Over the last few years I’ve written lots of articles relating to the inappropriately-named “greenhouse” effect and covered some topics in great depth. I’ve also seen lots of comments and questions which has helped me understand common confusion and misunderstandings.
This article, with huge apologies to regular long-suffering readers, covers familiar ground in simple terms. It’s a reference article. I’ve referenced other articles and series as places to go to understand a particular topic in more detail.
One of the challenges of writing a short simple explanation is it opens you up to the criticism of having omitted important technical details that you left out in order to keep it short. Remember this is the simple version..
Preamble
First of all, the “greenhouse” effect is not AGW. In maths, physics, engineering and other hard sciences, one block is built upon another block. AGW is built upon the “greenhouse” effect. If AGW is wrong, it doesn’t invalidate the greenhouse effect. If the greenhouse effect is wrong, it does invalidate AGW.
The greenhouse effect is built on very basic physics, proven for 100 years or so, that is not in any dispute in scientific circles. Fantasy climate blogs of course do dispute it.
Second, common experience of linearity in everyday life cause many people to question how a tiny proportion of “radiatively-active” molecules can have such a profound effect. Common experience is not a useful guide. Non-linearity is the norm in real science. Since the enlightenment at least, scientists have measured things rather than just assumed consequences based on everyday experience.
The Elements of the “Greenhouse” Effect
Atmospheric Absorption
1. The “radiatively-active” gases in the atmosphere:
- water vapor
- CO2
- CH4
- N2O
- O3
- and others
absorb radiation from the surface and transfer this energy via collision to the local atmosphere. Oxygen and nitrogen absorb such a tiny amount of terrestrial radiation that even though they constitute an overwhelming proportion of the atmosphere their radiative influence is insignificant (note 1).
How do we know all this? It’s basic spectroscopy, as detailed in exciting journals like the Journal of Quantitative Spectroscopy and Radiative Transfer over many decades. Shine radiation of a specific wavelength through a gas and measure the absorption. Simple stuff and irrefutable.
Atmospheric Emission
2. The “radiatively-active” gases in the atmosphere also emit radiation. Gases that absorb at a wavelength also emit at that wavelength. Gases that don’t absorb at that wavelength don’t emit at that wavelength. This is a consequence of Kirchhoff’s law.
The intensity of emission of radiation from a local portion of the atmosphere is set by the atmospheric emissivity and the temperature.
Convection
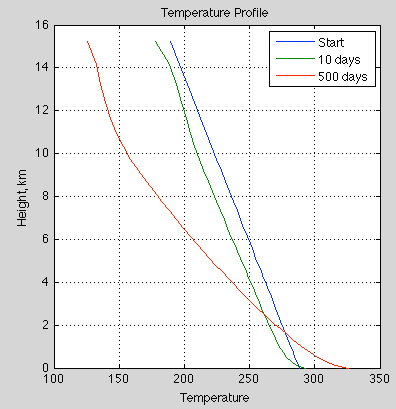
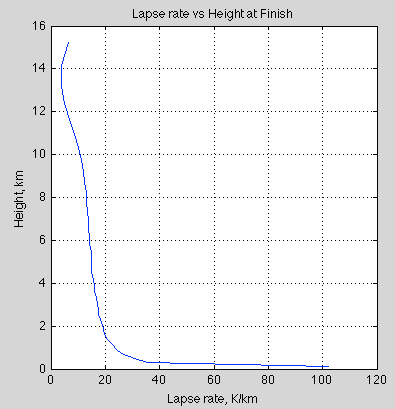
3. The transfer of heat within the troposphere is mostly by convection. The sun heats the surface of the earth through the (mostly) transparent atmosphere (note 2). The temperature profile, known as the “lapse rate”, is around 6K/km in the tropics. The lapse rate is principally determined by non-radiative factors – as a parcel of air ascends it expands into the lower pressure and cools during that expansion (note 3).
The important point is that the atmosphere is cooler the higher you go (within the troposphere).
Energy Balance
4. The overall energy in the climate system is determined by the absorbed solar radiation and the emitted radiation from the climate system. The absorbed solar radiation – globally annually averaged – is approximately 240 W/m² (note 4). Unsurprisingly, the emitted radiation from the climate system is also (globally annually averaged) approximately 240 W/m². Any change in this and the climate is cooling or warming.
Emission to Space
5. Most of the emission of radiation to space by the climate system is from the atmosphere, not from the surface of the earth. This is a key element of the “greenhouse” effect. The intensity of emission depends on the local atmosphere. So the temperature of the atmosphere from which the emission originates determines the amount of radiation.
If the place of emission of radiation – on average – moves upward for some reason then the intensity decreases. Why? Because it is cooler the higher up you go in the troposphere. Likewise, if the place of emission – on average – moves downward for some reason, then the intensity increases (note 5).
More GHGs
6. If we add more radiatively-active gases (like water vapor and CO2) then the atmosphere becomes more “opaque” to terrestrial radiation and the consequence is the emission to space from the atmosphere moves higher up (on average). Higher up is colder. See note 6.
So this reduces the intensity of emission of radiation, which reduces the outgoing radiation, which therefore adds energy into the climate system. And so the climate system warms (see note 7).
That’s it!
It’s as simple as that. The end.
A Few Common Questions
CO2 is Already Saturated
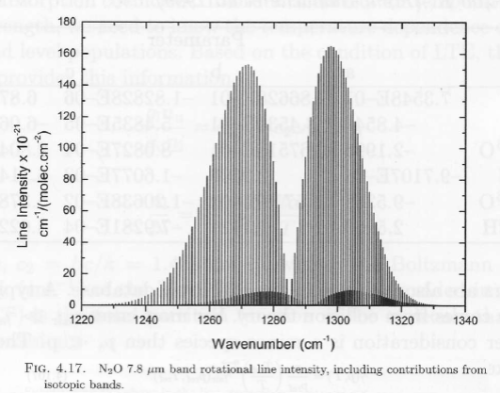
There are almost 315,000 individual absorption lines for CO2 recorded in the HITRAN database. Some absorption lines are stronger than others. At the strongest point of absorption – 14.98 μm (667.5 cm-1), 95% of radiation is absorbed in only 1m of the atmosphere (at standard temperature and pressure at the surface). That’s pretty impressive.
By contrast, from 570 – 600 cm-1 (16.7 – 17.5 μm) and 730 – 770 cm-1 (13.0 – 13.7 μm) the CO2 absorption through the atmosphere is nowhere near “saturated”. It’s more like 30% absorbed through a 1km path.
You can see the complexity of these results in many graphs in Atmospheric Radiation and the “Greenhouse” Effect – Part Nine – calculations of CO2 transmittance vs wavelength in the atmosphere using the 300,000 absorption lines from the HITRAN database, and see also Part Eight – interesting actual absorption values of CO2 in the atmosphere from Grant Petty’s book
The complete result combining absorption and emission is calculated in Visualizing Atmospheric Radiation – Part Seven – CO2 increases – changes to TOA in flux and spectrum as CO2 concentration is increased
CO2 Can’t Absorb Anything of Note Because it is Only .04% of the Atmosphere
See the point above. Many spectroscopy professionals have measured the absorptivity of CO2. It has a huge variability in absorption, but the most impressive is that 95% of 14.98 μm radiation is absorbed in just 1m. How can that happen? Are spectroscopy professionals charlatans? You need evidence, not incredulity. Science involves measuring things and this has definitely been done. See the HITRAN database.
Water Vapor Overwhelms CO2
This is an interesting point, although not correct when we consider energy balance for the climate. See Visualizing Atmospheric Radiation – Part Four – Water Vapor – results of surface (downward) radiation and upward radiation at TOA as water vapor is changed.
The key point behind all the detail is that the top of atmosphere radiation change (as CO2 changes) is the important one. The surface change (forcing) from increasing CO2 is not important, is definitely much weaker and is often insignificant. Surface radiation changes from CO2 will, in many cases, be overwhelmed by water vapor.
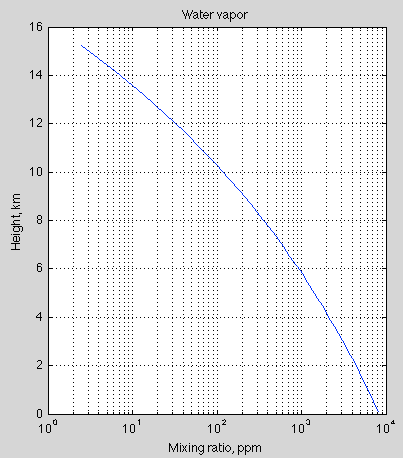
Water vapor does not overwhelm CO2 high up in the atmosphere because there is very little water vapor there – and the radiative effect of water vapor is dramatically impacted by its concentration, due to the “water vapor continuum”.
The Calculation of the “Greenhouse” Effect is based on “Average Surface Temperature” and there is No Such Thing
Simplified calculations of the “greenhouse” effect use some averages to make some points. They help to create a conceptual model.
Real calculations, using the equations of radiative transfer, don’t use an “average” surface temperature and don’t rely on a 33K “greenhouse” effect. Would the temperature decrease 33K if all of the GHGs were removed from the atmosphere? Almost certainly not. Because of feedbacks. We don’t know the effect of all of the feedbacks. But would the climate be colder? Definitely.
See The Rotational Effect – why the rotation of the earth has absolutely no effect on climate, or so a parody article explains..
The Second Law of Thermodynamics Prohibits the Greenhouse Effect, or so some Physicists Demonstrated..
See The Three Body Problem – a simple example with three bodies to demonstrate how a “with atmosphere” earth vs a “without atmosphere earth” will generate different equilibrium temperatures. Please review the entropy calculations and explain (you will be the first) where they are wrong or perhaps, or perhaps explain why entropy doesn’t matter (and revolutionize the field).
See Gerlich & Tscheuschner for the switch and bait routine by this operatic duo.
And see Kramm & Dlugi On Dodging the “Greenhouse” Bullet – Kramm & Dlugi demonstrate that the “greenhouse” effect doesn’t exist by writing a few words in a conclusion but carefully dodging the actual main point throughout their entire paper. However, they do recover Kepler’s laws and point out a few errors in a few websites. And note that one of the authors kindly showed up to comment on this article but never answered the important question asked of him. Probably just too busy.. Kramm & Dlugi also helpfully (unintentionally) explain that G&T were wrong, see Kramm & Dlugi On Illuminating the Confusion of the Unclear – Kramm & Dlugi step up as skeptics of the “greenhouse” effect, fans of Gerlich & Tscheuschner and yet clarify that colder atmospheric radiation is absorbed by the warmer earth..
And for more on that exciting subject, see Confusion over the Basics under the sub-heading The Second Law of Thermodynamics.
Feedbacks overwhelm the Greenhouse Effect
This is a totally different question. The “greenhouse” effect is the “greenhouse” effect. If the effect of more CO2 is totally countered by some feedback then that will be wonderful. But that is actually nothing to do with the “greenhouse” effect. It would be a consequence of increasing temperature.
As noted in the preamble, it is important to separate out the different building blocks in understanding climate.
Miskolczi proved that the Greenhouse Effect has no Effect
Miskolczi claimed that the greenhouse effect was true. He also claimed that more CO2 was balanced out by a corresponding decrease in water vapor. See the Miskolczi series for a tedious refutation of his paper that was based on imaginary laws of thermodynamics and questionable experimental evidence.
Once again, it is important to be able to separate out two ideas. Is the greenhouse effect false? Or is the greenhouse effect true but wiped out by a feedback?
If you don’t care, so long as you get the right result you will be in ‘good’ company (well, you will join an extremely large company of people). But this blog is about science. Not wishful thinking. Don’t mix the two up..
Convection “Short-Circuits” the Greenhouse Effect
Let’s assume that regardless of the amount of energy arriving at the earth’s surface, that the lapse rate stays constant and so the more heat arriving, the more heat leaves. That is, the temperature profile stays constant. (It’s a questionable assumption that also impacts the AGW question).
It doesn’t change the fact that with more GHGs, the radiation to space will be from a higher altitude. A higher altitude will be colder. Less radiation to space and so the climate warms – even with this “short-circuit”.
In a climate without convection, the surface temperature will start off higher, and the GHG effect from doubling CO2 will be higher. See Radiative Atmospheres with no Convection.
In summary, this isn’t an argument against the greenhouse effect, this is possibly an argument about feedbacks. The issue about feedbacks is a critical question in AGW, not a critical question for the “greenhouse” effect. Who can say whether the lapse rate will be constant in a warmer world?
Notes
Note 1 – An important exception is O2 absorbing solar radiation high up above the troposphere (lower atmosphere). But O2 does not absorb significant amounts of terrestrial radiation.
Note 2 – 99% of solar radiation has a wavelength <4μm. In these wavelengths, actually about 1/3 of solar radiation is absorbed in the atmosphere. By contrast, most of the terrestrial radiation, with a wavelength >4μm, is absorbed in the atmosphere.
Note 3 – see:
Density, Stability and Motion in Fluids – some basics about instability
Potential Temperature – explaining “potential temperature” and why the “potential temperature” increases with altitude
Temperature Profile in the Atmosphere – The Lapse Rate – lots more about the temperature profile in the atmosphere
Note 4 – see Earth’s Energy Budget – a series on the basics of the energy budget
Note 5 – the “place of emission” is a useful conceptual tool but in reality the emission of radiation takes place from everywhere between the surface and the stratosphere. See Visualizing Atmospheric Radiation – Part Three – Average Height of Emission – the complex subject of where the TOA radiation originated from, what is the “Average Height of Emission” and other questions.
Also, take a look at the complete series: Visualizing Atmospheric Radiation.
Note 6 – the balance between emission and absorption are found in the equations of radiative transfer. These are derived from fundamental physics – see Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations – the equations of radiative transfer including the plane parallel assumption and it’s nothing to do with blackbodies. The fundamental physics is not just proven in the lab, spectral measurements at top of atmosphere and the surface match the calculated values using the radiative transfer equations – see Theory and Experiment – Atmospheric Radiation – real values of total flux and spectra compared with the theory.
Also, take a look at the complete series: Atmospheric Radiation and the “Greenhouse” Effect
Note 7 – this calculation is under the assumption of “all other things being equal”. Of course, in the real climate system, all other things are not equal. However, to understand an effect “pre-feedback” we need to separate it from the responses to the system.