken at weather stations.
John Doe: Please skip the BS: There is no need to look for locations where the day/night temperature difference is 20 degC. In humid areas, the day/night temperature difference less than 10 degC (New Orleans, for example). In deserts, (Phoenix, for example, especially in the months with the least rain) the differences are greater than 10 degC. In Islamabad, the day/night temperature difference drops dramatically in the summer months when the monsoon arrives with humidity and clouds. In Baghdad in the driest summer months, the day/night temperature difference is 19 degC.
@Frank You are only trying to dodge the point. NASA and many others claim there would be extreme temperatures spreads in the desert, well beyond 20°C. “driver1100” repeats this claim, suggesting it was due to the lack of vapor and that it was “special” in the desert. However with clear skies we see such spreads also with moderate climates like in Europe or the US.
Extreme temperature spreads are only true for surface or sand temperatures in the desert, not for air temperatures. We know why that is, and it is not because of vapor (or the lack of it). So let me return the “Please skip the BS” to you, cause that is where it belongs 😉
John Doe: This page at this URL is discussing the climate of deserts. The page makes not mention of carbon dioxide, greenhouse gases or the greenhouse effect. 75 degF is a reasonable estimate for SEASONAL temperature change, not the diurnal (day/night) temperature change for an average day.
https://www.earthobservatory.nasa.gov/biome/biodesert.php
Q) Did you read your NASA link?
I’ve provided that very same link, in this discussion thread, to demonstrate the science behind my assertion, thanks for validating my view as supported by NASA.
NASA: “The temperature in the desert can change drastically from day to night because the air is so dry that heat escapes rapidly at night.”
@Frank
“driver1100” is right, that link just tells the same mistake I have discussed before. I mean it should be impossible to get the message as wrong as you do, at least without intention.
The links I provided are clear, water vapor traps heat. Just accept the science.
@driver1100
I already honored your ability to read (as opposed to “Frank”). That is not your ability to reflect..
Yeah, I know…
NASA must be wrong!
lol
Frank and driver1100,
You’re wasting your time.
@driver1100
NASA was certainly right in launching the Challenger despite too low temperatures. And NASA was doing a perfect job in not checking for potential damage on the Columbia. NASA is always right, especially when lifes are at stake.. “facepalm”
Your red herring is a significant indication that you know that you have lost the argument.
Thanks for admitting defeat.
Cheers! 🍻
Driver1100 and John Doe: I left a message for the webmaster of that NASA web page telling him 75 degF was being misinterpreted by some and suggested that they clarify. No response so far.
How can the stated 75°F day to night variance in temperature be “misinterpreted”?
lol
You are right, it is being misinterpreted by …NASA!!!
I am perfectly fine if they say soil temperatures show such a temperature spread. It is likely true. But that is due to sand having low thermal conductivity, you know. However it has NOTHING to do with the lack vapor. And that is all the point I am making.
The lack of water vapor is validated again
Deserts AND Mars
Read the section in the below link titled:
*The role of water*
https://phys.org/news/2019-11-climate-mars-cold-atmosphere-carbon.html
Good luck with your mission to correct the entire world on water vapor’s major role in retaining heat in the atmosphere.
lol
“However it has NOTHING to do with the lack vapor. And that is all the point I am making.”
Frank,
This is exactly why I told you that you were wasting time feeding this [mythical creature thought to live under bridges].
The Romans made ice in the ancient Judean desert (look it up). Try doing that where the humidity is higher.
driver1100 commented: “The question asked at the start was “Is CO2 an insignificant trace gas?” and the answer is no. CO2 and water vapor are very significant in the earth’s climate” Why is water vapor included in a defense of CO₂? Desert’s day/night temperature differential show the effect of water vapor (lack thereof) What demonstrates the effect of CO₂?”
We can’t demonstrate the effect of changing CO2 on radiation traveling through our atmosphere because we have no ability to quickly change the amount of CO2 in the atmosphere while keeping everything else the same. We focus on a different GHG, water vapor, because it changes naturally between deserts and more humid regions on the planet.
The right place to do experiments that show the effect of changes in CO2 on radiation is in the laboratory. We have been doing those experiments for a century. Those experiments measure an absorption spectrum and provide an absorption cross-section (o) which varies with wavelength (and modestly with pressure and temperature). That allows us to calculation how radiation is changed by both absorption and emission as it passes through our atmosphere. Those are called radiation transfer calculation. Modtran is an online program that automates radiative transfer calculations and allows you to vary CO2. Such radiation transfer calculations have been validated many times in the atmosphere by correctly predicting the radiative fluxes we observe under a wide variety of conditions. The quality of those predictions can be seen in this post.
If you could point me towards an experiment that demonstrates CO₂ back radiation (simply adding more CO₂ will warm the surface of an object more than a lesser amount) that would be great, thanks!
Driver: “Saying that CO2 back radiation warms the surface” is gross over-simplification, because the steady state temperature of any object (like the surface of our planet) depends on all of the fluxes that deliver and remove energy from that object. Here is a clearer statement: Radiation transfer calculations predict that a rise in CO2 will reduce the rate of radiative cooling to space (without changing incoming SWR). When combined with the law of conservation of energy, this means that the temperature of our climate system must warm until incoming and outgoing radiative fluxes are again equal.
The equations used in radiative transfer calculations arise from quantum mechanics and are supported by nearly a century of laboratory experiments studying the interactions between GHGs and thermal infrared. And these calculations have been validated by careful experiments in our atmosphere. Unfortunately, the equations used in radiation transfer are differential equations that must be numerically integrated along a path from the surface to space when calculating radiative cooling to space, so discussing those equations is unlikely to provide you with a better intuitive understanding of why rising GHGs reduce radiative cooling to space. There are a number of explanations that don’t involve radiative transfer calculations, but they are (IMO) all flawed.
Note that radiative transfer calculations and conservation of energy don’t predict where warming must occur (it isn’t required to be at the surface) OR how much warming must occur (that is climate sensitivity, the big unknown of climate science) OR how long it will take to restore balance, OR when warming can be unambiguously detected against the background of natural climate variability. However, since our atmosphere is efficiently mixed by winds, to a first approximation we might expect equal warming everywhere including the surface. These questions can only be addressed by AOGCMs, and these models aren’t very good.
Based on its temperature and emissivity, we know that the surface of our planet emits an average of about 390 W/m2 of thermal infrared upward. Measurements from space show that only about 240 W/m2 of thermal infrared is escaping to space. GHGs are by definition the only gases in our atmosphere that absorb and emit thermal IR, so they obviously must be the cause of this decrease in outward radiation. Radiative transfer calculations do a good job of predicting this large decrease and predict a comparatively small further decrease as GHGs rise.
Alternatively, if you want a real experiment “that demonstrates increasing CO₂ will warm the surface”, we have been and are continuing to do an experiment on our entire planet by raising CO2 (currently about 2 ppm/yr) and other GHGs. The surface of our planet seems to be rising about 0.2 degC/decade for the last half-century. And ARGO shows that the ocean has been accumulating about 0.7 W/m2 of heat that formerly escaped to space when CO2 was lower. However, this “experiment” is complicated by the co-emission of aerosols that reflect incoming sunlight and by natural variability in climate (like the LIA and MWP). The proxy record shows that 1 degC of unforced near-GLOBAL warming in a half-century is uncommon. Local warmng and cooling in Greenland ice cores has been larger.)
I’m glad that you have now admitted that CO₂ back radiation is an artificial construct with no experimental evidence to support it.
As for your excessively worded excuses, I will refer to just one of your multiple incorrect statements.
Greenland’s dramatic and very abrupt (ice core cooling/warming) climate changes being “local” couldn’t be farther from statements from noted paleoclimatologists such as Prof Jeff Severinghaus and Prof Stefan Rahmstorf.
Severinghaus said it was a “global phenomenon”
Rahmstorf said these abrupt changes are largescale climatic changes “confirmed by data from more than 170 locations around the planet, including New Zealand and the Antarctic”
Cheers!
🥂
Driver mis-represented what I wrote when he said: “I’m glad that you have now admitted that CO₂ back radiation is an artificial construct with no experimental evidence to support it.” Perhaps I need to stop feeding the troll.
When presenting GHG-driven global warming as a consequence of a GHG-mediated reduction in radiative cooling to space combined with conservation of energy, I didn’t NEED to get into the details of back-radiation (which is relatively trivial for radiation transfer calculations to handle), nor the problem of convection of simple and latent heat (which is currently impossible to model without grid cells and parameters that describe sub-grid processes.)
I took this approach I did so because long experience has taught me that many people who think that the 2LoT prevents a photon emitted by a GHG in the colder atmosphere from being absorbed by a molecule on the warmer surface. These idiots don’t realize that individual molecules have a kinetic energy that changes with collisions about 10^9 times per second, but they don’t have a “temperature”, In thermodynamics, temperature is defined as being proportional to the mean kinetic energy of a large groups of colliding molecules (so it doesn’t change 10^9 times per second). Consequently heat is defined as the net flux of energy between two large groups of molecules, one of which may be hotter and one may be colder. Thus the 2LoT doesn’t prevent thermal infrared emitted by GHGs in the colder atmosphere from being absorbed by the warmer surface, but the net flux between them will always be from warmer to colder.
Abrupt climate change (D-O events) did occur in Greenland during the last ice age. However, GHG-mediated global warming has mostly taken place in the last 50 years of the Holocene, a period with a much more stable climate. I was looking for precedent for recent warming during the stable Holocene. There are more than t50 half-century periods when natural variability – if it were large enough – could have produced 1 degC of global warming. The MWP, RWP and Minoan WP are found in Greenland ice cores, but not the global record. And natural variability in Greenland is inflated by Arctic amplification.
You still can’t point to one experiment that demonstrates CO₂ back radiation. It’s an artificial construct.
I already showed you that the abrupt warming (first seen in Greenland ice cores) were planet wide, not “local”
Please research Severinghaus, Rahmstorf, Alley on *abrupt climate changes* instead of being a science denier.
driver1100
The Amazing Case of “Back-Radiation”
The Amazing Case of “Back Radiation” – Part Two
Still no physics experiment that demonstrates that simply adding more CO₂ will warm the surface of an object more than a lesser amount.
We’re still stuck on demonstrating an artificial construct, why is it so hard to demonstrate?
Water vapor effect is easily demonstrated.
Dry sauna vs wet sauna
(deserts vs tropics)
Deserts can cool dramatically at night (droppng 75°F) because of a lack of water vapor, not for a lack of CO₂ (or even DWIR) as CO₂ is a well mixed gas in the atmosphere.
DWIR doesn’t avoid deserts, does it?
lol
driver1100,
If your reading comprehension is not up to par so you don’t believe the links SoD listed above, buy an infrared thermometer and point it at the clear sky, but obviously not towards the sun during the day. You will get a temperature reading. Note that the temperature reading can be correlated to the total column water vapor. See:
https://www.drroyspencer.com/2013/04/direct-evidence-of-earths-greenhouse-effect/
In the winter, you will need an IR thermometer that reads to -60C.
If there were no IR back radiation, an IR thermometer could not register a temperature. And that’s not to mention IR spectrophotometers that measure the spectrum of atmospheric radiation.
The total column water vapor experiment is detailed here:
https://journals.ametsoc.org/view/journals/bams/92/10/2011bams3215_1.xml
Water vapor column?
I’m asking for an experiment that demonstrates CO₂ back radiation.
(I hope you realize that water vapor isn’t CO₂)
The experiment I’m looking for:
Simply adding more CO₂ will warm the surface of an object more than a lesser amount.
A physics lab can control the parameters to isolate the effect of adding CO₂, right?
Driver: And I pointed you to the post below with experiments that show how well radiative transfer calculation predict the spectrum of DLR, which confirms that about half comes from CO2, not water vapor. Data such as this shows that radiative transfer calculations predict the radiation we observe in atmosphere with a high degree of accuracy.
Once again, I’m asking for a repeatable experiment that can demonstrate CO₂ back radiation – simply adding more CO₂ will warm the surface of an object more than a lesser amount (without introducing specific heat capacity of gases)
If you can’t point to one repeatable experiment that demonstrates CO₂ back radiation, just say so.
I’m ok if you say that you don’t know and that your aren’t aware of one.
Once again, I’m looking for a repeatable experiment that demonstrates CO₂ back radiation – simply adding more CO₂ will warm the surface more than a lesser amount.
If you can’t point to a repeatable experiment demonstrating CO₂ back radiation, just say so.
It’s perfectly ok to admit that you don’t know of one.
driver1100,
Lots of people don’t understand basic physics. Normally that’s fine. But in your case you appear to celebrate this lack of understanding.
We’ve demonstrated that back radiation exists. Your various comments indicate you don’t believe it exists (1), or that it if it exists it can’t affect the surface temperature (2). We aren’t sure whether you believe proposition 1, proposition 2, or something else.
You are in “good” company, as you will find if you peruse the comments on various articles on this blog – including the ones I linked to earlier.
Do you believe proposition 1?
Do you believe proposition 2 (aka, the first law of thermodynamics is flawed)?
Do you somehow believe that back radiation exists and the first law of thermodynamics and still somehow that back radiation doesn’t do anything? It disappears? Many confused people have grappled with these ideas in very entertaining ways on this blog.
Frank attempted to explain the more important consequence of CO2 absorption in the atmosphere. The simpler approach to explain atmospheric radiation.
More CO2 causes a reduction in outgoing longwave radiation, therefore certainly leading (via the first law of thermodynamics) to a warming of the climate and you were delighted (comment July 11, 2021 at 10:01 pm) that this somehow proved your point.
Your comment unfortunately demonstrated your lack of understanding of the conservation of energy. Or the consequences thereof.
On a positive note, you might even be in the majority. Celebrate that!
If you can write a comment that demonstrates some grasp of the possible consequences of the first law of thermodynamics (that’s “conservation of energy” if you aren’t sure) then your comment will get to stay.
Otherwise I’ll moderate it out and your succession of stupid comments (to date) can stay as a testament to your lack of physics basics.
Once again, I’m asking for a demonstration, not an explanation of something that can’t be demonstrated.
If it’s basic physics, why not demonstrate it or point to a demonstration or explain why it’s not possible to demonstrate ‘basic physics’
One needs an object that emits LWIR abd CO₂.
The good news, everything that’s above 0 kelvin emits LWIR (so that eliminates the potential problem of having a LWIR source)
CO₂ is readily available in many forms (tanks, cylinders etc) So CO₂ isn’t a problem.
Let’s see the warming of the LWIR emitting object from simply adding more CO₂.
(Svante Arrhenius suggested 15°C for the experiment demonstrating CO₂ back radiation)
Try 500ppm
5000ppm
50,000ppm
100,000ppm
250,000ppm
The surface of the object should warm more, on its own, with these higher and higher levels of CO₂ – if CO₂ back radiation is a thing.
Cheers! 🍻
Against my better judgement I approved your comment.
We can’t add 1000ppm of CO2 to the atmosphere. More CO2 in the atmosphere emits more radiation to the surface. And less radiation is emitted to outer space.
How do we know this?
We have lots of experiments with more and less water vapor. Because water vapor naturally varies in the atmosphere.
You can see plenty of measurements in numerous articles on this blog. These measurement demonstrates that radiation really functions how textbooks say it functions. Less water vapor results in more radiation to outer space from the surface and less radiation to the surface from the sky.
If you can absorb this important point and say something slightly sensible instead of just repeating your confusion your next comment will also be approved.
Sorry, comment not approved.
Good luck in your journey.
SoD: For the benefit of other readers, I’d like to have a go at explaining why
Driver1100’s challenge is MEANINGLESS: “I’m looking for a repeatable experiment that demonstrates CO₂ back radiation – simply adding more CO₂ will warm the surface more than a lesser amount.”
From the time of the Greeks until Galileo, our ideas about the laws that governed the motion of objects were grossly wrong. Then Galileo performed some experiments with metal balls on an inclined plane where little friction was present. He was able to show that acceleration of the balls was independent of their weight. (The legendary cannon balls dropped from the Leaning Tower of Pisa apparently were a thought experiment intended to illustrate the logical consequences of his experiments on inclined planes. If Driver1100 challenged Galileo to perform an experiment that showed acceleration on an inclined plane under water was independent of the weight of the balls, Galileo would have been unable to satisfy Driver1100’s demands. Apparently Galileo would have also been unable to measure the acceleration of cannon balls falling from the Leaning Tower of Pisa, because he lacked a clock accurate enough to measure acceleration under these circumstances.
Our inability to perform a particular complicated experiment doesn’t prove we can’t predict the outcome of that experiment. The right place to learn about how GHGs and thermal infrared interact and how temperature and energy interconvert is in carefully controlled experiments in a LABORATORY, not the complex situation that exists on the surface of our planet due to heat transfer by both radiation and convection.
When we put everything we know about absorption and emission of thermal IR by GHGs, we can correctly predict the radiation transfer we observe in our atmosphere and its response to “natural experiments” that change the temperature and water vapor (a GHG like CO2) in the atmosphere. We can’t induce these changes whenever we want to do an experiment, we are limited by what nature provides. Nature doesn’t provide significant changes in CO2 – unless you want to compare Earth, Venus and Mars, another natural experiment that comes with complication. Combined with the law of conservation of energy, we can be sure the rising CO2 will cause the planet to warm.
When you see someone DEMANDING “a repeatable experiment that demonstrates CO₂ back radiation – simply adding more CO₂ will warm the surface more than a lesser amount”, an experiment that can’t be done, you are looking at someone who does not WANT to know the the right answer. Someone who would also deny that Galileo’s experiments didn’t illustrate the correct laws of motion had he lived at that time.
A laboratory experiment on this has been done. Link Royal Society.
https://royalsocietypublishing.org/doi/pdf/10.1098/rsos.192075
Dinero,
There appears to be a possible flaw in that experiment. The molar heat capacity at constant pressure of CO2 (36.94 J/mol K) is significantly higher than for air (29.19). That appears to have been ignored in the calculations. Only the heat capacity of the filament was used in the calculations. That should mean that the CO2 balloon would cool more slowly than the air filled balloon. I don’t want to run the numbers myself to see if it would have been significant compared to the measured cooling rate, but it should have been mentioned and dealt with. I’m not impressed with the reviewers of this publication who obviously failed to note this obvious problem.
typo correction –
The radiative flux BETWEEN the two is equal but the heat loss FROM the CO2 is lower.
DeWitt asked: “Why do you keep saying the heat capacity of CO2 is lower than air in a balloon at the same pressure? The heat capacity pf CO2 on a per gram basis is lower than air, but not on a per mole basis. The volumetric (or molar) heat capacity of CO2 is significantly higher than air, not lower. That’s because the density at constant pressure is higher for CO2 than for air because CO2 has a molecular weight of 44 and air is about 29. So in the same size balloon, the CO2 filled balloon will weigh more than an air filled balloon, 52% more, in fact.”
You are misunderstand the point I am trying to make. Focusing first on radiative heat transfer, as the heating element is warming and cooling, its thermal IR warms only the balloon when it is filled with IR-transparent air. When filled with CO2, some fraction is absorbed by the CO2. The emission of thermal IR back towards the heating element (that slows its cooling) depends on the amount the balloon and the CO2 are warmed by the heating element and THAT depends on the heat capacities of the CO2 and the BALLOON. For radiative heat transfer, the heat capacity of the air is irrelevant, because the air absorbs no radiation.
Now, in the real experiment, convective heat transfer is also occurring and apparently dominates. In that situation, the relative heat capacities of air, CO2 and the balloon are important. However, I’m only interested in abstracting the lessons this experiment teaches about radiative forcing by GHGs. Those lessons are incomplete, because they ignore the importance of a temperature gradient – both in this experiment and in the atmosphere.
Dinero: You are correct that heat is conducted through the skin of the balloon to the ambient air in the room where the experiment is being done. This is one of many factors that is inadequately controlled in this experiment. I’m trying to focus only on the radiative heat transfer aspects of the experiment.
To summarize my objections to this paper, the authors conclude:
“The experimental and calculated results illustrate a consistent trend where the heater in the CO2-filled balloon has a slower cooling rate than the heater in the air-filled balloon. The slower cooling rate is a result of a decrease in the radiative heat transfer from the simulated Earth (the heater) that is a result
of the increased gas ABSORPTION in the CO2-filled balloon.”
In reality, the heater cools more slower, because the heater has warmed the CO2 more than the air and the WARMER CO2 radiates more thermal IR towards to the heater than air or walls of the balloon. All three component are also warmed by the main mechanism of heat transfer (convection) and warmer air doesn’t radiate towards the heater.
In our atmosphere, doubling CO2 slows the rate of radiative cooling to space BEFORE causing any temperature change. However, this slowing wouldn’t occur in an isothermal atmosphere. And the thermocouple in this experiment would cool at the same rate in air or CO2 if the gas in the balloon were isothermal.
This experiment does show that back radiation from CO2 can slow cooling of the thermocouple even though the CO2 is cooler than the thermocouple. This demonstrates the fallacy of the 2LoT argument against AGW and the origin of the GHE.
Finally, long experience has taught me to value DeWitt’s snippy comments and the time he has invested in trying to understand my comments and illuminate their potential fallacies. (There have been many over the years.) If my explanation of what would happen in this balloon experiment with an isothermal gas inside were wrong, the person most likely to help would be DeWitt.
DeWitt Payne,
The experiment is not a study of the temperature of the balloon, it studies the temperature of the heating element. That is stated at the fifth sentence of the abstract.
Dinero,
Duh!
But the temperature of the gas in the balloon has an effect on the temperature of the filament. The filament cools by radiation and convection/conduction.
Read much?
Again, I’m not saying that the difference in heat capacity invalidates the experiment. It probably doesn’t. But it should have been at least mentioned.
DeWitt Payne,
The subject of the experiment was not to compare the insulation properties of balloons of different gasses.
Its subject is to demonstrate the fundamental principle that gasses, ie CO2, can insulate an object that is surrounded by that gas.
A Demonstration of a principle that Driver1100 and many others have been looking for.
Someone pointed out that increasing the size of the balloon appeared to have more effect on the reduction of cooling than totally changing the contents of the balloon.
So in the real world a change in convection patterns may possibly have a greater effect than a change in the amount of a significant trace gas.
Great experiment either way.
jrm,
The size of the balloon and the internal pressure only made a difference in the cooling rate when the balloons were filled with CO2. There was no difference from different size balloons when filled with air. That tends to support the hypothesis that the cooling of the filament was dominated by radiation in the air filled balloon.
Dinero and DeWitt: I think it is possible this experiment is flawed by what happens as the heating element + thermocouple warm up to 50 degC. Let’s first imagine what happens with a “rigid” balloon under vacuum. In that case, all of the thermal IR emitted by these components in the center of the balloon ends up being absorbed by the walls of the balloon. (For simplicity, we can imagine that the walls of the balloon have a very high heat capacity so that their temperature doesn’t change during the experiment.) The rate at which the thermocouple absorbs thermal IR from the wall with depend upon the temperature of the walls of the balloon, the emissivity of the walls, and a host of other factors that don’t change when we add a GHG to the balloon (including the absorptivity/emissivity and temperature of the thermocouple + heating element.)
Filling the balloon with a gas that doesn’t absorb or emit thermal IR doesn’t change any of the radiative fluxes that would be present in a vacuum, but it does permit convective heat transfer. Since we only care about radiation, I’m going to assume that the heat transfer by convection will be the same for both GHGs and non-GHGs.
Now imagine adding a GHG to the empty chamber which has exactly the same temperature as the walls of the balloon. There can be no net radiative heat transfer between the GHG and the walls of the balloon because they have the same temperature. Therefore, for every thermal IR photon absorbed by the GHG, the GHG must be emitting a photon back towards the walls. When GHG molecules “block the view” of the thermocouple and absorb photons of thermal IR that would otherwise add energy to the thermocouple, the GHG must also be emitting exactly the same number of photons towards the thermocouple. The GHG has no “idea” idea of whether it is emitting photons towards the thermocouple and is always emitting as many photons as it absorbs from the walls. So my conclusion is that the thermocouple would cool at the same rate in the presence of a GHG or non-GHG AS LONG AS THE GAS HAD THE SAME TEMPERATURE AS THE WALLS. As noted above, the GHG can be warmed by the heating element as it warms to 50 degC, but with a non-GHG, that radiative energy would be absorbed by the walls.
It is a common misconception that the GHG exists because CO2 traps heat near the surface of the planet by absorption. This misconception ignores emission of thermal IR by GHGs. In reality, the GHE also depends on a lapse rate being present in the atmosphere. In Antarctica, on the average, there is no lapse rate in the atmosphere and no GHE (and negligible warming away the ocean so far). Likewise, inside this balloon in this experiment, the presence of a GHG will only effect the cooling rate if there is a temperature gradient between the walls and the thermocouple. (:))
Continuing my comment that went into moderation and now may show up out of order ….
In section 4.2 of this paper, the authors use some engineering equations from Hottel (a famous MIT professor who studied heat transfer in furnaces among other things). Arguing with famous MIT professors of heat transfer isn’t usually a smart idea. In this case, the exponent of 0.65 in one equation suggests to me that the equations were empirically derived to fit observations. An absorbing gas near a heat source is not likely to be isothermal in real world situations. And in the real world, both convection and radiation can play a role. The authors also calculate “convective heat flow” (thermal diffusion?), which I suspect is different from the bulk convective flow that controls the lapse rate in our atmosphere. In most of the troposphere, that lapse rate is unaffected by vertical heat transfer by radiation, because bulk convection moves heat faster vertically than radiation.
The fundamental question posed by this demonstration of reduced radiative cooling in the presence of “back radiation” from a GHG is: “How relevant is the experimental situation in the balloon to the situation that exists in the atmosphere. That debate could be lengthy.
When we calculate the instantaneous radiative forcing produced by increased GHGs, we do so assuming the temperature of the troposphere doesn’t change.
However, for those who believe that DLR from a cooler atmosphere can’t make the surface warmer that it would be without GHGs in the atmosphere, this experiment does demonstrate that radiation from cooler CO2 can slow down the rate at which the warmer thermocouple cools. In that sense, the experiment is a success and may satisfy Driver 1100.
Frank,
Hottel’s graphs/tables were indeed empirically derived. But they have been updated more recently. The new values are derived by integrating the absorbance using the path length and partial pressure (atm cm, atm m, atm feet, etc.) of the gas of interest over the wavelength range of interest. There are also corrections for spectral overlap. Someone who shall not be named misused Hottel by only looking at the numbers for a path length of one meter and a partial pressure of 0.4 millibars.
Needless to say, if you’re looking at atmospheric CO2, the integrated absorbance over a path length of one meter is quite small. Over the full depth of the atmosphere, equal to about 8,000m at one atmosphere, the path length at one atmosphere pressure of pure CO2 is about 3 meters and the integrated absorbance is about 0.22. It took me a while to figure out what he was doing. Needless to say, explaining it to him was not possible.
Dewitt: Thanks for the reply about Hottel’s work. Are the new values derived by assuming the gas is isothermal, or calculating how much hotter the gas is near the heat source. In my first comment, which is in moderation, I argue that as the heating element warms to 50 degC, it radiately warms the nearby CO2 but not the air. In the atmosphere, the existence of a GHE depends on a temperature gradient.
SOD: The above comment is caught in moderation.
Frank,
I do not think that reasoning is correct. The photon from the skin of the balloon
would go into the CO2 and a photon would go into the skin of the balloon. That is correct. However it is going into the skin of the balloon instead of moving away from the gas and balloon system. Overall Photons are constantly travelling between the gas and the skin instead of being lost from the balloon and CO2 system and therefore at any one time there are more photons available in the CO2 to occasionally go into the thermocouple. In summary the warm skin of the balloon adds to the reservoir of heat energy that is keeping the thermocouple warm, overall the CO2 does not block the photons
Dinero wrote: “Overall Photons are constantly travelling between the gas and the skin instead of being lost from the balloon and CO2 system and therefore at any one time there are more photons available in the CO2 to occasionally go into the thermocouple. In summary the warm skin of the balloon adds to the reservoir of heat energy that is keeping the thermocouple warm, overall the CO2 does not block the photons.”
With CO2 present, there are more photons being emitted, but those photons travel a shorter distance between emission and absorption. This is due to Kirchhoff’s Law, absorptivity equals emissivity at any wavelength. This ensures that there is no net flux of heat by radiation between two objects (including gases) that are at the same temperature.
Let’s first consider the balloon without the heating element and focus on the spherical shell of gas lying within one centimeter of the balloon. If they are at the same temperature, the radiative flux between the balloon and this shell of gas must be equal no matter what the gas is. Now, let’s consider the spherical shell of gas lying between 1 and 2 cm from the wall and the outermost shell of gas. Their fluxes must be equal too and equal to the flux from the balloon. Eventually we get to the sphere of gas in the center and still have the same flux. Now replace that gas in the center with the 50 degC heating element and thermocouple. The nature of the gas lying between the walls of the balloon and the thermocouple has no impact on the “back radiation” that is slowing the cooling of the thermocouple – as long as the gas has the same temperature as the walls of the balloon.
In the real world of experiments, you can’t have a balloon filled with a gas at room temperature and a heating element at 50 degC in the center. The gas nearest the heating element will be warmer. When that gas has high emissivity, its emission will slow the cooling of the thermocouple. When that gas doesn’t emit (or absorb) thermal IR, the only thermal IR slowing the cooling of the thermocouple will come from the balloon. The lower heat capacity of the CO2 near the heating element than the heat capacity of the balloon means that the temperature of the balloon changes less in the experiment than the temperature of the nearby gas.
FWIW, I struggled for a long time trying to understand how doubling CO2 could cause radiative forcing when it doubles the number of photons emitted and halves the average distance they travel. The answer is that the existence of radiative forcing (and the GHE) depends on the existence of a temperature gradient in the atmosphere. In our atmosphere, GHGs radiate thermal IR equally in all directions, but they absorb more upward than downward radiation because of the temperature gradient in the atmosphere. (Absorption is proportional to the intensity of the incoming radiation.)
Frank,
I believe the assumption is isothermal. The principal use is in furnaces or boilers where the path length is short and there’s a lot of turbulence. That being said, in most cases, the slope of the constant pressure-pathlength curves vs temp don’t change rapidly with temperature, as I remember. You do get changes when more vibration or stretch modes are activated as temperature increases so you get more degrees of freedom.
Frank,
The temperature of the skin of the balloon is higher than the ambient air.
Frank wrote
“This ensures that there is no net flux of heat by radiation between two objects (including gases) that are at the same temperature. ”
The radiative flux BETWEEN the two is equal but the heat loss FROM the two is lower.
typo correction –
The radiative flux BETWEEN the two is equal but the heat loss FROM the CO2 is lower.
Dinero wrote: The radiative flux BETWEEN the two is equal but the heat loss FROM the CO2 is lower.
By definition, radiative heat flow IS the difference between incoming radiation and outgoing radiation. When the temperature is the same, there is no difference and radiative heat flow is zero. However, for some reason, you seem to think that the temperature of the skin of the balloon differs from the “the ambient air”, whatever that may be.
At the start of the experiment, the temperature of the balloon, gas inside the balloon and the heating element + thermocouple are all the same. If the gas is transparent to thermal IR, the walls of the balloon absorb all of the thermal IR emitted by the heating element + thermocouple. The heating element + thermocouple absorb only a fraction of the thermal IR emitted by the walls of the balloon and the rest misses them and is absorbed by the wall of the balloon on the other side. This is called the viewing angle. For no radiative heat flux to occur when the balloon and the heating element + thermocouple are at the same temperature, the correction for viewing angle and any difference surface absorptivity/emissivity must result in zero net flux when both have the same temperature.
When you change the temperature of the heating element + thermocouple, it emits more thermal IR that is absorbed by the walls of the balloon. However the radiative flux from the balloon remains unchanged until the walls of the balloon are warmed by the flux from the heating element. The difference between these fluxes controls the rate at which the thermocouple cools.
What changes when the gas absorbs and emits thermal IR? Nothing, when the gas, balloon and heating element + thermocouple are all at the same temperature. However, some of the photons arriving at the surface of the balloon and heating element + thermocouple are emitted by CO2 molecules and some of the photons emitted by the walls, heating element and thermocouple are absorbed by the CO2. When all have the same temperature, the net flux between any component (including any layers of gas) is zero.
When the heating element gets hot, its outward flux increases. However, the inward flux toward it doesn’t change until the balloon AND the CO2 change temperature. Since the CO2 absorbs some of the photons that formerly reached the balloon, the CO2 warms and the balloon will warm more slowly than when an IR-transparent gas was present. And since the CO2 is closer to the heating element and has a lower heat capacity, moving some of the heat from the walls of the balloon to the CO2 near the thermocouple does increase the radiative flux towards the thermocouple and slow its cooling.
The abstract claims that “This investigation showed that the cooling rate of an object, with surface temperature akin to temperatures found on Earth, is reduced in a CO2-rich atmosphere because of the concomitant lower heat loss to its environment.” They should have added that this happens because the “CO2-rich atmosphere” gets warmer.
Radiative transfer calculations show that an instantaneous doubling CO2 in the real world would produce an instantaneous radiative forcing before any temperature change occurs. However, that radiative forcing depends on the presence of a temperature gradient in the atmosphere. In an isothermal atmosphere, there would be no radiative forcing and no GHE. So this balloon model obscures a key element of the GHE – the temperature gradient. Since they don’t monitor the temperature of the balloon nor the gas inside the balloon, they fail to inform the reader that warmer CO2 – not just CO2 itself – is responsible for the slower cooling.
In the troposphere, temperature is controlled by convection (the lapse rate) and radiative cooling from upper troposphere to space – not by the surface the surface radiatively warming the atmosphere. Both processes carry heat vertically, but if more CO2 were present near the surface, upward convection of heat would increase to compensate.
In their last paragraph, the authors say:
“The experimental and calculated results illustrate a consistent trend where the heater in the CO2-filled balloon has a slower cooling rate than the heater in the air-filled balloon. The slower cooling rate is a result of a decrease in the radiative heat transfer from the simulated Earth (the heater) that is a result
of the increased gas ABSORPTION in the CO2-filled balloon.”
The heater cools more slower, because the CO2 is warmer than air (from absorption) and the WARMER CO2 radiates more thermal IR towards to the heater. In the real world, doubling CO2 slows the rate of radiative cooling to space BEFORE causing any temperature change.
Frank ,
Ambient air, i.e. the air surrounding the outside of the balloon is cooler than the balloon skin , and so heat is lost to it via the balloon skin.
The Skin and the CO2 and thermocouple are the same temperature and in them, the heat content of the system is contained. The addition of the skin of the balloon to the CO2 and heating element increases the heat capacity of the combined system more than it increases the outer surface area radiating to the surrounding air. And so the time it takes for the whole system to cool is longer.
Frank,
Why do you keep saying the heat capacity of CO2 is lower than air in a balloon at the same pressure? The heat capacity pf CO2 on a per gram basis is lower than air, but not on a per mole basis. The volumetric (or molar) heat capacity of CO2 is significantly higher than air, not lower. That’s because the density at constant pressure is higher for CO2 than for air because CO2 has a molecular weight of 44 and air is about 29. So in the same size balloon, the CO2 filled balloon will weigh more than an air filled balloon, 52% more, in fact.
Dinero,
The balloon skin and the filament will only be at the same temperature when they are at the same temperature as the surrounding air. When the filament warms, you get a temperature gradient between the filament and the skin. It says so in the paper you linked far above. Didn’t you read the whole thing?
It also says that the dominant heat transfer mechanism is convection, not radiation.
So your idea that the interior of the balloon is isothermal when the heating element is on is completely and utterly wrong.
DeWitt Payne,
Why the snippy remarks. You are the one who is completely and utterly wrong.
You have come into a conversation half way through that you are not following. Franks’s concern is a particular case that HE has presented where the element, gas and balloon skin are the same temperature.
@scienceofdoom
“More CO2 in the atmosphere emits more radiation to the surface. And less radiation is emitted to outer space.”
Both are true statements. The problem is, both could cause the GHE, or enhance it respectively, and you will have to choose which one does. Is it because less radiation goes into space, or because more radiation is emitted to the surface??? It is a very trivial question, and if you are honest to yourself, you should be able to answer it. But if you try, you will find it is not that easy. That is because there are indeed two different GHE theories..
John Doe,
It is because less radiation goes into space.
The reason we end up talking about radiation to the surface is because “the confused” (who are many) think it’s impossible – “radiation to the surface can’t be emitted”, “can’t be absorbed”, confused mishmash of the two..
If it was possible – with more CO2 in the atmosphere – for less radiation to be emitted from the climate into space *and also* “radiation from the atmosphere to the surface can’t exist/can’t be emitted/can’t be absorbed/must magically both not conflict with the first law of thermodynamics yet at the same time gloriously do nothing” then there would be a conflict.
The first law of thermodynamics would be invalidated. It’s a complex field known as adding up.
For my simplest explanation of the inappropriately-named “greenhouse” effect, with many references to more complete answers, see:
The “Greenhouse” Effect Explained in Simple Terms
Right, it is because less radiation goes into space. But then that renders “back radiation” obsolete, and rightfully so. And of course there is confusion if most climate scientists explain the GHE in the wrong way.
Manabe, Strickler (1964) made the case for a multi-layered “back radiation” caused GHE, where each layer would add “back radiation” to the lower ones. They concluded that based on this theoretic model, the surface would yield a radiative equilibrium of 332.3K, which in practice was only mitigated by convection. The funny thing here is, that if this would work, you could easily fix “climate change”. One could build a device with multiple semi-transparent layers (of glass) and an obsorbing layer at the bottom. Then you could shine some light onto it from the top and you would get very high temperatures at the bottom. Just exploit these high temperatures to generate energy, and you have a perpetuum mobile..
The problem is, this non-sense is still considered true.
That’s a classic example of the logical fallacy known as a false dichotomy. You don’t have to choose one and reject the other. Both can happen.
The 33 Deg C greenhouse effect. In the land,oceans and atmosphere, how many Joules of heat content is that.
“If you do the maths (see the end of the post), you find that the equations say that the earth should be about -18°C (255K) when in fact it is an average +15°C across the globe.”
That’s a very clever lie.
1. Most sunshine hits clouds and never reaches ground.
2. So effective temp of clouds is (say) 255K.
3. It’s totally unscientific comparing effective temp of clouds with actual temp of surface.
4. The correct comparison is effective temp of clouds and actual temp of clouds.
5. If you do this correct comparison, the answer is zero difference, then you realize that the greenhouse effect is purely down to cloud altitude.
6. Take for example Mars. Thirty times as much CO2 as Earth but no ghe. Why? Because it has virtually no clouds.
7. Or take Venus. Clouds fifteen times high as on Earth, ghe is fifteen times as much.
Nope. The Earth’s albedo is ~0.3. That means that 70% of sunshine is not reflected by clouds or the surface and hence is absorbed by the surface. Not to mention that most of the surface is water, not ground.
The rest of your response is equally fallacious, but it’s not worth my time to point out the problems.
“The Earth’s albedo is ~0.3. That means that 70% of sunshine is not reflected by clouds or the surface and hence is absorbed by the surface. Not to mention that most of the surface is water, not ground.”
This is sleight of hand where you use two definitions of “surface”.
It helps if you look at clouds and sea level surface separately.
Clouds reflect 40% of the sun that hits them and absorb the rest.
Sea level surface reflects 10% of the sun that hits it and absorbs the rest.
So sea level surface does not absorb 70%. It never gets that much. But the average that is absorbed is indeed the 238 W/m2 as widely accepted.
Then a sensible scientist wanting to do a like for like comparison without sleight of hand, looks at outgoing IR from clouds and sea level surface.
Clouds are cooler 255K approx and have approx 70% emissivity, emit approx 168 W/m2 back to space.
Sea level surface is warmer 288K approx, 97% emissivity and emits approx 378 W/m2 upwards.
There has to be this temp difference between clouds and sea level surface because of the gravity induced lapse rate (G/C) and clouds are the altitude they are (relative humidity, partial pressure and all that stuff).
Of course, two-thirds of what sea level surface emits upwards never gets to space – it hits the underside of clouds, and in turn is absorbed by clouds or reflected back down. One-third gets directly to space.
Then take the weighted average of IR actually going back to space – 2/3 x 168 and 1/3 x 378 and unsurprisingly, you get approx 238 W/m2.
Therefore, the parts of the actual absorbing/emitting surface – that which can directly absorb from Sun or emit to space – clouds and cloud free sea level surface – are the correct temperature to emit as much as they receive. There is no Greenhouse Effect above and beyond this.
There seems to be no need to factor in “back radiation” from anything (apart from the separate balance between clouds and the sea level surface beneath them, of course clouds reflect some IR back down, but that is part of what maintains the lapse rate).
This neatly also explains
a) why the “effective emitting altitude” is as high as it is – 5km or so. It is the clouds doing a lot of the emitting, and they are just that high.
b) Why temps on Venus are what they are (very high 100% cloud cover + their altitude x lapse rate). Don’t pretend that the sun that hits the top of clouds and is not reflected is absorbed by hard surface – that is simply not true by orders of magnitude.
c) why Mars has no greenhouse effect despite its 95% CO2 atmosphere (it’s thin, but there is still more CO2 up there than there is water vapour + CO2 combined on Earth). Mars has no clouds!
Until somebody explains why this common sense, basic physics approach that quickly explains pretty much everything is somehow flawed, I refuse to take any of this CO2 stuff seriously. Sorry but there it is.
Mars has so little atmosphere that it’s effectively transparent in the thermal IR, so there’s only a very small greenhouse effect. The Venusian atmosphere is nearly opaque in the thermal IR so the amount of sunlight that does get to the surface, about the same intensity as at the Earth’s surface on a heavily cloudy day, is enough to keep the surface temperature at about 750K. Water clouds have an emissivity on nearly 1 in the thermal IR, not 0.7. That’s why the lower surface of cloud cover has almost the same temperature as the surface on a fully overcast day.
If most sunshine was reflected or absorbed by clouds and didn’t reach the surface, then solar panels wouldn’t work. But they do. Get a clue. Try looking at the data from the SURFRAD sites.
Bye.
“If most sunshine was reflected or absorbed by clouds and didn’t reach the surface, then solar panels wouldn’t work.”
Duh. Solar panels create far more electricity when the sun is shining on them than when it’s cloudy. So of course clouds absorb or reflect sunshine.
Your abject failure to address any of my points is telling.
IR emitted to space by clouds comes from their upper surface, which is at a much lower temperature than sea level surface..
You show your lack of grasp by telling me that the underside of low clouds is similar to temp of sea level surface. True, but irrelevant.
So you have confirmed my suspicion that the alleged 33 degree effect (sea level temp minus Teff) is a sleight of hand.
Thanks, I’m on to something here, aren’t I?
_“Until somebody explains why this common sense, basic physics approach“_
Your „commen sense“ does neglect that clouds do not only reflect or absorb sunlight, but let much sunlight simply pass through. Yoiu can see it on cloud covered day, it is still not nearly dark. Also do solar panels work with reduced effectivity, so they must be reached by direct sunlight that passed through the clouds.
Your entire calculation falls apart by just introducing the part of the sunlight that passes clouds. Basic physics demands that this passing part of the sunlight is accounted for.
So, this is a very basic physics approach, yet it is seemingly beyond your „common sense“.
That you again come up with the lapse rate on venus without giving an explanation how the vertical circulation necessary for a lapse rate is kept going without the radiative properties of the GHGs remains your personal secret.
“Your „commen sense“ does neglect that clouds do not only reflect or absorb sunlight, but let much sunlight simply pass through.”
Irrelevant. Sunlight is either reflected or absorbed by clouds/exposed oceans and land. That which is not reflected is absorbed. Whether by clouds or the surface beneath them is a secondary issue.
And my calculations show that clouds/exposed oceans and land emit as much IR to space, in total, as they absorb, in total.
“That you again come up with the lapse rate on venus without giving an explanation how the vertical circulation necessary for a lapse rate is kept going without the radiative properties of the GHGs remains your personal secret.”
Again, common sense. Lapse rate = G/Cp. There is a lapse rate in all large gas clouds or planets with atmosphere, regardless of the composition. Huge clouds of hydrogen floated around and became larger and heavier and compressed under their own gravity. This pressure and temperature at the centre is sufficient to trigger nuclear fusion.
If atmospheres were isothermal, please tell me, would they be as warm as the surface/centre beneath them, or as cold as outer space? Is assuming a gradual decline from surface to space not more realistic?
You are also ignoring my fundamental point that assuming that Earth’s surface has uniform temperature and 100% emissivity to arrive at effective temp 255K and then comparing it with sea level temp 288K to arrive at 33 degrees Greenhouse Effect is slipshod. It is a diagonal comparison, which has no place in science.
Clouds cover two-thirds of surface at typical altitude 5km, where their emissivity is average only 70%.
Go back and see if clouds/exposed oceans and land, in total between them, are emitting 235 to 240 W/m2 to space to match incoming solar. Yes, they are.
Everyday example – an aluminium frying pan in the sun reaches a much higher temperature than the pavement on which it is resting. This is not a Greenhouse Effect. This is because aluminium has very low emissivity. Same goes for clouds, which in turn dictates/is in strict relationship with temp of oceans and land below them, which in turn is much the same as temp of exposed (= not covered in cloud) oceans and land.
Here’s just one example of where you’re wrong in your post. Aluminum heats up in the sun because it’s emissivity and absorbtivity in the IR is lower than it’s absorbtivity in the solar energy range. Or, to put it another way, its reflectivity in the IR is higher than its reflectivity in the visible.
There’s a company that sells a coating for solar water heater pipes which has an absorptivity in the solar wavelength range of over 90% and emissivity in the thermal IR on the order of 5%.
That’s absolutely not true of clouds. Clouds have a very high IR emissivity, very close to 1. That’s one of the reasons why cloud tops are cold. And it’s also the reason why the cloud bottom temperature is very close to the surface temperature.
There’s none so blind as those who will not see. And since you don’t want to see, corresponding with you is a waste of my time.
“That’s absolutely not true of clouds. Clouds have a very high IR emissivity, very close to 1. That’s one of the reasons why cloud tops are cold. ”
Fact: cloud emissivity declines with their increasing altitude and falling temperature.
Fact: Average cloud top altitude 5km, average emissivity 70%. I have been told that your models assume emissivity nearly 100%, that’s a silly assumption, seeing as we know facts.
Fact: clouds are same temp as air at same altitude. cloud bases near ground are barely cooler than the ground, obvs. Cloud tops are cold because air at 5km is also cold.
The challenge is to work out what we expect surface temp to be, assuming solar sw absorbed and ir emitted to space is around 235 to 240 w/M2 average in the absence of GHGs. The answer is, as I have explained several times, 288k. Ergo, GHGs have no effect on temp.
„Fact: Average cloud top altitude 5km, average emissivity 70%“
Your value of 70% is not a fact, it is simply wrong.
Emissivity is a wavelength dependend quantity. And in the range of wavelengthes where clouds emit thermal radiation their emissivity is near to 1.
(my other comment is stuck in the moderation process 😦 )
OK, just to humour your claim that cloud emissivity is 1.0, I have redone the calculation on that assumption (it is only your assumption, scientists who actually measured this all say about 0.7). The expected sea level temperature as a result of this would fall from 288K to 276K. Still well above 255K.
Mark,
Please cite your source for your claim that scientists say that cloud top emissivity in the thermal IR (wavelength 5 micrometers or longer) is 0.7 and not 0.99+.
Yes!
Clouds, water vapor (latent heat) and atmospheric pressure “trap heat”
CO₂ is an innocent bystander that’s blamed for everything in our coupled non-linear chaotic climate.
“Despite the harsh conditions on the surface, the atmospheric pressure and temperature at about 50 km to 65 km above the surface of the planet is nearly the same as that of the Earth”
https://en.m.wikipedia.org/wiki/Atmosphere_of_Venus
Yes, exactly. We have to find a set of rules that we can apply to Earth, Venus and Mars and get sensible results.
No surprise that Venus at 50 to 65 km is similar to surface of earth 🙂
Markwardsworth: After many paragraphs of unsupported numbers and rational, you conclude: “There seems to be no need to factor in “back radiation” from anything…”
Given that molecules emit radiation equally in all directions, it is up to you to provide some explanation for why GHG’s that are known to emit radiation to space don’t also emit radiation toward the surface that is absorbed by the surface. People who don’t understand the concepts of temperature, heat and the Second Law of Thermodynamics must have heard many times that the 2LoT does NOT prevent photons emitted by the colder atmosphere (aka back radiation) from being absorbed by the warmer surface. As a past commenter here, you don’t have that excuse.
Temperature and heat flow are concepts that only apply to large groups of colliding molecules. Temperature is proportional to the mean kinetic energy of a group of molecules and heat is the NET energy flux between two such groups of molecules. Individual molecules and photons obey the laws of quantum mechanics, not thermodynamics. This is because a molecule can have a kinetic energy (which changes with each collision – about 10^9 times per second), but not a temperature – a property of a group of molecules that stays constant in the absence of heat transfer. The laws of quantum mechanics ensure that radiation flows both ways between two groups of colliding molecules (say the warmer surface and the colder atmosphere) so that the net flux *heat) is always from warmer to cooler.
Therefore, there must be an error in your long chain of deductions. As I am sure you are aware, consensus science has developed a budget for all average energy fluxes in the atmosphere that include about 324 W/m2 of back radiation flowing from the atmosphere (including clouds) to the surface. For example, see “K/T” energy budget of Kiehl and Trenberth or the excellent discussions here at SOD:
https://journals.ametsoc.org/view/journals/bams/78/2/1520-0477_1997_078_0197_eagmeb_2_0_co_2.xml?
With a little effort, it should be easy for you to track down your first mistakeS. Your second mistake is having the ego to presume that you have refuted science that has withstood careful scrutiny for more than a half-century (including years of scrutiny by many skeptics at this blog). The third mistake is made by you and your readers is confirmation bias: The human tendency to instinctively pay attention and not question information that confirms deeply held beliefs and to fail to assimilate information that contradicts those beliefs. A willingness to question our beliefs is what distinguishes science from religion, including the religions of global warming denial and catastrophic global warming.
Hint: I believe (but I’m not positive) that the IR emissivity of clouds is near unity (not your 70%) and the altitude of the bottom of clouds (that emit back radiation) may be far lower than you think. The online calculator below can provide you with some correct values for back radiation from cloudy and clear skies. Alternatively, you could collect a few dew points and ask yourself the condensation level would be at -6.5 K/km) IF air were rising in your vicinity. And I suspect you’ve totally ignored the vertical transfer of energy by latent and sensible heat in creating a need for compensating heat flow from “back radiation.”
http://climatemodels.uchicago.edu/modtran/
Frank, perhaps your misunderstanding can be traced back to the following statements of yours:
“I believe (but I’m not positive) that the IR emissivity of clouds is near unity (not your 70%)”
I have checked and cross checked this. A detailed study gave a range of 30% to nearly 100%, which seems to vary depending on cloud type, thickness (in metres), density (droplets per metre cubed), altitude and hence temperature.
I decided to use the accepted overall average of 70% from here
https://en.wikipedia.org/wiki/Cloud_physics#Parameters
“the altitude of the bottom of clouds (that emit back radiation) may be far lower than you think”
Yes of course, lower and hence their temp closer to sea level temp – I assume that clouds are a similar temp to air at that altitude. And clouds reflect upward IR in the same way as they reflect visible. This is everyday experience (warm cloudy nights etc).
While this is relevant to the temp balance and altitude of clouds (a relationship which is far more complicated than you can imagine – gravity/specific heat capacity, laten heat of evaporation, IR reflected and emitted in both directions, relative humidity, partial pressure etc) the overall outcome is that the UPPER surface of clouds is at altitude about 5 km or 5.5 km (halfway up troposphere). That is what is relevant here.
I am comparing like with like in a proper scientific way (change as few variables as possible when making a comparison),
a) when we calculate solar being absorbed, we take clouds and their albedo into account. Solar not reflected is absorbed.
b) we know that solar absorbed = IR going to space.
c) when we calculate upward IR that can possibly get to space, we must only take into account IR emitted by clouds and that one-third of IR emitted by sea level surface.
d) when looking at overall solar in/IR out to space, the separate rules that apply between clouds and sea level surface beneath them are irrelevant. Like, if my house has 100% perfect insulation and is warm inside, that does not affect IR to space.
e) So… we take into account actual temperature and emissivity of UPPER surface of clouds and cloud free sea level surface, take a weighted average and unsurprisingly this is equal to solar in.
f) the simplistic “sea level emits 390 W/km2 and only 255 W/m2 gets to space” comparison – and hence a 33 degree GHE is only tenable if you completely ignore clouds on the way out!
Mark: Thanks for the reply and reference. The most obvious error in your calculation is the the last one I thought of and mentioned: You have ignored the upward transport of latent and sensible heat from the surface. When you include those, you need a lot of DLR to have a balance between incoming and outgoing energy at the surface. The KT energy budget can help show you when you other assumptions may be off. The budget was put together to create one consistent picture of all of the data we have accumulated from observing the planet from space. That budget was refined about a decade later.
For many purposes, scientists estimate cloud top temperatures and altitudes from space by assuming a cloud emissivity of 1:
“For satellite-based cloud height retrievals based on passive IR measurements, the radiative emission level is regarded as the cloud top. When the emissivity is 1, the cloud is emitting as a blackbody and the cloud top is at, or close to, the actual cloud’s upper boundary. As the emissivity decreases, the cloud top inferred from IR measurements will be lower than the actual cloud-top level.” https://amt.copernicus.org/articles/12/5039/2019/ Third paragraph in introduction.
However, as our satellites and instruments have become more sophisticated and make measurements at many different wavelengths and different angles, you are correct that scientists are now measuring cloud emissivities that are below 1. Today the percentage of the sky covered by “clouds” depends on how optically thick a cloud must be to be called defined as being cloud. There are “non-visible” cirrus clouds that can only be detected in the IR channel. Clouds made of ice are more problematic than ones with water droplets.
When it comes to radiative transfer calculations, the simple assumption that clouds have an IR emissivity of 1 may often be used. If you check out the online MODTRAN calculator, you’ll see that it predicts almost as much DLR from tropical nimbostratus cloud with a base at 0.16 km (453 W/m2) as OLR from a tropical surface (445 W/m2). I removed all of the GHGs for this calculation. So the calculator must be assuming the emissivity of these clouds is near unity. The emission towards the TOA from the top of altostratus clouds (base 2.4 km and top 3.0) is slightly less than the reference curve for 280 K, which is also appropriate for an emissivity near 1 (with no GHGs in the atmosphere). The emission from cirrus clouds appears to be well below unity, but altitude (and therefore temperature) are provided with these clouds.
In AOGCMs, clouds have an emissivity that depends on the cloud water path (g/m2), the fraction of the cloud that is ice and possible the size of the water droplets.
https://www.cesm.ucar.edu/models/atm-cam/docs/cam2.0/description/node84.html
Frank, re your reply 15/9/2022 at 1.16 am.
You are missing the fundamental point I am making. So let’s go back to a basic physics class. Please tell me which paragraph you disagree with. Simply saying ‘but you are ignoring back radiation’ won’t cut it anymore, I have made this clear where I am ignoring it and why, purely as an academic exercise.
1. Solar absorbed by Earth, as viewed from space must be equal to IR emitted Earth back to space.
2. You are told, as seen from space, Earth’s surface is 2/3 clouds with albedo 0.4 and one-third sea/land with albedo 0.1. Calculate absorbed solar.
Answer = 240 W/m2 average.
3. You are asked to calculate likely temp of a planet which is emitting 240 W/m2 average i.e. its effective temp. You are told to ignore ‘back radiation’ and assume that surface is a uniform temp and has emissivity 1.0.
Answer = 255K.
I hope that we are in agreement on those first three, seeing as I have learned all this from sites such as yours.
4. Now, a bit more advanced. Instead of a hypothetical idealised black body planet with uniform temp etc, we look at actual Earth in all its glory:
You are told that emissions to space are two-thirds from upper surface of clouds and one-third from cloud free sea/land.
Clouds are 33 deg cooler than sea/land (5km altitude x 6.5 K/km lapse rate) and have emissivity 0.7. Sea/land has emissivity 0.97.*
Calculate likely temp of clouds and sea/land such that on average, they are emitting 240 W/m2 to space.
As in part 3, you are told to ignore ‘back radiation’.
Answer (by interpolation and trial and error, or a clever Excel sheet) = sea/land 288K and upper surface of clouds 255K. I have already done the workings and they appear above.
* I am aware that each of my variables in this list in 4. are just my best estimates of mid-points of ranges pulled from a variety of sources and I would not stand and die for them. But the overall approach is sound and gives a very plausible and correct answer, so can’t be far off.
5. You are then told that as a matter of fact, sea/land is average 288K and upper surface of clouds is average temp 255K, so you are quite happy with your result from 3.
6. You are then given the relevant numbers for Venus and Mars (and the amount of incoming solar before albedo adjustment) and calculate hard surface temps of about 200K – 210K and about 700K – 750K.
Again, these tie in with actual observations.
End of lesson. It seems strange to me that a bit of basic physics gets you pretty good answers in each case if you ignore ‘back radiation’. Conclusion – you can ignore ‘back radiation’ when estimating what sea/land temp ‘should’ be. There is no missing 33 degrees, there is no missing 150 W/m2.
7. For really advanced, we can discuss the temp/energy exchanges between sea/land and clouds. This is heinously complicated but not relevant to q’s 1 to 6. We would also have to discuss radiation being reflected, absorbed and re-emitting up and down, the gravito-thermal lapse rate, how latent heat of evaporation affects this, specific heat capacity of sea/land, air itself and water vapour, estimate likely altitude of clouds based on the fine balance of temp, pressure, density, relative humidity and work out the ‘Goldilocks zone’ where clouds can exist (and not evaporate again or condense and fall as hail or rain).
Mark: There are two energy balances you need to deal with: the energy balance across the TOA and the energy balance at the surface. The KT energy budget has both. The existence of DLR is only important to the surface energy balance. You can only “prove” DLR doesn’t exist or transfer heat to the surface by studying the energy balance AT THE SURFACE.
Energy balance across the TOA is the simplest because you only need to deal with radiation, which in principle can be calculated given the known temperature and composition of the atmosphere. That is why we quantify forcing across the TOA (even though we care about global warming at the surface). Measurements from space tell us how much SWR is reflected and scattered back to space and how much LWR escapes to space (239 or 240 W/m2). DLR doesn’t directly enter into this calculation.
Calculating the energy balance at the surface is more complicated, because you need to add convection of latent and simple heat and DLR to the energy fluxes arriving at the surface. You completely ignore latent and sensible heat, and therefore CAN’T DRAW ANY CONCLUSIONS ABOUT DLR!
Average precipitation of about 1 m/year gives us a decent estimate of how much latent heat is removed from the surface due to evaporation and convection (but you need to correct for the fraction that comes down as snow). The convection that carries water vapor also carries some sensible heat that is difficult to quantify. Given the known composition and changing temperature with altitude of the atmosphere, the amount of DLR arriving at the surface is relatively simple to calculate for those who aren’t confused by the 2LoT. The amount of SWR is reaching the surface is poorly known because scattering and absorption by clouds has some uncertainty. (AOGCMs apparently differ by more than 10 W/m2 about this.) Best estimates for all of these values are found in the KT energy balance diagram.
Mark says: “3. You are asked to calculate likely temp of a planet which is emitting 240 W/m2 average i.e. its effective temp. You are told to ignore ‘back radiation’ and assume that surface is a uniform temp and has emissivity 1.0. Answer = 255K.”
This is a crude blackbody MODEL for our planet that has some uses, but in no way properly describes how our planet behaves. The temperature of our planet varies from 190 to 310 K, so the assumption of a uniform temperature is grossly wrong. Emission varies with the fourth power of temperature, making blackbody emission vary 7-fold. GHGs emit at only certain wavelengths, not like blackbodies. You can also construct a grey body MODEL (emissivity 0.615, temperature 288 K) that has some uses, but also in no way accurately describes how our planet behaves. And you can create a variety of AOGC MODELS for our planet that do a better of describing how our planet behaves, because they break it up into a million grid cells with realistic temperature and composition and apply the laws of physics to them. Those models also have flaws and uncertainty because the full physics of clouds, condensation and convection can’t be properly modeled in these grid cells. These MODELS contain parameters to model these components that are then TUNED to agree with observations, a process that does not reliably lead to a unique and optimum set of parameters.
The KT energy balance model is mostly composed of things we can and do MEASURE. This is why you should compare your calculations with the measured values in KT energy balance diagram. You need to look up what values are measured (and their references if need be) and which values are calculated.
FWIW, radiative transfer calculations have been validated by proving they are just as accurate as the best measurements we can make in the atmosphere. You can point a sophisticated IR thermometer at the night sky and measure the same amount of DLR as you would calculate if you sent up a weather balloon to measure the temperature and humidity at various altitudes overhead and used the data in a radiative transfer calculation. A reference in the Wikipedia article you kindly pointed out leads to papers showing scientists doing the same thing for cloudy skies, where you need to know the IR emissivity of various cloud types. IIRC, studies on the IR emissivity of clouds go back to at least the 1970’s.
Mark also wrote: ‘Clouds are 33 deg cooler than sea/land (5km altitude x 6.5 K/km lapse rate) and have emissivity 0.7. Sea/land has emissivity 0.97.* Calculate likely temp of clouds and sea/land such that on average, they are emitting 240 W/m2 to space…. e) So… we take into account actual temperature and emissivity of UPPER surface of clouds and cloud free sea level surface, take a weighted average and unsurprisingly this is equal to solar in.
This in incomplete. Like radiation emitted by the surface, radiation emitted from cloud tops is modified by absorption and emission by the GHGs as it travels to space. It is easy to demonstrate with MODTRAN that GHGs dramatically reduce the amount of radiation leaving cloud tops that succeeds in reaching space. Averaging emission from marine boundary layer clouds with tops below 2 km with that of thin cirrus clouds is dubious. Thanks to compensating errors, your values are similar to the OBSERVATIONS in the KT energy balance paper (Table 2): 235 W/m2 crossing the TOA above cloudy skies and 265 above clear skies.
Finally Mark writes: “f) the simplistic “sea level emits 390 W/km2 and only 255 W/m2 [240 W/m2] gets to space” comparison – and hence a 33 degree GHE is only tenable if you completely ignore clouds on the way out!
Here I agree with Mark. If you rely on a lousy blackbody MODEL, you will calculate 33 degK. I prefer to say that the GHE is 150 W/m2 – a measured value that doesn’t depend on any MODEL. However, those who want to talk about the warming caused by the GHE insist on quantifying it in terms of temperature. Converting a GHE or forcing measured in W/m2 into a change in temperature is the biggest challenge in climate science.
Mark revised what I wrote to say: “CLOUDS in the atmosphere… ensure that the average photon escaping to space is emitted from well above the surface of the Earth, where it is much colder.”
I will revise that to say BOTH CLOUDS AND GHGs in the atmosphere ensure that the average photon escaping to space is emitted from well above the surface of the Earth, where it is much colder. Colder means BOTH clouds and GHGs emit less radiation than the surface – whatever the emissivity of the cloud tops.
For once, stop talking and go to the online MODTRAN radiation calculator and see what theory predicts.
http://climatemodels.uchicago.edu/modtran/
For simplicity, take a Tropical Atmosphere with altostratus clouds with cloud tops at 3.0 km. Looking down from the TOA (70 km), 269 W/m2 are escaping to space. Notice that in the atmospheric window where no GHGs emit (750-1000 cm-1), the spectral intensity is appropriate for a blackbody at 280 K, which is the temperature 3 km above a surface at 300 K with a lapse rate of 6.5 K/km. Now set CO2 to 0 ppm (emission rises to 295 W/m2) and then water vapor scale to zero (rises to 344 W/m2). Now, except for the wavelengths where the minor GHGs absorb, the intensity of emission across the TOA is exactly what Planck’s Law predicts for a blackbody at 280 K. Water and CO2 above the cloud tops are absorbing outgoing OLR emitted by the cloud tops and emitting less thermal IR than they absorb BECAUSE THEY ARE COLDER THAN THE CLOUD TOPS. When you remove the minor GHGs, emission across the TOA rises another 6 W/m2 to 350 W/m2. Remove the clouds and emission rises to 444 W/m2 and falls just below the blackbody emission spectrum for 300 K because the model isn’t using a surface emissivity of 1.
I’ll repeat, BOTH CLOUDS AND GHGs in the atmosphere ensure that the average photon escaping to space is emitted from well above the surface of the Earth, where it is much colder. (Since the wavelengths absorbed by GHGs and clouds overlap, the order in which these absorbers are removed changes how much they individually enhance the GHE.)
Mark then said: “OK, you all dispute that clouds have overall average emissivity of around 70% and insist it’s 99%+, but that’s not my problem.”
No, I didn’t dispute this recent reference. I THANKED YOU for the reference. The people who crudely added clouds to this online calculator long ago need to adjust the emissivity of their clouds. However, the climate scientists who put together the Earth’s Energy Budget didn’t calculate the emission from cloud tops of various types with differing emissivities at various altitudes and temperatures. They got their values for the emission from cloud tops from OBSERVATIONS made from space. Therefore, they didn’t need to worry about their exact altitude and emissivity. Measurements made in the atmospheric window give their effective radiating temperature, but we now know that their real temperatures are higher and their altitudes and emissivities are lower. The published Earth’s Energy Budgets are mostly based on OBSERVATIONS made from space and require NO ADJUSTMENT because we now know cloud emissivity is significantly lower than unity. Our host has many excellent discussions of these OBSERVATIONS in his “Clouds and Water Vapor” series, beginning with Part One (which was revised). Read further than this early post and learn something about the real planet.
“Clouds reflect solar radiation by 48 W/m2 but reduce the outgoing longwave radiation (OLR) by 30 W/m2, therefore the average net effect of clouds – over this period at least – is to cool the climate by 18 W/m2. Note that these values are the global annual average.”
AOGCM’s are a different matter. The properties of their clouds are tuned to produce the same flux of LWR across the TOA as we observe from space. The SWR reflectivity of these clouds and presumably their LWR emissivity can be calculated from their liquid water path and probably droplet size (controlled by the number of condensation nuclei), but I’ve never looked into these details. I do know that the most crucial parameter in any tuned model is the “entrainment parameter” that controls the local turbulent mixing of moist rising air with drier subsiding air. This parameter must have a huge impact on the altitude of cloud tops. (This may be the reason why observations of the lapse rate in the upper tropical troposphere appears to disagree with the predictions of models.) The entrainment parameter has the biggest impact on climate sensitivity.
Responsible climate skeptics assert that AOGCMs can’t accurately model the effect of rising global temperature on clouds and therefore can’t reliably predict climate sensitivity. They aren’t conspiracy theorists who assert that their flawed calculations have uncovered a fundamental error that has been hidden for the past half-century. As I noted below, your calculations “proving” that DLR can’t contribute to the surface energy balance are grossly incorrect because they ignore about 100 W/m2 of heat loss via latent and sensible heat!
Mark wrote: “Fact: cloud emissivity declines with their increasing altitude and falling temperature.”
If you take the same cloud (same number of droplets with the same size distribution) at different altitudes/temperatures, emission falls with temperature and emissivity remains constant. Emissivity is a property of a material that remains constant over modest changes in temperature.
If you changes the properties of the clouds in question when you are changing their altitude and temperature, emissivity may change modestly too – or a lot if liquid clouds turn into ice.
Mark raised an interesting point about Mars. According to Wikipedia, the atmospheric pressure on the surface of Mars is 0.088 psi and the atmosphere is 95% CO2, giving a CO2 partial pressure of 0.0836 psi. On Earth, surface pressure is 14.7 psi, but the atmosphere is only 0.04% CO2, giving a CO2 partial pressure of 0.00588 psi. So Mars has about 34-fold more CO2. Why doesn’t it have a larger GHE than Earth?
First, let’s consider the GHE as measured in W/m2. Earth emits only about 240 W/m2 of thermal IR through the TOA, but it has an average surface temperature high enough to emit an average 355 W/m2 (assuming the latest value for emissivity, 0.91). The difference, 115 W/m2, is the best way to quantify the GHE.
(To quantify the GHE in terms of temperature, one needs to choose a model for the Earth without GHGs. 33 degC comes from assuming the Earth without GHGs behaves like a blackbody. 255 K is the blackbody equivalent temperature that emits 240 W/m2, but there is no reason to assume Earth with GHGs will emit like a blackbody – it’s just a MODEL.)
The reason 115 W/m2 less thermal infrared crosses the TOA than is emitted by the average surface is due to the presence of GHGs AND CLOUDS in the atmosphere. They ensure that the average photon escaping to space is emitted from well above the surface of the Earth, where it is much colder.
According to MODTRAN, a US Standard Atmosphere (surface 288 K) with 400 ppm CO2 and no other GHGs or clouds would emit 345 W/m2 across the TOA. 34-times as much CO2, 13,600 ppm, would reduce TOA emission to only 323 W/m2, an enhancement to the GHE of only 22 W/m2.
The US Standard Atmosphere with no clouds and 400 ppm CO2 and standard values for the other GHGs emits 268 W/m2 through the TOA. Removing the CO2 allows 296 W/m2 to cross the TOA. So today’s 400 ppm of CO2 enhances the GHE by 28 W/m2.
Removing water vapor has a much bigger impact than removing CO2: 335 W/m2 escapes across the TOA. Water vapor enhances the GHE by 67 W/m2.
The rest of the 28 W/m2 of GHE that reduces 268 W/m2 to 240 W/m2 would be due to clouds – if the US Standard Atmosphere were a good model for the average Earth atmosphere (which it may not be). If we add a layer of stratus/stratocumulus clouds (0.66 to 2 km) to the Standard US Atmosphere with normal GHGs, emission across the TOA drops to 243 W/m2. So these clouds covering 100% of the sky enhance the GHE by 25 W/m2. Higher altocumulus clouds (2.4 to 3.0 km) reduce TOA emission to 228 W/m2 enhancing the GHE by 30 W/m2 for 100% clouds. So 28 W/m2 is a reasonable estimate for the enhanced GHE due to clouds but it might be a little high.
So, if we remove water vapor and clouds from the Earth’s atmosphere, but raise CO2 to 13,600 ppm, we would lose about 67 (water vapor) + 28 W/m2 of GHE enhancement
and gain 22 W/m2 (CO2) for a net loss of 73 W/m2 of GHE enhancement (out of 115 W/m2). So we should expect Mars to have a much smaller GHE than the Earth. Using the blackbody equivalent temperature model, Mars GHE is 5 degC and the Earth 33 degC.
(FWIW, the above calculations are crude approximations that ignore the fact that some absorption bands overlap and therefore their effect on emission crossing the TOA isn’t additive. Planetary rotation rate and heat capacity also have an also impact surface temperature. The average surface temperature of the Moon with no clouds or GHGs is not its Blackbody Equivalent Temperature. Despite these problems, it is clear that Mars should have a much smaller GHE than the Earth.
Frank: “CLOUDS in the atmosphere… ensure that the average photon escaping to space is emitted from well above the surface of the Earth, where it is much colder.”
I’ve tweaked your sentence and now would wholeheartedly agree with it. It’s not just that clouds are emitting “colder” (i.e. less radiation) due to low temperature, they are emitting even less than that because of low emissivity.
OK, you all dispute that clouds have overall average emissivity of around 70% and insist it’s 99%+, but that’s not my problem. I have not been so rude to ask for evidence for this (as you did to me, which I duly provided and it was ignored), everybody is entitled to their own beliefs, even if wrong.
Don’t be so condescending, it’s not a good look or even polite.
For your benefit, I will clarify.
1. 30% is reflected, 70% is absorbed. Most of the 70% that is absorbed is absorbed by clouds. You know that, I know that, don’t waste everybody’s time by playing dumb and pretending you thought I didn’t.
2. When you say “the surface” in everyday conversation, a normal person assumes you mean the land or ocean surface.
3. When you say “the surface” when discussing albedo and effective temps, we assume you mean “whatever is the top layer which is visible from space”.
For example – albedo of Venus is high because of cloud cover. It would not make any difference to its albedo whether the land surface of Venus were black or white. We can’t see the land surface colour of Venus from space – it is irrelevant for eff temp purposes.
4. This top visible layer which we look at through telescopes to work out albedo is “the effective emitting layer”. The physical layer that emits the visible light going to space is pretty much the same as the layer that emits the longwave radiation going to space.
5. This top layer is also “whatever the sunlight hits first” i.e. the effective absorbing layer. If a ray of light hits a cloud first, it cannot also hit the land beneath it (with anything like the same intensity)
6. So when we calculate the effective temp of a planet or moon, we are calculating – and can only calculate – the effective temp of the visible – absorbing – emitting – top layer.
In other words, for Mars and Moon, we are calculating eff temp of actual hard surface – there are no clouds so the hard surface is visible from space. For Venus and Earth, we are calculating eff temp of the cloud cover, that being the only thing visible from space.
OK, Earth is only 2/3 cloud cover so I’m rounding up for simplification. It’s far better than what sites like this do which is pretend that clouds aren’t there are at all.
7. To sum up, the clever lie adopted by Mann back in the 1980 to support his theories was switching between the two different meanings of surface – he gambled on the general public not noticing the clever switching between the two meanings of “surface” explained in 2 and 3 above. It appears that you are as gullible as the general public on this.
8. Once you spot this sleight of hand, it’s all very simple to understand. The 33 degree difference is simply the difference between cloud temp and sea level temp.
That’s down to the lapse rate – i.e. the trade off between thermal energy and potential energy – energy cannot be created or destroyed, just transformed from one state to another etc.
The logic and maths stacks up perfectly on Venus and Mars too. Even though Mars has thirty times as much CO2 per surface area than Earth it has no clouds (apart from a few dust clouds) and virtually no GHE.
(sorry if this is double post but your site accepts, rejects and hides comments pretty much at random).
I’m not the one playing dumb here. The majority of solar energy that isn’t reflected is absorbed by the surface, ~67%, not clouds.
In TFK2008, Earth’s Global Energy Budget, we have 341.3 W/m² annual average insolation. Of that 102W/m² is reflected, 79W/m² by clouds and 23W/m² by the surface. 161W/m² is absorbed by the surface and 79W/m² is absorbed by the atmosphere and clouds. So more than twice as much solar energy that isn’t reflected is absorbed by the surface rather than by clouds.
Please cite references to support your assertion.
Do the math.
Earth’s effective emitting/absorbing surface, as far as exchange of radiation with outer space is concerned, is…
a) Two-thirds clouds, typical albedo 0.37 meaning that clouds absorb 63% x 2/3 of sunlight reaching Earth = 42% overall.
b) One-third land or ocean, typical albedo 0.16 meaning that land or ocean absorb 84% x 1/3 of sunlight reaching Earth = 28% overall
42% + 28% = 70% total absorbed and 30% reflected.
42% is “most” of 70% (well a lot more than half at least).
But explaining the maths wasn’t my main point.
My point was that people like you use “surface” interchangeably to mean either
a) “land and ocean surface” or
b) “the effective emitting/absorbing surface” which is two-thirds clouds and one-third land or ocean.
Inevitably, surface a) is warmer than surface b) by about 33 degrees, that’s to do with the lapse rate, the trade off between thermal and potential energy. It has nothing to do with radiation.
PS, when you refer to TFK2008, that reminds me of arguing with Christians and Creationists, when you try to drag them back to reality and point out all the flaws and contradictions in their belief systems, they just have a mental block and keep saying “but the Book of XYZ says so” or “you just have to have faith”.
„that’s to do with the lapse rate, the trade off between thermal and potential energy. It has nothing to do with radiation.“
The lapse rate does not occur out of nothing. It is a consequence of radiation entering the system at lower heights (land and ocean surface) but leaving higher up.
There would be near to no lapse rate if those localities were not spatially separated – uprisen air parcels need to cool nonadiabatically by losing energy to space via radiation.
To say this „has nothing to do with radiation“ has nothing to do with basic physics.
Chris, it’s back to school for you.
1. The lapse rate is the trade of between TE and PE. That’s why you can calculate it as G/Cp. You can work this out yourself if you think about it sensibly.
2. You can use this formula to calculate the likely lapse rates for Venus, Earth and Mars (adjusting Earth’s for latent heat of evaporation, of course) and they come out fairly close.
3. This is despite the fact that on Venus, all the sunlight is absorbed by clouds 50km+ above the surface. On Mars, there are no clouds to speak of so all sunlight does hit the surface (like in your simplistic model). On earth, two-thirds of sunlight hits the cloud surface and one-third hits land/ocean surface.
4. Air is not constantly churning up and down vertically. Even on a completely still day, there will be a lapse rate. And even in a completely still night, the surface and atmosphere will be cooling (i.e. radiating to space).
5. As a bonus, what you find is that the surface which the sunlight actually hits is actually pretty close to effective temp of the planet as a whole. So on Venus, temp at top of clouds = effective temp. On Mars, temp of surface = effective temp. And on Earth, weighted average of temp of top of clouds and land/ocean surface = effective temp.
You need to think about WHY a lapse rate occurs in atmospheres in the first place.
Only after that you can show off with your ability to use a simple equation.
And the WHY is quite simple: For a lapse rate to be present you need a dp/dt.
This means the cooling of uprising parcels of air / heating up of down coming parcels.
Once you answered the question WHY parcels are moving around vertically all the time ( and why you observe inversed lapse rates at literally so called „inversed weathers“ ) you know what you need radiation to space from the upper atmosphere for.
Hint: In a hypothetical vertical completely non-mixed column of air there will be no dp/dt, thus no lapse rate regardless of what the pressure gradient between top and bottom of the column is.
On the other hand: If gas somewhere up in a column can radiate energy out of the column it will never stop vertical mixing.
Chris A,
You’re wasting your time.
mark,
I’m violating my principle of not feeding tr011s, but here goes.
70% of the Earth’s surface is ocean. The albedo of the ocean is a lot less than 0.16. The average albedo of the land surface is also less than 0.16. And your calculation ignores the absorption of sunlight that is transmitted through the clouds. TFK is based on measurements, not assertions. You also don’t cite any references for your assertions. They’re likely all numbers you pulled out of the air to fit your narrative.
@Scienceofdoom
Thanks for putting in the effort of explaining a very complex topic
I don’t know if you have an interest in improving further this page, but I have found a few things that could help
making it even clearer
– give a number to graphs so that there is no chance of misunderstanding when referring to them (it would avoid potential confusion in a sentence like “as shown in the 3rd and 4th graphs above”. 3rd and 4th starting from where?)
– on the graph “from Vardavas & Taylor” it is very difficult to distinguish the solar irradiation curve from the black body curve (I suspect they are very close)
– the HITRANS linear graphs showing absorption characteristics of CO2 and H2O : it took me a while to notice the small “H2O” and “CO2” in the top right
– the most confusing bit to me is the diagram “Absorption of different wavelength radiation in the earth’s atmosphere”. Are “absorption” and “atmospheric opacity” supposed to be the same thing? I think this diagram describes what radiation is observed at the earth’s surface (which doesn’t seem to work well with “best observed from space”), but it took me some thinking. Is it that some of what we observe is due to reflection (lower wavelength), some to absorption within the atmosphere? Clearly I struggle with this diagram
All these things are probably very minor for someone with brains or experience. But those little extra hurdles can be the difference between persevering and giving up for someone with limitations like myself.
Hope that helps
[…] Climate School – [EN] Ce que j’ai trouvé de plus complet (en 8 parties !) : CO2 – An Insignificant Trace Gas? Part One – [EN] Sur la question de la saturation du CO2: Part II: What Ångström […]
[…] Climate School– [EN] Ce que j’ai trouvé de plus complet (en 8 parties !) : CO2 – An Insignificant Trace Gas? Part One– [EN] Sur la question de la saturation du CO2: Part II: What Ångström didn’t […]
thanks. a very very descriptive analysis.