In A Challenge for Bryan I put up a simple heat transfer problem and asked for the equations. Bryan elected not to provide these equations. So I provide the answer, but also attempt some enlightenment for people who don’t think the answer can be correct.
As DeWitt Payne noted, a post with a similar problem posted on Wattsupwiththat managed to gather some (unintentionally) hilarious comments.
Here’s the problem again:
Case 1
Spherical body, A, of radius ra, with an emissivity, εa =1. The sphere is in the vacuum of space.
It is internally heated by a mystery power source (let’s say nuclear, but it doesn’t matter), with power input = P.
The sphere radiates into deep space, let’s say the temperature of deep space = 0K to make the maths simpler.
1. What is the equation for the equilibrium surface temperature of the sphere, Ta?
Case 2
The condition of case A, but now body A is surrounded by a slightly larger spherical shell, B, which of course is itself now surrounded by deep space at 0K.
B has a radius rb, with an emissivity, εb =1. This shell is highly conductive and very thin.
2a. What is the equation for the new equilibrium surface temperature, Ta’?
2b. What is the equation for the equilibrium temperature, Tb, of shell B?
Notes:
The reason for the “slightly larger shell” is to avoid “complex” view factor issues. Of course, I’m happy to relax the requirement for “slightly larger” and let Bryan provide the more general answer.
The reason for the “highly conductive” and “thin” outer shell, B, is to avoid any temperature difference between the inside and the outside surfaces of the shell. That is, we can assume the outside surface is at the same temperature as the inside surface – both at temperature, Tb.
This kind of problem is a staple of introductory heat transfer. This is a “find the equilibrium” problem.
How do we solve these kinds of problems? It’s pretty easy once you understand the tools.
The first tool is the first law of thermodynamics. Steady state means temperatures have stabilized and so energy in = energy out. We draw a “boundary” around each body and apply the “boundary condition” of the first law.
The second tool is the set of equations that govern the movement of energy. These are the equations for conduction, convection and radiation. In this case we just have radiation to consider.
For people who see the solution, shake their heads and say, this can’t be, stay on to the end and I will try and shed some light on possible conceptual problems. Of course, if it’s wrong, you should easily be able to provide the correct equations – or even if you can’t write equations you should be able to explain the flaw in the formulation of the equation.
In the original article I put some numbers down – “For anyone who wants to visualize some numbers: ra=1m, P=1000W, rb=1.01m“. I will use these to calculate an answer from the equations. I realize many readers aren’t comfortable with equations and so the answers will help illuminate the meaning of the equations.
I go through the equations in tedious detail, again for people who would like to follow the maths but don’t find maths easy.
Case 1
Energy in, Ein = Energy out, Eout : in Watts (Joules per second).
Ein = P
Eout = emission of thermal radiation per unit area x area
The first part is given by the Stefan-Boltzmann equation (σTa4, where σ = 5.67×10-8), and the second part by the equation for the surface area of a sphere (4πra²)
Eout = 4πra² x σTa4 …..[eqn 1]
Therefore, P = 4πra²σTa4 ….[eqn 2]
We have to rearrange the equation to see how Ta changes with the other factors:
Ta = [P / (4πra²σ)]1/4 ….[eqn 3]
If you aren’t comfortable with maths this might seem a little daunting. Let’s put the numbers in:
Ta = 194K (-80ºC)
Now we haven’t said anything about how long it takes to reach this temperature. We don’t have enough information for that. That’s the nice thing about steady state calculations, they are easier than dynamic calculations. We will look at that at the end.
Probably everyone is happy with this equation. Energy is conserved. No surprises and nothing controversial.
Now we will apply the exact same approach to the second case.
Case 2
First we consider “body A”. Given that it is enclosed by another “body” – the shell B – we have to consider any energy being transferred by radiation from B to A. If it turns out to be zero, of course it won’t affect the temperature of body A.
Ein(a) = P + Eb-a ….[eqn 4], where Eb-a is a value we don’t yet know. It is the radiation from B absorbed by A.
Eout(a) = 4πra² x σTa4 ….[eqn 5]- this is the same as in case 1. Emission of radiation from a body only depends on its temperature (and emissivity and area but these aren’t changing between the two cases)
– we will look at shell B and come back to the last term in eqn 4.
Now the shell outer surface:
Radiates out to space
We set space at absolute zero so no radiation is received by the outer surface
Shell inner surface:
Radiates in to A (in fact almost all of the radiation emitted from the inner surface is absorbed by A and for now we will treat it as all) – this was the term Eb-a
Absorbs all of the radiation emitted by A, this is Eout(a)
And we made the shell thin and highly conductive so there is no temperature difference between the two surfaces. Let’s collect the heat transfer terms for shell B under steady state:
Ein(b) = Eout(a) + 0 …..[eqn 6] – energy in is all from the sphere A, and nothing from outside
= 4πra² x σTa4 ….[eqn 6a] – we just took the value from eqn 5
Eout(b) = 4πrb² x σTb4 + 4πrb² x σTb4 …..[eqn 7] – energy out is the emitted radiation from the inner surface + emitted radiation from the outer surface
= 2 x 4πrb² x σTb4 ….[eqn 7a]
And we know that for shell B, Ein = Eout so we equate 6a and 7a:
4πra² x σTa4 = 2 x 4πrb² x σTb4 ….[eqn 8]
and now we can cancel a lot of the common terms:
ra² x Ta4 = 2 x rb² x Tb4 ….[eqn 8a]
and re-arrange to get Ta in terms of Tb:
Ta4 = 2rb²/ra² x Tb4 ….[eqn 8b]
Ta = [2rb²/ra²]1/4 x Tb ….[eqn 8b]
or we can write it the other way round:
Tb = [ra²/2rb²]1/4 x Ta ….[eqn 8c]
Using the numbers given, Ta = 1.2 Tb. So the sphere is 20% warmer than the shell (actually 2 to the power 1/4).
We need to use Ein=Eout for the sphere A to be able to get the full solution. We wrote down: Ein(a) = P + Eb-a ….[eqn 4]. Now we know “Eb-a” – this is one of the terms in eqn 7.
So:
Ein(a) = P + 4πrb² x σTb4 ….[eqn 9]
and Ein(a) = Eout(a), so:
P + 4πrb² x σTb4 = 4πra² x σTa4 ….[eqn 9]
we can substitute the equation for Tb:
P + 4πra² /2 x σTa4 = 4πra² x σTa4 ….[eqn 9a]
the 2nd term on the left and the right hand side can be combined:
P = 2πra² x σTa4 ….[eqn 9a]
And so, voila:
T’a = [P / (2πra²σ)]1/4 ….[eqn 10] – I added a dash to Ta so we can compare it with the original value before the shell arrived.
T’a = 21/4 Ta ….[eqn 11] – that is, the temperature of the sphere A is about 20% warmer in case 2 compared with case 1.
Using the numbers, T’a = 230 K (-43ºC). And Tb = 193 K (-81ºC)
Explaining the Results
In case 2, the inner sphere, A, has its temperature increase by 36K even though the same energy production takes place inside. Obviously, this can’t be right because we have created energy??.. let’s come back to that shortly.
Notice something very important – Tb in case 2 is almost identical to Ta in case 1. The difference is actually only due to the slight difference in surface area. Why?
The system has an energy production, P, in both cases.
- In case 1, the sphere A is the boundary transferring energy to space and so its equilibrium temperature must be determined by P
- In case 2, the shell B is the boundary transferring energy to space and so its equilibrium temperature must be determined by P
Now let’s confirm the mystery unphysical totally fake invented energy.
Let’s compare the flux emitted from A in case 1 and case 2. I’ll call it R.
- R(case 1) = 80 W/m²
- R(case 2) = 159 W/m²
This is obviously rubbish. The same energy source inside the sphere and we doubled the sphere’s energy production!!! Get this idiot to take down this post, he has no idea what he is writing..
Yet if we check the energy balance we find that 80 W/m² is being “created” by our power source, and the “extra mystery” energy of 79 W/m² is coming from our outer shell. In any given second no energy is created.
The Mystery Invented Energy – Revealed
When we snapped the outer shell over the sphere we made it harder for heat to get out of the system. Energy in = energy out, in steady state. When we are not in steady state: energy in – energy out = energy retained. Energy retained is internal energy which is manifested as temperature.
We made it hard for heat to get out, which accumulated energy, which increased temperature.. until finally the inner sphere A was hot enough for all of the internally generated energy, P, to get out of the system.
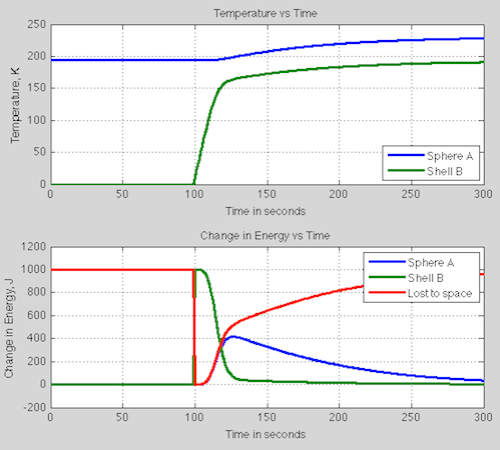
Let’s add some information about the system: the heat capacity of the sphere = 1000 J/K; the heat capacity of the shell = 100 J/K. It doesn’t much matter what they are, it’s just to calculate the transients. We snap the shell – originally at 0K – around the sphere at time t=100 seconds and see what happens.
The top graph shows temperature, the bottom graph shows change in energy of the two objects and how much energy is leaving the system:
At 100 seconds we see that instead of our steady state 1000W leaving the system, instead 0W leaves the system. This is the important part of the mystery energy puzzle.
We put a 0K shell around the sphere. This absorbs all the energy from the sphere. At time t=100s the shell is still at 0K so it emits 0W/m². It heats up pretty quickly, but remember that emission of radiation is not linear with temperature so you don’t see a linear relationship between the temperature of shell B and the energy leaving to space. For example at 100K, the outward emission is 6 W/m², at 150K it is 29 W/m² and at its final temperature of 193K, it is 79 W/m² (=1000 W in total).
As the shell heats up it emits more and more radiation inwards, heating up the sphere A.
The mystery energy has been revealed. The addition of a radiation barrier stopped energy leaving, which stored heat. The way equilibrium is finally restored is due to the temperature increase of the sphere.
Of course, for some strange reason an army of people thinks this is totally false. Well, produce your equations.. (this never happens)
All we have done here is used conservation of energy and the Stefan Boltzmann law of emission of thermal radiation.


If we take into account view factors – Ta drops a little in relation to Tb.
Without view factors we had (eqn 8b)
Ta4 = Tb4.2rb²/ra²
With view factors we get:
Ta4 = Tb4.(rb²/ra² +1)
The view factor means that some of the radiation emitted from the inside surface of the shell is absorbed by the shell instead of by the sphere. Whereas all of the radiation emitted by the sphere is absorbed by the shell. It’s just a geometry thing.
So it’s like some radiation “leaks” straight back to the shell and therefore the temperature of the sphere can’t be as high when we take it into account.
Following through with this line of thought, the answer to the problem I defined at
is:
(Tn)^4 = (P/σ)*(1/Ao + 1/A1 + 1/A2 + … + 1/An)
where:
An = 4π*(Rn)^2
for n = 0, 1, 2, …
Its a great honour to headline two posts in your prestigious blog
The reason is no doubt when I pointed out your misrepresentation of the G&T paper two threads ago when I said
The little puzzle presented above was already answered early on in the G&T paper
” It is an interesting point that the thermal conductivity of CO2 is only one half of that of nitrogen or oxygen. In a 100 percent CO2 atmosphere a conventional light bulb shines brighter than in a nitrogen-oxygen atmosphere due to the lowered thermal conductivity of its environment.”
Page 12
Click to access 0707.1161.pdf
So if you increase the insulation around a powered source the source equilibrium temperature will rise.”
The normal reaction would be ‘thank you Bryan I will make a note of that.’
However the Willis model of steel dome is worth revisiting and I can see nothing wrong with his conclusions.
You must admit that readers of your blog are unlikely to have the graphic and mathematical texting tools that you have.
But why do G&T write about the thermal conductivity of CO2, when it has virtually no influence on anything in the atmosphere.
First of all the total thermal conductivity of air affects noticeably only the heat transfer from the surface to the air, and even major changes in the conductivity have an negligible influence on the temperature difference over the boundary between the air and the liquid/solid surface.
Secondly for the conductivity the influence of CO2 is really as small as its concentration.
It’s obvious that G&T write about that only to confuse, most probably the same is true also for Bryan, but it’s also possible that he really doesn’t understand the issue at all.
I could add that G&T statement about light bulbs in a 100% CO2 atmosphere is totally undocumented: no references and no equations. Would you care to show us how to calculate how much hotter/brighter a 100 W light bulb will be in 100% CO2 vs ordinary air?
Frank
This would be a very easy experiment to set up.
All you need is a light meter, a suitable container, a thermometer and say a CO2 fire extinguisher,light bulb, voltmeter and ammeter
I’m sure I don’t have to spell it out for you.
More simple experiments of this kind are required before nonsense is fed into climate models.
I fear that ‘climate science’ is an enormous superstructure built on sand.
Bryan,
The main influence of more CO2 on the brightness would almost certainly come from more IR radiation from CO2 to the filament and the exterior glass of the bulb rather than from changes in conductive/convective heat losses.
Thus making the experiment and concluding what you claim is impossible. Only a detailed calculation based on models might perhaps tell more. Such calculations of heat transfer over relevant distances are very difficult.
Pekka
Eli Rabett reports that he has carried out the same experiment but with O2 as the surrounding gas.
The filament increased in temperature and melted .
So I think we can rule out radiation
Pekka
What G&T allude to is apparently something that is generally known so that they unfortunately they did not give a reference.
However fortunately the experiment linked below gives some evidence to support their statement
Click to access Wagoner%20AJP%202010.pdf
How did the Argon gas heat up?
Some people think you are a self opinionated fossil but I could not possibly comment
That experiment has no relevance to the behavior of the atmosphere (as noted also by the authors in the conclusions). It’s dominated by phenomena that are important for a small closed setup, but negligible in the free atmosphere. Absorption in the gas is less important than the absorption by the surfaces of the enclosure even for CO2.
What they observe correctly is that some proposed class room demonstrations of GHE have little to do with atmospheric GHE. (G&T have claimed that their paper was also written to show only that some simplistic arguments are erroneous, but their paper contains also numerous explicitly untrue claims. Thus this post hoc statement does not save them.)
The same basic physics can explain (in principle) all the various small scale experiments and the basic properties of the atmosphere, but different parts of that physics dominate in different cases. I wrote “in principle”, because many of the experiments are difficult to analyze. It’s often possible to use commercial CFD software to do the calculation, but even that may require so much work that it’s usually not done.
Flat plate solar collectors are perhaps the most interesting example where effects in some way similar to the GHE lead to spectacular results. The selective coatings used have analogous (but not identical) properties with the atmosphere. Multiple glazing windows have also similarities with the shell model used in this challenge, while their relationship with the atmosphere is more remote.
The difference is that the Wagoner paper is about a system coupled to the outside world by conduction… whereas the planet is surrounded by vacuum. If one could suspend the Wagoner bottle in a little vacuum and then repeat, the argon would presumably have little difference. This is because in a case where you have conduction or convection contact with the outside world, then, obviously the conductive properties of the gas matter. If the only contact you have with the outside world is radiative (as in the case of the planet we live on), then only the radiative properties of the gas matter. (as a first approximation, anyway).
-MMM
SoD
The steel shelled metal cored planet in reality would imply a shell with almost no heat capacity and a core with a massive heat capacity.
Also metals expand when heated.
The shell is also specified to be very close to the core.
What would then happen is the core would expand and touch the shell and remain clamped there for some time before cooling and unclamping.
But as specified with no expanding metal your analysis is fine
Bryan’s further contribution on this blog will be confined to this and the last thread.
He has had plenty of opportunity, but prefers not to engage. For whatever reason.
Perhaps G&T have taught him well.
Comments from Bryan in the future will be removed and parked in this thread. The reason – he has demonstrated that he is unable/unwilling to engage on the subject of actual physics and prefers to recite litanies that don’t relate to the question at hand.
In the off chance that he says something different and useful they might get to stay in an original thread.
This chance is extremely slim.
He can always look upon his immense contribution in this thread as the pinnacle of his achievement. And look back with pride on his many years of “scientific” endeavour on this blog.
which was really in time…
I suspected that this was the point of this thread. I almost posted a comment to Bryan earlier suggesting that he should take this challenge as seriously as a final exam.
Perhaps he simply can’t do the math.
DeWitt
It looks like Nasif S. Nahle was right all along.
Wood has been confirmed not just by experiment the paper does a calculation of the radiative effect expected.
A few tenths of a degree.
Try the maths for yourself and I will of course help out as usual if you get stuck
Bryan,
You really know how to pick your references. Nasif is never right.
Did you actually look at the picture of his experimental apparatus? I’ll help you out: http://i165.photobucket.com/albums/u43/gplracerx/NahlePicture06.jpg Can you guess which box had the polyethylene film cover? Here’s a clue: It’s the one that’s insulated with glass wool. He later shows that insulation of that thickness raises the temperature inside the box by 10°C. The measured temperature of the insulated box with a polyethylene film cover was the same as the non-insulated box with a glass cover. So, in fact, he fails to replicate Wood’s result. When this was pointed out to him, he continued to defend his conclusion, much like you.
NN has made howlers in his calculations before. A fundamental one is that he thinks that in this formula from Modest, the term p_aL is equal to p_a where p_a is the partial pressure of the gas and L is the path length. That’s why he maintains that the emissivity of CO2 in the atmosphere is insignificant. In his mind p_aL for the full vertical column of the atmosphere for 400 ppmv CO2 is 0.0004 atm m instead of 3.2 atm m. He once questioned the fact that the mass of air above a square meter at sea level was ~10,000kg.
If you could actually help with math, you would have posted the correct equations for the surface temperatures of the inner and outer spheres. If you think that NN has made a correct calculation, that proves your incompetence.
Up to a point, questions based on erroneous arguments are helpful for writing explanations – but only up to a point.
Reblogged this on Centinel2012 and commented:
Good summary of what is actually going on!
View angle is irrelevant in this case. The temperature of the outside shell is just calculated from total power divided by area (heat flux) and S-B, assuming zero sky temperature. The inner surface temperature is just the power divided by the inside area and S-B against a ‘sky temperature’ equal to the temperature calculated for the outer shell. Angles don’t ever have to be considered because they all cancel out.
Steve:
That’s true for convex shapes; but replacing the inner spherical shell by the “spikey ball” head of a medieval morning star will produce a lot of area that does not fit that formula.
editorial update to above:
I mean an “embellished” morning star, which would look like an iron sea urchin.
No Neal, that is only true if limited conductivity of the inner body makes significant temperature differences possible. At constant surface temperature, the angle of view factors always cancel out; think about what your hypothetical spikes can ‘see’ compared to a smooth surface…. They see lots of things, including other spikes. I am pretty sure the geometry of the inner surface is irrelevant.
Steve,
An irregularly shaped inner body leads to an nonuniform temperature distribution of the outer shell as well, if it’s conductivity is not infinite.
The case is simple for the spherical case only.
Steve:
Three cases, all assuming an outer spherical shell of radius Ro and internal heating element of power Po, but with different housings for the heating element. The housings have different shapes:
1) Sphere of radius R1:
This corresponds to SoD’s case with ra = R1, and rb = Ro.
My formulation:
Po = power radiated from outer sphere = (4πRo^2)σ (To)^4
Conservation of energy in steady state for inner sphere:
power radiated from inner sphere to outer sphere = Power(1 => 0)
power radiated from outer shell to inner sphere = Power(0 => 1)
Power(1 => 0) – Power(0 => 1) = Po
Power(1 => 0) = (4πR1^2)σ (T1)^4
because the inner sphere is convex, in the sense that every point of the surface has an unobstructed visibility to the outer sphere (i.e., over the solid angle 2π for emission)
Power(0 => 1) = (view_factor)*(4πRo^2)σ (To)^4
because only a fraction of what is emitted from the inner surface of the outer shell lands on the inner housing. This can be estimated as the ratio of the solid angle of the cross-section of the inner sphere to 2π: but this gives the ratio as (1 – cosθ), where sinθ = R1/Ro; but if the calculation is done in consideration of Lambert’s law, the ratio is (sinθ)^2 = (R1/Ro)^2. Thus:
Power(0 => 1) = (R1/Ro)^2 *(4πRo^2)σ (To)^4
= (4πR1^2)σ (To)^4
Hence:
(4πR1^2)σ (T1)^4 – (4πR1^2)σ (To)^4 = Po
(T1)^4 – (To)^4 = Po/(4πσR1^2)
(T1)^4 = Po/(4πσR1^2) + (To)^4
= Po/(4πσR1^2) + Po/(4πσRo^2)
= (Po/σ) * [1/(4πR1^2) + 1/(4πRo^2)] [eqn (1)]
For easier comparison with SoD’s formulation:
(T1)^4 = (Po/(4πσRo^2)) * [1 + (Ro/R1)^2)]
= Po *[1 + (Ro/R1)^2)]/(4πσRo^2), or
Ta^4 = Tb^4 *[1 + (rb/ra)^2)]
which is SoD’s last equation at 11 August, 5:12 am.
Now, you could argue that another way of calculating P(0 => 1) would be to consider the principle of detailed balance in the case that To = T1 (with Po = 0); or to consider the emission from the inside of sphere-o to the outside of sphere-1 as the time-reversal of emission from sphere-1 to sphere-o. I would go back to Planck’s formulation of thermal radiation, which gives the radiative power emitted from differential area da_o to da_1 as
dPower = (da_o)*(r.n_o)(r.n_1)*(da_1)/|r|^4
n_o: unit vector normal to da_o
n_1: unit vector normal to da_1
r: vector displacement from da_o to da_1
If you do the integral over da_o first, it turns into the solid-angle calculation (which is easy because the view is “open sky” from da_1’s perspective); the integration over da_1 gives (4πR1^2).
Whereas if you do the integral over da_1 first, you get the view_factor (R1/Ro)^2, and then the factor (4πRo^2).
So in this case, you can side-step the view_factor calculation and still get the answer. Is that always the case? Let’s consider:
2) Cube of side S:
Power(1 => 0) = 6 *(S^2)*σ*T1^4
Power(0 => 1):
– Using the “open sky” argument, this should be 6 *(S^2)*σ*To^4
– Using the view_factor approach, this gets hard, because the cross-section of each face of the cube looks different from different positions on the inside of the sphere-o shell.
So let’s just the answer the easy way, and then:
Power(1 => 1) – Power(0 => 1) = Po
6 *(S^2)*σ*T1^4 – 6 *(S^2)*σ*To^4 = Po
6 *(S^2)*σ*T1^4 = Po + 6 *(S^2)*σ*To^4
Since
Po = (4πRo^2)σ*To^4
6 *(S^2)*σ*T1^4 = Po + (6*(S^2)/(4πRo^2))*Po
T1^4 = (Po/σ)*[1/(6*(S^2)) + 1/(4πRo^2)]
This bears a family resemblance to eqn(1), and they can be generalized as:
T1^4 = (Po/σ)*[1/Ao + 1/A1] eqn(2)
where
Ao = 4πRo^2
A1 = 6*(S^2)
So it would seem that we can always side-step the view_factor calculation by using the “open sky” approach. But is it true?
3) The Iron Sea Urchin:
Let the inner housing be a sphere with a multitude of spines, everything being made of a perfectly heat-conducting black substance. The basic sphere itself has radius R1, but each spine has length R1, and is conic in shape, with cross-section at base of radius rc. Each spine thus covers area π(rc)^2 of the basic sphere, but adds area π(rc)*sqrt(R1^2 + (rc)^2); so each spine added increases the area of the housing by the amount:
π(rc)*[sqrt(R1^2 + (rc)^2) – rc] = π(rc)^2 * [sqrt((R1/rc)^2 + 1) – 1]
So if N spines are added, the new area will be:
4πR1^2 + N*π(rc)^2 * [sqrt((R1/rc)^2 + 1) – 1]
If we make rc/R1 very small, we should be able to approach the closest-packing ratio for a plane, which would give a coverage ratio of
π/(2*sqrt(3)) ~ 0.9069
Thus
N*π(rc)^2 = π/(2*sqrt(3)) * 4πR1^2
= (2π^2/sqrt(3))*R1^2
and so the total area is:
4πR1^2 + (2π^2/sqrt(3))*R1^2 * [sqrt((R1/rc)^2 + 1) – 1]
~ 4πR1^2 + (2π^2/sqrt(3))*R1^3/rc
= 4πR1^2 *[1 + (πR1/rc)/(2sqrt(3)) ]
~ 4πR1^2 * (π/2sqrt(3))(R1/rc)
Defining
M = (π/2sqrt(3))(R1/rc)
we see that the total area is
A1 = M*4πR1^2
and M can be made arbitrarily large by reducing rc/R1
So if we apply the approach that worked for us before, eqn(2) gives:
T1^4 = (Po/σ)*[1/Ao + 1/(M*4πR1^2)]
= To^4 *(1 + (Ro/(M*R1))^2)
Now this is a remarkable result: By choosing M arbitrarily large (adding lots of radiating spines to the inner iron sea urchin), we seem to be able to reduce the inner temperature to being arbitrarily close to To.
The basic argument is that by increasing the area of the inner housing of the heating element, we’re able to arrange for the power radiated from it to the outer sphere to be sufficient to provide the required Po – and this allows us to house the heating element at an arbitrarily tiny temperature margin over To.
This is obviously implausible. So what’s the problem?
The “clear sky” no longer applies, so it’s hard to calculate Power(1 => 0) by doing the integral over da_o first. Instead, do the integral over da_1 first: then the approximation that suggests itself is that the view_factor of sphere–1 as seen by an inside point of shell-o is 2R1/Ro, because the sea urchin will be radiating pretty much as a sphere of radius 2R1. (It will be slightly less, because at the tips you’ll be able to see between the tips, transversely.)
So the equation will still hold, with A1 ~ 4*4πR1^2 :
T1^4 ~ To^4 *(1 + (Ro/(2R1))^2)
should be a slight over-estimate.
Neal,
I figured you didn’t want everything in bold so just added an unbold after each subheading..
SoD,
Thanks. I really miss the ability to revise & correct.
Steve,
Here’s how Ta(case 2) varies as ra/rb.
I’ve expressed it as the ratio of with shell/without shell:
The formula for case 2, taking into account view factor:
Ta4=P. [1+(ra/rb)2]/(4πra2σ)
So as a ratio vs the no shell condition:
Ta(case 2)/Ta(case 1)=[1+(ra/rb)2]1/4
Your conceptual description is correct.
And the two extremes..
With the shell diameter almost equal to the sphere the ratio is 21/4, i.e. double the power expressed as a temperature..
With the shell diameter out to infinity the temperature of the shell is down to zero and so it’s the same as case 1.
SoD:
I think you have an error in the last equation of your comment posted August 12, 4:37: It should be:
Ta(case 2)/Ta(case 1) = (1 + (rb/ra)^2)^1/4
The graph accordingly is wrong, too: It indicates that as ra (the inner sphere’s radius) goes to zero, it’s temperature goes to a constant. But constant temperature and zero area gives zero power, not P.
Right now, your equation here contradicts your final equation of August 11, 3:47; which I believe to be correct.
Neal,
I’m always amazed how easily I can mess up simple algebra. But here’s my working..
In case 1, Ta4 = P/(4πra2σ) …..[1]
In case 2, Tb4 = P/(4πrb2σ) ….[2]
energy balance for sphere A, gives T’a:
P + (view factor) x total emitted radiation from inner surface B = energy emitted from A
so, P + (ra/rb)2.4πrb2σTb4 = 4πra2σTa4 ….[3]
substitute the value for Tb4 (eqn 2) into eqn 3:
P [1 + (ra/rb)2] = 4πra2σT’a4 …..[eqn 4]
so T’a4 = P [1 + (ra/rb)2] / (4πra2σ) …..[4a]
Now we want to look at the before and after ratio for Ta, so:
T’a4/Ta4 = [1 + (ra/rb)2]
And if we look at the ratio of T’a to Tb:
T’a4/Tb4 = (rb/ra)2 [1 + (ra/rb)2] = [ (rb/ra)2+1]
– that’s all totally unreadable with html tags so let’s see how it looks when it turns into a comment, and sorry to everyone else that there is no preview function. Hosted WordPress doesn’t have that feature.
That seems to be what I wrote down on my piece of paper.
So the case in the latest graph is comparing before and after for A, not A to B.
So as ra → 0 the ratio of T’a/Tb → ∞ – which is correct because zero area for fixed power implies infinite power per unit area and infinite temperature.
And as a result, the ratio of T’a/Ta → 1 – i.e., it doesn’t make any difference about the shell being added.
My first reaction looking at the curve was the same as Neals’s, but then I realized that the natural way to look at the result is to keep ra constant and to increase rb. I didn’t check the details of the algebra, but at least the limits are correct for that case.
SoD:
– Your reasoning is right, and your most recent equation
(T’a/Tb)^4 = (rb/ra)2 [1 + (ra/rb)2] = [ (rb/ra)2+1]
is equivalent to what you wrote down at the end of August 11, 3:47.
– But what you wrote down below the graph is:
Ta(case 2)/Ta(case 1)=[1+(ra/rb)2]1/4 , or
(Ta(case 2)/Ta(case 1))^4 = [1 + (ra/rb)^2]
These are not the same, and you graphed the second one.
Pekka:
As is easily shown (see above), another way of writing the correct answer is:
(T(inner))^4 = T1^4 = (Po/(4πσ))*[1/(R(outer))^2 + 1/(R(inner))^2)]
So for Po fixed and R(inner) => 0, T(inner) => infinity, regardless of R(outer). This makes sense (because you’re getting finite energy out of zero area), but is not reflected in the graph. It should be sloping down from infinity at x = 0.
“So the case in the latest graph is comparing before and after for A, not A to B.”
But B is just an implementation of A-before: It has the same radius and it is emitting (to space) the same power. So comparing A-after to A-before is the same as comparing A to B.
Neal,
I discussed the case of changing ra in this comment. Now I realized that keeping ra fixed and varying rb leads to the simpler analysis. The alternative is keeping rb fixed and changing both ra and P in the way that P/ra^2 is constant, i.e. P is proportional to the surface area of the inner sphere.
Neal,
Can you point out where in the equations I go wrong. Your argument is from intuition:
– and I think your intuition is wrong. But that’s my intuition – which might be wrong. So let’s use the first law and see where the problem lies.
I’m quite prepared to accept I’ve messed up some maths, I usually do, especially when mixed with alcohol.
My intuition, probably informed by my inconsistent equation manipulation, says that the ratio of Ta(case 2) to Ta(case 1) is bound to be 1 at ra/rb=0.
Because in case 1 there is no rb, so it all seems obvious – we are comparing the case of
EITHER:
– some “normal” radius of A with a massive outer shell (so massive it is the size of the universe) with the same “normal” radius of A with deep space
– and this = 1
OR
– some infinitesimally tiny radius of A with a normal outer shell with the some equally infinitesimally tiny radius of A with deep space
– and this = 1
– because temperature is tending to infinity in both cases due to the infinitesimally tiny area
Probably best to resolve with the maths. It doesn’t get confused.
SoD,
The equations you present under the curve are correct assuming that P/ra^2 is constant, otherwise the second equation is not correct. You don’t specify that this condition must be true.
SoD:
I think part of the problem has been a bit of confusion in the equations because of neglecting the view_factor issue in the beginning; and I also think that focusing on the temperature ratios instead of the temperatures themselves has not been helpful. All this has, I think, confused the terminology.
It’s going to be tough to argue intuition, because we’ll have to pin down exactly what corresponds to what. Also, sometimes intuition is malleable!
Let me try something easier first: If you look at my comment of August 13, 2014 at 9:32 am:
– Notice that I point out that I agree with your results almost until the very end; but
– In your last equation, you made a false move and are inconsistent with yourself. Basically, you have written “(ra/rb)^2” where, algebraicly, you should have written “(rb/ra)^2”.
Can you identify in your own write-up the last equation I agree with, and the equations that I don’t?
If not, we can go back and redefine a common terminology.
Pekka:
I think it will be easier to focus on the actual values of Ta and Tb rather than on ratios. We can always calculate ratios once we have the values; but when you try to do the reverse, there is the possibility of not being quite clear with respect to what the ratio is being taken.
For the absolute value of Ta the formula I like best is
Ta^4 = P/(4πσ) . (ra^-2 + rb^-2)
That can be generalized nicely to more shells by adding similar terms to ra^-2 + rb^-2.
I don’t remember, whether Latex works here. Thus I try to present the formula for Ta using Latex
Seems to work, but the formula contained a typing error. Here the error is corrected:
Pekka:
Yes, that’s a very clear and “unbiased” form of the solution. If SoD is willing to start from there, it should only be a matter of getting the terminology straight.
But I would prefer it with “T^4” than with “( )^.25”.
It’s easier to look at.
I agree with Pekka’s formula – from my comment of August 13, 2014 at 3:19 am that is equation 4a, with 1/Ra2 moved inside the brackets.
[Likewise the equation in the comment of August 12, 2014 at 4:37 am under the graph].
How do I do a Latex equation? They are so much easier to read..
The code for the equation is
$ latex T_a = (\frac{P}{4 \pi \sigma} (\frac{1}{r_a^2} + \frac{1}{r_b^2}))^{1/4} &s=2$
In the above I added a blank between the first $ and the word latex. That should be enough to leave tho code visible as code. The combination of these parts and the final $ bracket the code, &s=2 specifies size 2, the rest is the equation.
One place to get further advice on using LaTeX on WordPress is
http://en.support.wordpress.com/latex/
Making errors is so easy that it’s better to test the equation before posting. I do that on my own site using the preview tool that’s available, because the site is not hosted by WordPress but by myself at my internet service provider. Anyone of you can use my site for testing as it’s not necessary to proceed to posting, the preview tells about the success. That requires, however, that you register, which requires giving a email address to receive the password.
Thanks Pekka.
There are also WYSIWYG (or WYSIWYM) editors for LaTeX, which may help composing valid expressions. According to the Wiki page http://en.wikipedia.org/wiki/Comparison_of_TeX_editors only few are free. One such is LyX (http://www.lyx.org/), which is a relatively heavy package, but seems to be easy to use. The full formula can be built there. The code requires then only the addition of the word latex after the initial $, and perhaps the size code like &s=2 at the end.
The browser based service at https://www.writelatex.com/ might also be a good choice, but I haven’t tried it.
OK, we can start with the “unbiased” equation. Let’s set up the terminology.
I would like to start with Case 2:
– Outer sphere of radius R_o, radiating into 0-degree cosmic background
– Perfectly black, perfectly conductive
– Steady-state temperature: T_o
–Inner sphere of radius R_i, within the o-sphere; powered internally by P.
– Perfectly black, perfectly conductive
– Steady-state temperature: T_i
– R_i < R_o
As we have agreed:
(T_i)^4 = (P/(4πσ)) * (1/(R_i)^2 + 1/(R_o)^2)
and as we have seen by now many times before, from conservation of energy:
(T_o)^4 = (P/(4πσ)) * (1/(R_o)^2)
= P/(4πσ(R_o)^2)
Case 1:
– Unique sphere of radius R_u, radiating into 0-degree cosmic background
– Perfectly black, perfectly conductive
– Powered internally by P
– Steady-state temperature: T_u
From conservation of energy, we get the same equation as for o-sphere in Case 2:
(T_u)^4 = P/(4πσ(R_u)^2)
Observations:
a) Within Case 2:
(T_i)^4/(T_o)^4 = (1/(R_i)^2 + 1/(R_o)^2)/(1/(R_o)^2)
= ((R_o)^2)/(R_i)^2) + (R_o)^2)/(R_o)^2))
= ((R_o)^2)/(R_i)^2) + 1)
= ((R_o/R_i)^2 + 1)
or:
(T_i/T_o)^4 = ((R_o/R_i)^2 + 1)
b) Comparing o-sphere (Case 2) with u-sphere (Case 1):
– Both are radiating P to the 0-degree background, so:
(4πσ)(R_o)^2 * (T_o)^4 = P = (4πσ)(R_u)^2* (T_u)^4
(T_o/T_u)^4 = (R_u/R_o)^2
(T_o/T_u) = sqrt((R_u/R_o))
c) Comparing i-sphere (Case 2) with u-sphere (Case 1):
– Imagine starting with Case 1 and then covering it with the o-sphere: What happens?
– R_u = R_i < R_o
(T_i)^4 = (P/(4πσ)) * (1/(R_i)^2 + 1/(R_o)^2)
(T_u)^4 = (P/(4πσ)) * (1/(R_u)^2)
= (P/(4πσ)) * (1/(R_i)^2)
(T_i/T_u)^4 = (1 + (R_i/R_o)^2)
So now let’s compare the comparisons:
(T_i/T_o)^4 = ((R_o/R_i)^2 + 1) eqn (A)
– as (R_i/R_o) => 0, (T_i/T_o) => ∞
– as (R_i/R_o) => 1, (T_i/T_o) => 4thrt(2)
(T_i/T_u)^4 = (1 + (R_i/R_o)^2) eqn (B)
– as (R_i/R_o) => 0, (T_i/T_o) => 1
– as (R_i/R_o) => 1, (T_i/T_o) => 4thrt(2)
Why are these results so different for the case (R_i/R_o) => 0 ?
– For the case of eqn (A), we are looking into a two-sphere arrangement and asking what happens if the inner sphere is shrunk (R_i => 0), but the outer sphere is to be maintained in all ways. The answer is that the inner sphere must take on a higher temperature to maintain the power output. The outer shell, which is unchanged, is taken as the reference.
– For the case of eqn (B), we are looking at a one-sphere arrangement and then asking what happens if we add an enclosing shell, whilst maintaining the power input.
(R_i/R_o) => 0 means that the outer shell radius R_o => ∞, and hence becomes less and less important. The original temperature of the inner shell, which is itself unchanged, is taken as the reference.
– Both cases are valid, but by the choice of reference temperature, we are imposing a different meaning on the procedure that takes (R_i/R_o) => 0.
Neal,
I agree.
Not easy writing it into the comment non-WYSIWYG format!
SOD: Now that you have dealt with Bryan, I’d like to point out that this heat transfer problem has little or nothing to do with the atmospheric greenhouse effect – despite its being cited by Halperin et al in their published reply to G&T. Yes, the model does show that internally-heated objects get warmer when some type of “insulation” reduces the rate at which they lose heat – even though the insulation is colder than the object itself.
The difficulty arises when Halperin et al try a little “bait and switch”, replacing a thin, highly-conducting, opaque shells with infrared-opaque atmospheric layers that “omit blackbody radiation at their temperature”. The temperature of a shell is determined by radiative equilibrium, but the temperature of a layer of atmosphere is determined (at least partially) by the lapse rate and convection. Gases don’t emit blackbody radiation, especially a layer of gas thin enough to have approximately the same temperature on both the top and bottom of the layer. Heat is conducted through a shell, but convected or radiated through a layer of atmosphere. (Radiating heat through a infrared opaque layer seems inherently self-contradictory.)
In fact, even your highly conducting, very thin shell has a problem – it can’t conduct heat unless there is a temperature difference between its two surfaces. Your 1000 W of power can’t escape without a temperature gradient or “infinite conductivity” or “infinite thinness”. Radiation will pass directly through an “infinitely thin” shell. Your series on Heat Transfer Basics properly discussed heat transfer through real objects, particularly this post: https://scienceofdoom.com/2010/09/12/heat-transfer-basics-part-zero/ Overly-simplified models can lead to sloppy thinking by your readers, but I can never “catch” you overlooking these problems. Under “Notes” above, you discuss the reason why you made your shell thin and highly conducting. The problem is easier to solve if the temperature difference between the inside and outside to be NEGLIGIBLE and you can solve for one shell temperature, not two.
Rejection of G&T and Kramm must rely on more realistic models than shells or atmospheric layers that behave like shells. And, to avoid nitpicking about the 2LoT, it should start with the statement that earth is “warmed” or “heated” only by the sun (and radioactive decay), even though the long-term surface energy balance depends on radiation from the atmosphere.
Frank,
No, it’s not. See for example Rosseland Radiation Model. Rosseland diffusion is useful for modeling solar atmospheres or fossil fuel fired furnaces.
You don’t need a temperature difference within a layer, you need a temperature difference between layers. There is very little difference, except in calculation time, between a model with many optically thin layers and a model with fewer, optically thick layers.
DeWitt: Please note I said “seems inherently self-contradictory”. I realize stellar atmospheres are very opaque to the radiation they emit, but heat still escapes to space. However, the “diffusion time” for energy to escape the sun’s core is something like 100,000 years.
If the mean-free path of a photon on Mars (ca 100 atm CO2 vs 0.0004 atm on Earth) may be only a few cm, then you need layers this thin. The temperature difference between such layers is almost negligible, so the upwards and downwards fluxes will be nearly equal. So it is really hard to radiate HEAT through an opaque atmosphere. On Mars, I do realize that the difference between the upward and downward LWR fluxes at any altitude must be equal to SWR – convection, but this could be very near zero.
In all cases, heat transfer still depends on the existence of a temperature gradient, something that is postulated not to exist in shell models or optically thick layers of atmosphere.
Frank,
Do you really mean Mars, not Venus.
Mars atmosphere is very thin (0.6% of Earth atmosphere), 96% CO2 meaning that the amount of CO2 is more than 10 times that on Earth. Due to the low pressure the lines are narrow and the Mars atmosphere is highly transparent for most wavelengths, but absorbs very strongly at the center of the main CO2 lines.
Frank,
I’ve done the radiative transfer calculations for Venus near the surface using Spectralcalc. You don’t need layers the thickness of the photon mean free path. The calculation works just fine with much thicker layers, just as it does in the Earth’s atmosphere. While the temperature is quite high, the temperature gradient is about the same. If you were correct, one would need to use layers no more than about 2m thick for the Earth’s atmosphere instead of the 1000m layer thickness that MODTRAN uses for the lower atmosphere.
While radiative transfer becomes slower as the opacity increases, it’s still much faster than conduction.
Pekka: Yes, I meant Venus. At one point I knew something about the mean free path of photons on Venus. The lines are very broad.
To put it another way, opaque in a gas does not mean a transmissivity of identically zero as it can in a solid where the surface only absorbs or reflects. For a gas, the transmissivity is small, but not nonexistent and the reflectivity is zero.
The question is how many layers do you need. Two explains the principle, as SoD has done here, line by line calculations have many. GCMs integrate convection as well as radiation over multiple layers.
Rejection of G&T, Kramm and Bryan only requires showing that their models are nonsense, such as the stuff about atmospheric conduction and Feynman diagrams being necessary to discuss heat transfer.
That seems like a standard issue of numerical analysis; it will depend on the accuracy required.
Pretty much Neal, except that there is a fork on accuracy needed, one direction is for understanding, the other for modeling. In a lot of cases you don’t want high accuracy to extract the maximum understanding but the best model may require high accuracy. OTOH, one of the skills of model making is to only keep what is necessary.
Eli:
So how much accuracy do you think is needed?
SOD,
Good luck with this path. In my blog travels, I discovered that there are many who don’t want to believe anything which leads to admission of a C02 greenhouse effect. Layer after layer of sophistry between you and physics reason. I haven’t read the thread but checked out the post.
Jeff,
It seemed to work at The Blackboard for a poster notorious for his many sock puppets and whose last name is the same as a common natural fabric fiber.
Jeff,
Around the mid 90’s I dug a copy of Leon Festinger’s When Prophecy Fails out of the city library archives. It was very illuminating and I learnt a lot. Probably one of the most illuminating books I’ve read, although it was my first on cognitive dissonance. More recently, I read Mistake’s Were Made But Not By Me among a few others. Very well written and probably much more useful than Festinger’s book as an introduction..
Lots of people are trying to win an argument. But lots are trying to understand climate basics and have conceptual problems understanding.
If breaking apart the basics into detail has helped some people that’s a good thing.
If not, at least it keeps me off the streets.
I checked customer reviews of the book of Tavis and Aronson at Amazon. There were really many positive (148 ***** and 55 ****) and very few negative reviews. Thus I read the first lengthy review that gave ***** and all the eight that gave * or **. In this case the reasons given for not liking the book made me think that I wouldn’t like it either. Most common reason was that it would have been a good article, but making it a book added nothing except pages. The other complaint was that the book was critical of one side of the political spectrum only.
Pekka,
The three star reviews raised the same points as the one and two star reviews, they just liked the book a little better. It doesn’t sound like a book I want to read. I’m already aware, for example, of the disaster known as Recovered Memory Syndrome.
I also just solved a very similar thought experiment (halfway through this comment). I solved Dr. Spencer’s thought experiment for blackbodies, then generalized to graybodies, then accounted for the larger enclosing shell area. I also accounted for the finite conductivity of an aluminum shell rather than a thermal superconductor shell, but haven’t yet posted those calculations because the discussion became unpleasant and unproductive. As always.
After deciding a simpler thought experiment might help, I asked if a plate electrically heated to an equilibrium temperature of 150F would warm if the surrounding vacuum chamber walls were warmed from 0F to 149F, while keeping electrical heating power constant. This simple question just involves noticing that the left hand side of an equation increases, and asking if we can agree that the right hand side also increases. I’m still waiting for an answer. As always.
I’d also like to apologize on behalf of Bryans everywhere. We’re not all like the Bryan who posts here. Some Bryans understand basic physics and can answer direct questions without absurd evasions. I’m glad to hear that this Bryan won’t be spamming future SoD threads. Enough is enough.
This is really a nice thought experiment.
Much of the muddle is rooted in classical thermodynamics which, of course, has some difficulties with thermal energy exchange between two bodies. In the classical view, the heat defined as the net flows one way, well enough, but that does not mean that there are no thermal energy flows in both directions.
This must have been a huge discussion post Gibbs and Boltzmann. It would not surprise Eli to hear that Planck had a nice resolution thereof.
Eli:
I don’t know what Planck thought about the back & forth of conducted heat, but he was very clear about the interchange of heat radiation: a differential area radiates to another differential area, and the fraction that is absorbed depends on the absorption coefficient (no mention of the relative temperatures of the two differential areas). Thus, radiative heat transfer incorporates 2-way interchange of thermal radiation.
To quote myself (somewhere above):
I would go back to Planck’s formulation of thermal radiation, which gives the radiative power emitted from differential area da_o to da_1 as
dPower = (da_o)*(r.n_o)(r.n_1)*(da_1)/|r|^4
n_o: unit vector normal to da_o
n_1: unit vector normal to da_1
r: vector displacement from da_o to da_1
Note:
– I omitted to explain that (r.n_o) means the inner product (dot product) of the vectors r and n_o.
– Looks like I left out the factor of the specific intensity of the source. Oops! that’s the factor that makes one emission greater than the other: If integrated over frequency, that would be the T^4 factor.
– This is taken from Planck’s The Theory of Heat Radiation, 2nd edition; eqn (17).
Neil, exactly what Eli was talking about. When you get into these things with the Bryans, G&Ts and Kramms of the world, it is best to talk about the interchange of thermal radiation rather than the interchange of heat or heat radiation because that stops them from telling you that heat only flows from hotter to colder, second law, etc.
Still, there must have been some serious discussion in the late 19th century about this new idea rooted in the atomic theory and stat mech.
Eli,
Wishful thinking on your part. They’ll manage to bring it up themselves. Bryan, for example, appears to think that thermal radiation ≡ heat flow.
I tried to point out to Bryan that the classical thermodynamic concept of heat as being the amount of energy exchanged between a warmer and colder body to bring them to the same temperature is not actually necessary. You only need energy and entropy. Also, the term ‘heat’ has multiple meanings depending on context. He didn’t take it well.
Have you looked at John Denker’s Modern Thermodynamics? Bryan, of course, thinks he’s a cr@ckp0t. Which is rich considering who he thinks are reliable sources, e.g. G&T.
DeWitt,
I feel it really painful to read someone telling, how others have done all wrong, and how he is the one, who gets it right.
I feel that way even if his presentation is correct, as it’s virtually certain that his presentation is not really superior to all others.
Pekka,
That’s not how I read it, although his style can be annoyingly arrogant and I can see how it could be taken that way. I do think he raises many valid points.
DeWitt,
It’s possible that he raises valid points, but any superiority is relative to some specific alternatives and according to some specific rules.
The issue is not really, what’s correct physics, but how to explain or teach correct physics, and for this there aren’t any well defined rules. What works in one case doesn’t work in another. What someone finds clarifying only confuses another.
He spoils much by the arrogant style.
Eli is basically with Prekka here. Denker wastes too much time and space preening. The best intro to classical thermo may (IEHO of course) be Fermi. Elegant and concise. Maybe the Feynman Lectures (again IEHO) are the only things that can compete with it.
Feynman doesn’t have a whole lot on thermodynamics; he was more interested in statistical mechanics. (He once said that thermodynamics is a topic in which you can only remember the equations if you are taking the course, or giving the course.)
Fermi is excellent, but brief.
I remember Pippard as being an excellent exposition on classical thermodynamics.
The thing I remember Feynman saying about thermo (I was one of the test subjects, i.e. a Freshman and Sophomore at Caltech, for the Feynman Lectures ) was that physicists liked the equations holding volume constant because they were more elegant. Chemists, OTOH, had to live in the real world where constant pressure, outside an autoclave anyway, was the reality.
DeWitt:
That’s interesting. That remark is still in the book, more or less.
There seems to be some question as to how well the lectures came across to the intended audience. An acquaintance of mine, John Clauser, claimed that he didn’t think anybody got the lectures, except for the professors sneaking into the room, and nobody was able to do the homework. I think he was in the class before. Of course, he’s not a fan of Feynman anyway.
What was your impression? What % of the students appreciated the lectures, versus those who might have felt they’d gotten on the wrong roller coaster?
Neal,
Think the old saw about Chinese food. When you were listening to him lecture, at least the first year and part of second, it seemed perfectly clear. An hour later, not so much. He went very fast and generally had all the blackboards in the lecture hall covered with equations. A significant fraction of the time, he’d get to the end and realize that there had been a sign error or a factor of pi left out or something. But it would be corrected in the lecture notes that were handed out later. The lecture notes were the basis of the books.
By the end of the second year, though, only a few undergraduates were still attending lectures. It was all professors and graduate students. Everybody else just read the notes. A while back, I tried reading through the books again. I wasn’t able to get through Volume III. I didn’t have the math skills any more, if I ever had.
Neal,
It’s not like you had a choice. The class was required for all students. But I did figure out very quickly that Physics was not for me. Only about 2/3 of the entering students graduated. Much of that was people deciding that science was not for them. They even started offering a degree in economics to try to retain more students.
My impression was that Feynman was very popular among the undergraduates. On the rare occasions when he dined in one of the student houses, the place was packed because after dinner he would tell the most wonderful stories. I think most of them were even true, although embellished over the years.
I know someone who worked on uranium isotope separation at Oak Ridge for the Manhattan Project, and he credits Feynman with saving lives by calculating the amount of 235U that could safely be stored in one location. That included how much could be held in how much volume of solution before there was a danger of a chain reaction. A variation of that involving the gaseous diffusion plant at Oak Ridge was one of the good stories Feynman told.
DeWitt:
Yes, the lectures are very inspiring, but when you try to do problems, you find that a lot of the time, Feynman has side-stepped an issue by considering a special case. So he gets across the insight he wants to share, but you still don’t have the “working tools” you need to do the problem generally. The professors love it, of course: They know the standard way to do the problem, so they’re just looking for the insights.
In QM problems, he also plays tricks that just barely work. I studied his papers on superfluid Helium, and several times, when he was playing around with the “wavefunction of all the Helium atoms in the liquid”, he would advance the argument by reference to a mental image of this wavefunction. My first reaction was, “You can’t visualize a wavefunction in 3n-dimensional configuration space like that, it’s not legitimate.” But if you thought very carefully, you could conclude that if you did it in just this special case, and only to the extent that he did it, that it was actually – but barely – OK. And he got the equations he wanted for superfluid Helium and they fit, no free parameters. Un-effing-believable.
But there were definitely times when I got thrown off the horse. I sat in on the QED class he taught, and I remember him wanting to prove the solution to:
(m^2 + Laplacian) f(r) = 4pi*delta(r)
So he wrote down something simple about the Fourier Transform of (1/r) (which is the solution when m = 0), and then he looked at it from one angle and the looked at it from another angle, and concluded the answer was (exp(-mr))/r. At the time, about half the students were taking notes, and the half were not: You could tell, because when he did this trick, it was over and done in less than 10 seconds; and the people that had been following with eyes & ears went “Woah!”; and the folks taking notes went “Whaaat??”
Unfortunately, I had been in the “Whaat?” group. So I took the problem to Pf. Matthews, and he eventually figured out what Feynman had been doing, and explained it to me straightforwardly. The explanation took 15 or 20 minutes.
Well yes, to the comment about holding volume or pressure constant, that is exactly how chemists teach thermo (except those who synthesize in pressure cookers).
DeWitt’s comment about the math in Volume III mystifies Eli tho. Compared to the plug and chug traditional way of teaching quantum, Volume III is very light on math, ok, you do need some linear algebra, which may have been lacking for sophomores, but Volume III was a sea change in teaching the subject.
I think the problem arises when you face a problem defined in the standard way from the orientation that Feynman uses. Eventually, you can get the hang of it; but perhaps not immediately.
I think that a genius generally makes a bad undergraduate lecturer.
A great undergraduate lecturer is usually someone who has struggled with the subject themselves in their time, has been confused over what seems obvious now, and spends their time trying to make a difficult subject easy – because they remember what it was like.
A genius on the other hand, found the undergraduate material instantly trivial, can barely comprehend that an 8 year old could struggle with the undergrad material and shakes their head in amazement when someone says “I didn’t understand a word of that lecture”.
A Martian walking into the lecture hall would be a little easier to get their head around.
Their aim is to inspire the audience into post-doctoral research which will solve the great, as yet unsolved, problems of the field of interest.
“How many students took 15-20 hours to do the homework I set last week?”
Looks around. No hands. Nods, as this was the expected result. Writes “0” next to 15-20 hours on the chalkboard.
“How many took 10-15 hours?”
No hands. Encouraged, nods some more. Writes “0” next to 10-15 hours.
“How many took 5-10 hours?”
Someone calls out from the back – “who’s done it?” and there is a huge wave of laughter.
Disconcerted, the lecturer asks, “Well, who has done the homework?”
One hand, out of 50+ students goes up. Of course, that guy ended up top of the class and went on to do a PhD.
Correction: In my post at August 19, 2014 at 2:44 am above, I wrongly remembered the problem Feynman solved: I think now it was something like getting the answer expressed as a an FT. Anyway, it all happened very fast.
SoD:
Actually, Feynman was an incredible lecturer. But what he communicated was insight, and a kind of bravado; not problem-solving ability – that came too easy to him. I think there was a case where a freshman asked about rotational invariance, and Feynman launched into the idea that every continuous symmetry – one that can be implemented a little bit at a time – implies that there is some conserved quantity. Essentially Noether’s theorem. It was great; but the kid would never have been able to do a conservation-of-angular-momentum problem based on that.
Neal,
Yep. To learn how to actually solve problems, you needed to spend a lot of time with the TA. Unfortunately, I didn’t. Something I now regret, but which didn’t bother me at the time.
DeWitt:
I got started in physics under a radically different approach:
– The professor broke the material down into self-study units, with specific readings, problems, and mastery tests.
– The students progressed through them at will; the TAs were available according to a schedule, to answer questions and help with the problems and quizzes.
– Lab sections were handled traditionally.
– There was a normal midterm and final, but no lectures.
I’m not sure how well it worked for everyone else, but I had a blast. By the end of the first year, I knew half of the first-year graduate students through osmosis, and terrorized them with conceptual questions that I (and usually they) didn’t understand as well as might have been hoped.
Steve Chu was one of my TAs.
Eli,
Try totally absent. In fact, it’s still nearly totally absent for me. The inspiration for learning what little I know now has been learning about inertial navigation for vehicle dynamics data logging. One approach uses cosine direction matrices to transform coordinates from the Earth frame to the vehicle frame and back. You have accelerometer and rotation rate data in the vehicle frame and GPS data in the Earth frame.
Because the inexpensive MEMS devices in smart phones are noisy, integration to get velocity, position and rotation leads to random walks. You need to compare to GPS data to correct for drift. That requires reference frame transformation. You could do it with quaternions too, but CDM’s, for me, are easier to deal with. Plus there’s code already written for DIY drones that uses CDM’s.
The math for QM is not much different than that for coordinate transforms, and probably considerably less involved than for quaternions (from what little I know of quaternions).
The mathematics needed for understanding the ideas of QM is not technically demanding, but the whole approach is so different from what most are used to that many students have great problems in making the necessary steps in changing their thinking about physics.
Practical calculations based on QM are often tedious and demanding, but those are not needed for the understanding of the ideas.
Every teacher seems to have her or his own ideas about the right way of helping the students to understand. For me the lectures were of little help and the main source of learning was one of the classics: Dirac’s Quantum Mechanics. When I lectured the course a few years later, I used to a significant extent my own lecture notes influenced by the approach of Dirac, but a standard textbook of the time by Schiff (https://archive.org/details/QuantumMechanics_500) formed the background.
Prekka, Eli read Dirac after Feynmann III and Mertzbacher (king of plug and chug) and Goldstein (CM). At that point it cemented the concepts. Today Dirac probably would not help much because CM has been so de-emphasized. The insight in Dirac is practically all by analogy with CM, which is a sign of its age.
Eli,
That was not, what I learned from it. To me it’s main message was in some sense the opposite, i.e. switching totally from the classical concept of state to the quantum mechanical concept.
I wrote above that, what works for one, is often quite different from what works for another. That’s particularly true, when a break must be made in the thinking and a fresh start taken.
I continued reading many other texts including von Neumann, whose approach was again very different from most presentations of QM.
One of the more generic messages of all that was that equivalent theories of physics can often be formulated in very different ways.
It is remarkable that the three main approaches could be developed and accepted as equivalent in essentially one year. Although in use they are not equivalent: It seems that the wave-function approach is most practical for detailed calculations.
Mehra’s 9-volume history of QM claims that only one person, aside from Dirac, has used his formalism to solve a research problem: Van Vleck.
What is still of interest to me: Given that the mode of thinking for QM is so different than for CM, how did they come to agree so quickly to the formalism? The interpretation took much longer (and arguably is still not 100% agreed); but nobody argues about how to calculate QM: You do it the right way or you don’t pass the class.
Eli,
Perhaps we were thinking on different books. I meant the monograph The Principles of Quantum Mechanics, while your comment might apply better to Lectures on Quantum Mechanics.
Eli:
What I am able to find, in my library and on the internet, is not deep enough in the history of thermal physics that I’m able to find a source that I trust.
I had a look a while back, and found a textbook on the history of heat which was quite interesting. It didn’t have any focus on how the great minds of 1870-1910 resolved the “problem” of 2-way thermal radiation in the light of classical thinking on heat.
It was a curiosity to me, more than anything else, to see what their thinking was. Perhaps it didn’t occupy them any more than it occupies anyone who goes to a few lectures on radiation and sees how the basics work.
There were a lot more interesting problems like the relationship between “work” and “heat”, and the classical ultraviolet catastrophe problem. I’ve read most of Max Planck’s “Theory of Heat Radiation”. I don’t think the “G&T problem” was a problem he spent any time on.
No one was looking out for Bryan back then.
And when you cite 6 textbooks with “here is radiative heat transfer 101” and are told you are cherry picking it’s clear the problem won’t be solved by a review paper from Max Planck in 1910.
Likewise on other blogs I’ve cited extracts from standard undergraduate heat transfer textbooks and been told I should stop quoting climate scientists and get with real heat transfer.
SoD
Its a pity that you did not mention Mark Zemansky’s Heat and Thermodynamics.
This was the book recommended by Feynman (lectures 45-1)
This was the thermodynamics textbook used in my university.
You could learn a lot from it.
I remember Zemansky’s book: boring as hell.
Pippard was shorter and more insightful.
Zemansky is an engineering textbook.
Classical thermodynamics has an unique set of properties as a field of physics:
1) It’s a highly restricted theory built on a small set of ad hoc axioms.
2) It’s an abstract mathematical theory.
3) It describes extremely well many physical phenomena of great practical importance.
Very many people have been educated to use the formulas of classical thermodynamics in engineering and chemistry, but only a small fraction of them understands classical thermodynamics well enough to answer correctly somewhat more complex questions, which go beyond the range of applications they solve regularly. I’m pretty sure this is the experience of everybody who gets contacted for advice, when others get confused.
Radiative heat transfer is an additional mechanism that must have had little weight in the development of classical thermodynamics. It differs from more typical forms of heat transfer in providing a mechanism of transferring energy directly in one step over a distance and in a way that makes it possible to observe separately the components of energy transfer that proceed in opposite directions. It’s heat transfer, not more generally energy transfer, in the sense that the energy is taken from the heat of the source and delivered to the heat of the target and also in the sense that work is not involved in the process.
DumbScientist:
Last year, after getting sucked into arguments with Bryan types at Roy Spencer’s blog, I presented at WUWT some very simple experiments with light bulbs surrounded by transparent, absorptive, and radiative shells. (I didn’t have a vacuum chamber, so I controlled for the conductive/convective losses in the different cases.) The write-up can be found here:
As SoD likes to say, hilarity ensued in the comments (and you should have seen what was said in the comments that were deleted…)
Since then, I have done further experiments that refined my original ones, but I haven’t written them up yet. I’ve been encouraged to submit a write-up to the American Journal of Physics by some physicists who think they could be good labs for high school and undergraduate students. I’m slowing progressing on that.
The presented experiments showed bulb temperature with constant power input under the different shells. Subsequent experiments showed the required power to hold the same bulb temperature under the different shells, and the rates of cooling under the different shells once power has been removed. (A lot of people get these three cases confused.)
I also did the tests with a bulb spray-painted black so the bulb surface is the only outwardly radiating surface, and did careful electrical measurements of the filament to determine its resistance and calculate its temperature. I found that I could increase the filament temperature about 10K by wrapping aluminum foil tightly around the bulb at full power.
Did that a while ago, but just with the aluminum foil and not the other things. It is instructive. AJP would be a good place for you.
Curt: I contemplated suspending a 9 W bulb and temperature sensor surrounded by foil inside an enclosure (coffee can?) partially or fully submerged inside a water bath. This would have the advantage of allowing one to directly control the temperature of the enclosure – rather than allowing the enclosure to reach equilibrium with the surroundings. The temperature of the suspended bulb should vary with the temperature of the cooler enclosure.
Changing the emissivity of the enclosure (spray paint?) would show that the emissivity of the enclosure changes the temperature of the suspended light bulb. This phenomena would be extremely hard to attribute to convection or conduction. I liked the way you used an IR thermometer to demonstrate differences in emissivity.
I didn’t have the patience to wade through the 400+ comments at WUWT to see if this version of your experiment would address any serious criticism of your experiment.
SoD
Your criticism of Gerlich & Tscheuschner has focused on (as you see it) three errors.
1. Radiation from a colder object cannot spontaneously move in the direction of a hotter object.
2. If by any chance such radiation gets there it cannot be absorbed.
Both these ‘errors’ you have illustrated in your diagram
3. A constantly powered source with a equilibrium temperature cannot have this temperature increased by an increase in any form of insulation.
Theres a big problem here.!
G&T never said anything of the kind.
Give a direct quote from either of their papers to back up any of your allegations.
G&T may have made errors and if so its a good idea to expose them but not the ‘made up’ errors you have quoted.
SoD
My previous post has been on moderation for 3hours.
Will I resubmit it?
SoD
Your criticism of Gerlich & Tscheuschner has focused on (as you see it) three errors.
1. Radiation from a colder object cannot spontaneously move in the direction of a hotter object.
2. If by any chance such radiation gets there it cannot be absorbed.
Both these ‘errors’ you have illustrated in your diagram
3. A constantly powered source with a equilibrium temperature cannot have this temperature increased by an increase in any form of insulation.
Theres a big problem here.!
G&T never said anything of the kind.
Give a direct quote from either of their papers to back up any of your allegations.
G&T may have made errors and if so its a good idea to expose them but not the ‘made up’ errors you have quoted.
Bryan,
In Does the surface temperature change with “back radiation”? Kramm vs Gerlich I showed this figure from their paper:
And I did comment:
I could assume they were falsifying imagined problems in atmospheric physics.
Of course, I took that paper at face value and therefore it’s hard to know what they really believe:
– There is no downward radiation?
– There is no first law of thermodynamics?
– The radiation is all reflected?
– Instantly re-emitted?
– Internal energy doesn’t increase temperature when it has come from a colder body?
Who would have thought that they actually believe that radiation from a colder atmosphere increasing the surface temperature of the earth is not a violation of the second law of thermodynamics?
In fact, no one. Everyone who has read their paper thinks that they attempted to prove such a temperature increase would be in violation of the second law of thermodynamics. In their reply paper they confirm such a violation. (But they do clarify that downward radiation exists so we can strike the top suggestion off the list).
Now you have confirmed that you believe there is no second law violation in this amazingly simple concept.
But I don’t see any statement from G&T.
Perhaps you can get them to confirm what you yourself have finally confirmed, that a colder atmosphere increasing the surface temperature of the warmer earth is not a violation of the second law of thermodynamics and then the illuminati of the blogosphere will be either better educated or will condemn G&T for their heresy.
Either one would be very entertaining.
G&T were having a laugh when they wrote their paper. And they still are.
SoD
Notice that the diagram specified heat transfer rather than energy transfer.
The energy transfer up would include radiation convection and conduction and possibly latent heat.
The energy transfer down would include radiation and the others but all to a lesser extent in all cases.
Heat is the net of all the energy transfers and is always spontaneously from a higher to a lower temperature as the second law indicates.
Any such diagrams that show heat transfer spontaneously from lower temperature object to a higher temperature object are contradicting the second law.
Perhaps G&T overestimated the comprehension level of readers but I can assure you there is nothing wrong with the G&T diagram as anyone with a physics degree will confirm
When I say there is nothing wrong with the diagram I of course mean that it is showing an impossible heat transfer as G&T say.
Where’s this diagram taken from?
They are correct that this fictional diagram represents something impossible.
But it’s fictional.
That’s what’s wrong with it.
On the Falsification of Fictional Ideas within the Frame of Physics by Gerlich and Tscheuschner.
I am not criticizing G&T for pointing out that their diagram is impossible. I am criticizing G&T for inventing a diagram, attributing it to the field of atmospheric physics and claiming that atmospheric physics makes impossible claims.
I am clearly overestimating your comprehension level of clear English. Or your [moderator deletion].
Its good that you realise the diagram is impossible.
However it might shock you to find that several such diagrams were quite common a few years ago.
Some of them illustrating CO2 heating up the atmosphere.
Arrows with the label HEAT pointing from the atmosphere to Earth surface
SoD says
“Please supply the reference for a one-way energy transfer from the colder atmosphere to the warmer surface from an atmospheric physics textbook or an atmospheric physics paper.”
The Halpern paper shows heat transfer two ways between hot and cold surfaces and see G&T for text examples of repeating the same error.
Also G&T point out that this is only a subset of errors.
SoD says
“Don’t forget to get that reference from G&T where they confirm what they really believe about the cold atmosphere increasing the surface temperature of the earth.”
Any insulator will have the same effect.
If there is no constant power input an insulator will simply slow down the temperature drop of the hotter object. not increase its original temperature.
As G&T say
“Once again, we never claimed — allegedly with reference to Clausius — that a colder body does not send radiation to a warmer one. Rather, we cite a paper, in which Clausius treats the radiative exchange. The correct question is, whether the colder body that radiates less intensively than the warmer body warms up the warmer one. The answer is: It does not.”
However the Earth has a constant power input and the insulator restricts the flow of heat forcing the surface temperature higher.
Note the constant energy from the Sun accumulating in the system forces the temperature higher not the insulating layer.
The insulating layer is not an independent heat source
There is no doubt that the Earths atmosphere moderates the temperature swings that the Moon suffers.
It acts as an insulator preventing a purely radiative heat loss from the surface and results in a habitable surface for most of the planet.
The addition of the trace gas CO2 attributed to humans will not have a significant effect on the Earths surface temperature.
The increase in CO2 partial pressure effect on the air is negligible.
Other much more significant heat transfer methods exist in parallel.
Note the emphasis is on negligible.
This means having little or no measurable effect.
Not an Earth surface exclusion zone for radiation from the atmosphere.
Just as Pekka says about conduction of heat by gases; it exists but has little effect (although I don’t fully agree with him on that).
Its a pity that because of a misunderstanding of the difference between heat and energy that the critique of the G&T paper here is misdirected.
It almost wholly deals with things they did not say.
As a result it has been given a free ride.
a much better approach would be to have a critical look at other aspects of their paper.
Pekka gave a list of possible problems (as he sees it) but nothing in any depth.
Might be worth examining in more detail.
This topic as Frank says is rather stale.
It feels like Groundhog Day when it comes up
Bryan: You say, “However the Earth has a constant power input and the insulator restricts the flow of heat forcing the surface temperature higher.”
Yup! But the thing is that the only reason there is an insulator is that the atmosphere has radiatively active gases that absorb radiation from the earth’s surface. In other words, greenhouse gases.
You have just given a very succinct one-sentence description of the greenhouse effect. Congratulations!
(Because the atmosphere cannot transfer power to the vacuum of space through conductive/convective means, it provides no conductive/convective insulation.)
Curt,
Indeed, GHGs form all the insulation, convection makes that insulation leak more than it would leak without. Latent heat transfer can be considered part of convection, conduction adds also to the leaks, but so little that it can be neglected almost everywhere.
Curt
I see the atmosphere as an object that can absorb heat.
Placed between the Earth and Sun it has both cooling and heating aspects.
It will moderate the Eaths temperature swings.
The Earth surface at the equator in direct sunlight could reach 121C like the Moon if there was no cooling effect
At night likewise the atmosphere restricts heat loss from the Earth surface so that the Moons temperature drop is not experienced.
All methods of heat transfer are involved not just radiation.
For instance on a cloudy night the cloud temperature may be little more than that of the Earth surface.
This means that convection (the most effective heat transfer method) is curtailed.
Convection is proportional to delta T.
However the cloud also reduces the (Ts^4 – Tc^4 ) radiative loss
The N2 and O2 part of the atmosphere cannot lose energy (except by collision with CO2 and H2O molecules causing radiation) so they retain their KE to a large extent.
Latent heat also plays a big part in the atmospheric temperature smoothing effect.
IPCC type science overemphasizes the Radiative aspect with an agenda of demonising CO2.
The ‘greenhouse effect’ is the popular name for this description.
Some folk who post here expect to find almost an almost 33K effect in a 3m tall glasshouse.
Even the radiative transfer calculation would expect less than one Kelvin degree
Bryan:
You are mixing up the capacitive effects and insulating effects of an atmosphere. A transparent atmosphere does have thermal capacitance — it would absorb energy from the surface during the day and return it to the surface at night — but it has no insulating capability. The surface would radiate directly to space, and the only important long-term energy balance is that the surface be at a temperature that would radiate as much to space as it absorbs from the sun.
You say, “IPCC type science overemphasizes the Radiative aspect with an agenda of demonising CO2.”
The Kiehl and Trenberth diagram, which is “IPCC type science”, shows 63 W/m^2 average radiative losses from the surface (396-333), and 97 W/m^2 from evaporative and conductive/convective losses — 50% greater than radiative! Do you think that these quantities are horribly wrong?
Curt
There is no sharp distinction between capacitive and insulative storage of thermal energy.
Even an electrical capacitor does not give back exactly the same energy as is used to charge it
Latent heat I suppose is at the capacitive end of spectrum and gas conductivity at the insulator end
The atmosphere is heated by conduction at the surface as well as by convection, latent heat and radiation.
It does not rely only on radiation from the surface although that is important.
Its not an either or situation.
The IR gases certainly thermalise surface radiation and some thermalised
energy moves up by convection and some part returns back to the surface as radiation.
I call this effect radiative insulation.
The pole to equator temperature difference and tidal effects also distribute the Sun’s thermal energy .
You ask
“Do you think that these quantities are horribly wrong?”
I think that the radiative fraction back to the surface is much smaller than in K&T.
I wrote this on another thread
……………………………
From memory (so numbers could be inaccurate)
In a pure CO2 atmosphere at atmospheric temperatures about 5% will be radiatively active.
Emission is as likely as absorption so no thermal effect
In an air atmosphere the CO2 will thermalise the 2500 non radiatively active molecules.
a 15um photon has about 200 times the translational energy of an ‘air molecule’ at that temperature so the thermalisation is significant.
Convection will move excess thermal energy upward.
I picture a barrel of water filling with a hose representing the upward radiation field.
A pipe leaves the barrel midway taking part of the water away this represents the convective effect
However the water level rises beyond this point till near the top some holes cause leakage and the water level stays constant.
The water escaping through the holes represent 15um re-emission up and down.
This in steady state represents LTE
We now add water vapour to the air.
This now supplies many more radiation possibilities to the air.
Result is now additional holes in the barrel much lower down, much greater in number .
These represent longer wave photons much more energetically likely to radiate than 15mm photons.
This in steady state represents a new LTE
Net result much less 15um photons going down and instead more longer wave photons going down
.
This accounts for ‘bite’ around 15um.
More CO2 (more holes at top) would have a negligible effect.
……………………………………………
Why don’t you read what Dr. Pierre-Marie Robitaille says about these laws of radiation.
He has made big advances in the field of NMR scanners.
The wavelength of interest in this field is similar to climate science at around 10um
http://hockeyschtick.blogspot.co.uk/2014/05/new-paper-questions-basic-physics.html
He has been recently getting a lot of traction and was a speaker at a recent APS meeting.
The video is worth watching
Bryan:
You remain completely confused on very basic concepts. You say: “There is no sharp distinction between capacitive and insulative storage of thermal energy.”
There is no such thing as insulative (resistive) storage of energy. Resistance is dissipative, not conservative. You point out that a real electrical capacitor has electrical resistance, but in analyzing this capacitor, you analyze it as a capacitive element that stores energy and a dissipating resistive element that does not. I do this all the time in my real work.
In a transparent atmosphere, the radiative resistance is zero. The conductive/convective resistance is irrelevant, because there is nothing for the top of the atmosphere to conduct/convect to. (It is like having an electrical resistor that is connected only at one end. It will reach the voltage of the connected end, but it will not transfer or dissipate any energy.)
It isn’t until you have some radiatively active gases that can absorb radiative power from the surface (more at the bottom than the top) and emit radiative power to space (more at the top than the bottom) that you get interesting dynamics in the atmosphere. The atmosphere will generally be at a lower temperature than the surface, so there will be conductive/convective transfers from the surface to the atmosphere as well as radiative. But this is only because this atmosphere, because of its “greenhouse gases”, can transfer power to space.
I took a look at the Robitaille presentation you linked. Its entire premise that atmospheric science treats the atmosphere as a blackbody is totally wrong – a complete strawman. Not worth going further into it.
Curt,
I see you’ve noticed that Bryan is very good at deflection and straw men. Have fun.
Curt says in quotation marks
“There is no such thing as insulative (resistive) storage of energy. Resistance is dissipative, not conservative. ”
Dissipative means turning to heat
The walls of your house are insulators and heat up.
Bricks were used in electrical storage hearers
“You point out that a real electrical capacitor has electrical resistance”
No I didn’t, read this
Click to access twocaps.pdf
“It isn’t until you have some radiatively active gases that can absorb radiative power from the surface (more at the bottom than the top) and emit radiative power to space (more at the top than the bottom) that you get interesting dynamics in the atmosphere.”
The atmosphere can heat up (and cool down) by conduction, convection,latent heat and radiation
“The atmosphere will generally be at a lower temperature than the surface, so there will be conductive/convective transfers from the surface to the atmosphere as well as radiative. But this is only because this atmosphere, because of its “greenhouse gases”, can transfer power to space.”
The surface also radiates through the atmospheric window
“I took a look at the Robitaille presentation you linked. Its entire premise that atmospheric science treats the atmosphere as a blackbody is totally wrong – a complete strawman. Not worth going further into it.”
Robitaille doesn’t say anything about the atmosphere just some general comments about solids liquids and gases.
His main point is about the Kirchhoff law being non general hence problems for Stephan Boltzmann equation Plancks and Wein laws.
There is an instrument called the pyrgeometer which is supposed to measure DLWR and uses the Stephan Boltzmann Law in its calibration.
For 70 years they have tried to get it to work reliably but have failed.
I think that that is a good experimental test showing that there is something wrong with the climate science approach to radiative transfer
Bryan:
You are still not getting it. Dissipative elements are non-conservative and non-reversible — entropy producing.
The temperature of thermal resistance elements, such as the insulation in your walls, will be in between what is on either side of it. They could cool just as much as heat up. And just as real-world capacitors have resistance, real-world resistors (thermal, electric, mechanical) have capacitance (I thought this should go without saying…)
Bricks would be used in electrical storage heaters for their thermal capacitance, not their resistance.
You said upstream that “Even an electrical capacitor does not give back exactly the same energy as is used to charge it.” I guess I gave you too much credit in thinking that you realized that this would be because of power dissipated due to resistance. The Princeton problem you cite simply argues that even if you don’t explicitly put any conductive resistance (actually an impossibility, but we will let that go) between the capacitors you suddenly connect, that there will be effective resistance due to radiative losses from the current surge.
You continue to evade my main point that without radiatively active (aka “greenhouse”) gases, there can be no ongoing power transfer by other means from the surface to the atmosphere. The entire spectrum would be the “atmospheric window” to space.
I have now wasted my time watching most of the Robitaille video you linked. So far, nothing new, unusual, or even provocative. He says that gases are not close to being blackbodies, so equations describing blackbody response are not appropriate to use for gases. Hold the presses!!!
Pyrgeometers are in wide and successful use in many applications around the world. Simple and cheap ones like your kitchen infrared thermometer make an assumption of high emissivity (typically ~95%) in the narrow frequency band they let in. Still, they are very good for most things you would want to use them for (but not polished metals, which have a very low emissivity in this band).
But even your dirt cheap kitchen infrared thermometer pointed at the sky can provide a reasonably accurate measurement of humidity (“total column water vapor”):
Click to access mims_BAMS11_PrecipWaterVaporIR.pdf
Pyrgeometers measure the difference between outgoing and incoming radiative flux in the frequency band by comparing the temperature of the element under the lens to one hidden from the lens. Any assumptions about emissivity are only used in subsequent processing to try to derive a temperature of the object pointed at.
Curt you say in “….”
“Dissipative elements are non-conservative and non-reversible — entropy producing”
Agreed just like the bricks in an electric storage heater..
“The temperature of thermal resistance elements, such as the insulation in your walls, will be in between what is on either side of it. They could cool just as much as heat up. ”
Agreed just like the atmosphere.
Both can store thermal energy to smooth out the diurnal cycle when they next receive a fresh supply of energy
Electrical in the case of storage heater, the Sun in the case of the atmosphere
“Bricks would be used in electrical storage heaters for their thermal capacitance, not their resistance.”
I think you are getting muddled up
The bricks have thermal capacity and thermal resistance
Both above examples are of temperature smoothing to produce more habitable environments
Non dissipative examples would be photosynthesis and the biosphere etc
“You continue to evade my main point that without radiatively active (aka “greenhouse”) gases, there can be no ongoing power transfer by other means from the surface to the atmosphere.”
You cannot be serious!
Conduction at surface and elsewhere
Convection
Latent heat
Wind ,tidal, I could go on and on
“I have now wasted my time watching most of the Robitaille video you linked. So far, nothing new, unusual, or even provocative.”
You had better watch again!
“Pyrgeometers are in wide and successful use in many applications around the world.”
Im not talking about cheap toys.
In a recent report(2008) two expensive research pyrgeometers made by reputable companies like Eppley and calibrated at Davos gave readings that differed by 40 to 60 W/m2 when pointed to same object.
This is an 70 year old ongoing problem and underlines the departure of climate science from reality.
Scientologists also have a device called an e-meter and it gives them much pleasure looking at the readings.
Outsiders are not impressed by either device.
This is all I have time for at the moment.
One thing I think we can agree on and applaud is your decision to carry out some experiments on aspects of heat transfer related to climate.
I look forward to reading them in the future.
Bryan,
Once again I see you have managed to deflect the topic away from the main point, radiative transfer in the atmosphere leading to the greenhouse effect.
What do bricks have to do with insulation? Brick doesn’t conduct heat like a metal, but it’s insulating properties aren’t that good. The thermal conductivity of a standard wall brick, 0.6-1, is about the same window glass , 0.96 W/(m K). Wood has a much lower thermal conductivity than brick, 0.1-0.2.
Good insulators have low density as well as low thermal conductivity so the volumetric heat capacity is low. Brick is fairly dense and has average specific heat capacity, 0.9 kJ/(kg K). It can also stand high temperature so it’s a good energy storage medium. The relatively high thermal conductivity of brick is also important for energy storage.
But all that, as I’m sure you’re well aware, has precisely nothing to do with the radiative transfer properties of the atmosphere.
Bryan:
Let’s focus on my central point, “that without radiatively active (aka “greenhouse”) gases, there can be no ongoing power transfer by other means from the surface to the atmosphere.”
You say that I cannot be serious, but I am completely serious, and it is trivial to demonstrate at the most introductory level of thermodynamics.
A transparent atmosphere has no mechanisms for energy transfer with the sun or with space. It has only the possibility of condcutive/convective transfer with the planetary surface. In the steady state, where its internal energy is constant, so energy in must equal energy out, if there is no energy in or out between the atmosphere and the sun or space, its energy transfer with the surface must be zero. There is no other conclusion consistent with the 1st law of thermodynamics.
But the surface is receiving energy from the sun. What keeps it from heating up continually? Unlike a transparent atmosphere, it can radiate energy to space. It reaches steady state when it is radiating energy to space at the same rate it receives it.
Since the atmosphere in the steady state cannot have any net transfer to or from the surface, it must be at the same temperature as the surface.
Now this is a very simple energy balance problem like you would get in the opening weeks of an introductory thermodynamics or heat transfer course. Any student would be expected to go through logic equivalent to what I have presented, and with a couple of numerical values provided, come up with a numerical answer. It is obvious to me that you would really struggle in such a course.
Curt says
“Let’s focus on my central point, “that without radiatively active (aka “greenhouse”) gases, there can be no ongoing power transfer by other means from the surface to the atmosphere.”
OK then one point at a time
Are you saying that if N2 and O2 molecules land on the warmer Earth surface they cannot leave the surface at a higher temperature or more correctly at a higher translational speed?
Secondly all molecules radiate above zero Kelvin, some more than others eg CO2.
So its not either or as I said in an earlier post.
Its impossible to specify non radiating molecules just to fit in with some theoretical justification for the greenhouse theory.
It looks as if the greenhouse theory has monopolised the fact that all molecules naturally radiate.
Next we will be informed that because we recognise all molecules radiate then we must believe in the greenhouse theory.
Bryan:
You ask: “Are you saying that if N2 and O2 molecules land on the warmer Earth surface they cannot leave the surface at a higher temperature or more correctly at a higher translational speed?”
No, I am saying that, in steady state conditions, the (transparent) atmoshpere will reach the temperature of the surface so, on average at least, the collisions of the gas molecules with the surface will not change the kinetic energy of the molecules. In a macroscopic sense, there will be no ongoing energy transfer between the surface and the atmosphere.
You are effectively arguing that, even with no way of transferring energy to space, a transparent atmosphere will continue to have energy transferred to it from the surface. This means that the internal energy of the atmosphere, and therefore its temperature, would increase without end, and so would continue even when the atmosphere has a higher temperature than the surface — a blatant 2nd law violation.
Look, if you want to understand the effect of radiatively active gases, you have to consider what would happen without them. That is what you are failing to come to grips with.
You then say: “Secondly all molecules radiate above zero Kelvin, some more than others eg CO2.” If you look up the emissivity of N2, O2, and Ar in the wavelengths corresponding to thermal emissions at earth temperatures, you would find that they are so many orders of magnitude below those of “greenhouses gases” like H2O and CO2 that they can be considered to be zero without any noticeable loss of accuracy of calculations.
(Upthread, you pointed me to a presentation whose major point was that gases do not behave like blackbodies or even graybodies. Now you want me to consider gases to behave like these…)
Until you get a fundamental understanding of very basic thermodynamic concepts like energy balance analysis, you will not be able to get a handle on even the simplest systems, and you will be totally lost on complex systems.
Curt you say “……..”
“No, I am saying that, in steady state conditions, the (transparent) atmoshpere will reach the temperature of the surface ”
Yes I know that the isothermal atmosphere is suggested by some for this hypothetical situation.
But even accepting totally non radiating air molecules there are many who dispute your conclusion.
For instance DeWitt Payne holds that because of pole to equator different surface temperatures and different local surface temperatures etc causing winds and tidal effects there will be a turbulent atmosphere which is non isothermal.
But he is better at explaining his position
“You are effectively arguing that, even with no way of transferring energy to space, a transparent atmosphere will continue to have energy transferred to it from the surface.”
No I don’t accept that is my position
The surface still radiates in this hypothetical situation and the surface is not isothermal.
If there is still an atmosphere it will have a heat capacity and will perhaps by conduction and convection be a net absorber from the surface during daytime and a net heat source to the surface at night if it has a higher temperature.
Evaporation, then the energy of latent heat condensing gives another conduit
The scattering atmosphere has yet to be factored in
“Until you get a fundamental understanding of very basic thermodynamic concepts like energy balance analysis, you will not be able to get a handle on even the simplest systems, and you will be totally lost on complex systems.”
No I think that even in simplified hypothetical situations your analysis does not hold up.
Of course the Earth radiates to space with the same average energy it absorbs.
The atmosphere oceans and land have a thermal capacities and these smoothe out temperature excesses quite independent of any greenhouse effect
Bryan:
This is completely pointless. You either can’t or won’t engage the central point, and continue to go off on irrelevant tangents. This seems to be a pattern, as it’s exactly what you did with SoD’s challenge to you in the last post.
The central point is this: A transparent atmosphere has no method of exchanging energy with anything but the earth’s surface, so over the long term to be in even approximately steady state condition, it will have no net transfers with the earth’s surface either. All the exchanges you talk about balance out over time and area to zero.
(By the way, I said nothing about the vertical profile of temperature in the atmosphere in this steady state. The temperature of the atmosphere at the surface would be equal to the surface temperature in the steady state case with no energy transfer. For the purpose of this analysis, it does not matter what the temperature profile above the surface is.
Since there is no long-term average energy transfer from the surface to the atmosphere in this case, the only way the surface can achieve energy balance is for it to radiate directly to space (through the transparent atmosphere) as much energy as it receives from the sun. This will result in lower temperature levels than if the atmosphere absorbed some of the surface’s radiation.
Your statement that started all of this off was “the Earth has a constant power input and the insulator restricts the flow of heat forcing the surface temperature higher.” But it is only the radiative absorption of the real atmosphere that provides this effect.
Curt wrote: “This is completely pointless. You either can’t or won’t engage the central point, and continue to go off on irrelevant tangents. This seems to be a pattern, as it’s exactly what you did with SoD’s challenge to you in the last post.”
Congratulations, you figured it out.
Curt,
See #4 here.
Curt you say ..”…..”
“This is completely pointless.”
I agree the greenhouse theory paradigm is is so embedded that rational discussion fails to weaken it for true believers.
Despite the historical evidence that atmospheric CO2 lags temperature. by hundreds of years.
Despite the recent evidence that atmospheric CO2 increase has no effect on global temperatures.
However I still have some hope for you in that you are proposing to do some actual experiments rather than computer models.
Here is a the record of an experiment carried out over two years.
Click to access penn_state_plastic_study.pdf
Sit down when you read this paper because the hilarity might be overwhelming and lead to irreversible life threatening giggling.
DeWitt Payne said I should read it because it was a PROOF OF THE GREENHOUSE THEORY.
Very embarrassing.
Still dodging and weaving, going off on irrelevancies. Obviously don’t have the technical chops to deal with the basic scientific issues…
Curt says about my previous post
“going off on irrelevancies.”
Because presumably he would like an endless examination of a planet where atmospheric molecules did not radiate.
Perhaps he realises that the historical and recent temperature record contradicts the greenhouse theory and so thats obviously irrelevant.
I encouraged him to do real experiments as the only real test of a theory
The experiment from Pen State supports the experiment of R W Wood rather than the greenhouse theory.
DeWitt Payne it would appear has difficulty reading plain text , this seems to be a general condition among greenhouse theory advocates .
Here are more things (that do not happen) that Curt might want to examine
1 A planet where atmospheric molecules have no mass
2.A planet where time runs backward
3 A planet where atmospheric photons have an electric charge
4 A planet where cold objects heat or warm warmer objects
5.A planet where water flows uphill
Oh, how clumsy of me, number 4 must in fact be true, its a cornerstone of the greenhouse theory
Every time I’m tempted to walk away from this train wreck, Bryan surprises me by exposing new depths of his confusion.
Today we learn that he cannot distinguish between a scenario that is physically possible (e.g. a transparent atmosphere) and one that is not (e.g. a no-mass atmosphere).
And no matter how many times he is reminded of it, he simply cannot understand that if you want to analyze a phenomenon, you have to consider cases with and without the phenomenon to understand the difference. Has he never heard of the “control” in an experiment?
He also reveals that he cannot distinguish between the level (value) of something and its rate of change (derivative). Without the (poorly named) greenhouse effect from radiative gases, the earth’s energy balance would be out of whack by over 150 W/m2. This is a completely separate issue from how much the energy balance changes with small additional increments of a radiatively active gas – the derivative of this effect. The arguments here are over imbalances of a fraction of a single W/m2. Here the uncertainties in the measurements are likely bigger than the proposed increment.
He states as a physical impossibility “[a] planet where cold objects heat or warm warmer objects.” But upthread he states “the Earth has a constant power input and the insulator restricts the flow of heat forcing the surface temperature higher.” Whether he thinks this insulator is conductive, convective, or radiative insulation, it is colder than the surface, yet he believes its presence leads to higher surface temperatures than it would have in the absence of this cold object. That’s all the planetary greenhouse theory is.
Curt says “…….”
“I’m tempted to walk away from this train wreck”
Hyperbole, Gaza and Ukraine are train wrecks this is light hearted banter
“He states as a physical impossibility “[a] planet where cold objects heat or warm warmer objects.” But upthread he states “the Earth has a constant power input and the insulator restricts the flow of heat forcing the surface temperature higher.”
What drives the temperature higher is the constant power input not the colder object.
If this is removed both hotter and colder objects will seek a new equilibrium temperature higher than the colder and lower than the higher.
Take an adiabatically enclosed box containing say a copper block at 400K and an iron block at 300K
Put actual numbers in if you like.
Both will eventually reach the same temperature >300K and <400K
Zeroth law of Thermodynamics
The heat lost by the copper block is exactly equal to the heat gained by the iron block
First law of Thermodynamics
The direction of heat transfer is from higher to lower temperature.
Second law of Thermodynamics
Cold objects never, ever, heat up or warm hotter objects.
Now you’re just arguing semantics. If you have an object with a steady power input in a cold ambient, it will reach a certain steady state temperature when its losses to ambient match its power input. Now if you put some insulation – conductive, convective, or radiative – between the object and the ambient sink – the temperature of the object will rise until it reaches a new steady state.
The presence of the insulation, other things equal, can be said to “drive the temperature higher”.
Of course, it would not do so without a separate power source. But no one here is arguing that it would. All people are saying is that the presence of radiatively active gases provides radiative insulation that drives the temperature of the surface of the earth (with its constant power input) higher than it would be without this insulation.
Curt says
“Now you’re just arguing semantics.”
Yes it would appear that on the fundamentals discussed there is little difference between us.
However I think that Roy Spencer and others stand language on its head when saying cold objects warm hotter objects.
To me its like saying a thief can make you richer.
For instance Thief A only takes your cash
Thief B takes cash ,your watch, credit cards and any valuables.
You will be richer after meeting A than B
I suppose that its easy for folk like Roy and others in climate science to lapse into this usage since they must deal with empty space at near absolute zero Kelvin all the time.
For others outside the climate science community the usage is very odd..
Where you learn your thermodynamics is also important.
If from a physics background the second law will be met via the Carnot Cycle and you will be interested in the quality of radiation as much as the quantity.
You will also be interested in the philosophical aspects of the second law.
Outside the physics community the results of physics are very important to engineers,chemists,climate science and others.
There is so much in the curriculum that quite often only the bare minimum physics is taught.
I asked Judith Curry on her blog if climate science students at her college are taught the Carnot Cycle.
She answered that climate science students are taught the thermodynamics relevant to the climate.
I took this to be a no (perhaps I was wrong).
For instance I am almost certain SoD did not learn his thermodynamics via physics 101 and 201.
He keeps his background very secret and thats fine.
He strikes me as very clever and is a wizard with equations but has his blind spots particularly with regard to the second law.
I on the other hand certainly have no background in climate science.
I became interested in the topic only after ‘climategate’
Two aspects interest me and here perhaps I can make a contribution
1. The basic thermodynamics
2. The pyrgeometer.
I was told early on that the pyrgeometer could measure the magnitude of the radiation stream from the cold atmosphere.
I thought that this if true it was news to me and if accurate would make the greenhouse effect more plausible.
I must say after reading up on the instrument I am far from impressed.
Bryan:
If I had had more time yesterday, I would have used a financial analogy too, but one I think is better.
Let’s say you have a steady job, and every week you deposit your paycheck in your bank account. For fun, let’s call it $240 per week. Every week you spend $240 out of your bank account. So the account balance in your bank account stays constant over the long term.
Now let’s say that one of the stores you spend your money at starts issuing rebates on your purchases, so every week you get a $60 rebate check from the store, which you deposit in your account.
Is it really wrong to say that these rebates are causing your bank account balance to increase (by $60 a week in this example), even though these rebates are not independent of your expenditures, and they are always less than your expenditures?
But this is exactly analogous to the situation we are talking about here, where the non-rebate situation is equivalent to the transparent atmosphere, and the with-rebate situation is equivalent to the radiatively active atmosphere, and the rebates equivalent to the “back radiation”.
And even if you don’t like the terminolgy, saying you have reduced your expenses to $180 per week, so the fact that your income, which is your only real source of money, exceeds expenses by $60 a week is what is causing your bank account to increase, the net result is the same. That is why I say you only have a semantic quibble.
Back to thermodynamics: I studied my thermodynamics through physics, physical chemistry, and mechanical engineering departments. Every time we covered radiative heat transfer, it was explained through the idea of “radiative exchange” between objects, with the resultant heat transfer being the difference between the energy carried by the two radiative “streams”. That’s the way my textbooks explained it, and SoD has quoted many more texts that explain it that way.
So when I started looking into climate science issues, I was not at all taken aback by the idea of “back radiation” from the atmosphere. It is just one half of the radiative exchange between earth and atmosphere.
But even if you prefer to think of a one-way “heat flow”, as the old fans of caloric theory did, it makes no real difference at this energy balance level of analysis. Whether you consider the earth radiating up 396 W/m2 and the atmosphere radiating down 333 W/m2, as in the K&T diagram, or instead that there is a single 63 W/m2 heat flux upwards, you end up with the same result.
(As an aside, if you get into the details of radiative absorption, they don’t make sense without understanding the quantized nature of photon/molecular interaction. And that doesn’t make sense without considering the heat transfer as resulting from a 2-way exchange of electromagnetic radiation. But as I said, at the higher level energy balance we are talking about here, it really does not matter which way you think about it.)
My kitchen infrared thermometer, a very very basic pyrgeometer, does a fine job reporting the temperature of most objects both hotter and colder than it is (polished metal surfaces aside). Before I trusted it, I compared what it reported to other methods of temperature measurement. I got very close agreement.
Please supply the reference for a one-way energy transfer from the colder atmosphere to the warmer surface from an atmospheric physics textbook or an atmospheric physics paper.
Don’t forget to get that reference from G&T where they confirm what they really believe about the cold atmosphere increasing the surface temperature of the earth.
They claim it would be a violation of the 2nd law of thermodynamics. I checked their followup reply paper:
Yet you agree with the calculation here. (Even though you were not able or willing to supply it yourself).
G&T think this cannot happen.
No one reading their original paper or their reply can conclude any different.
So either:
– there is a flaw in the above calculation, i.e., the first law of thermodynamics is falsified
– G&T can’t do simple maths, or
– they are deliberately attempting to confuse the illuminati
SOD: As best I can tell, G&T come up with the fictional Figure showing heat transfer from the colder stratosphere to the warmer ground using the following sleight-of-hand: Descriptions of the greenhouse effect involve radiation transferring ENERGY from the cooler atmosphere to the warmer surface – a process that doesn’t violate the 2LoT. G&T assert (correctly as far as I can tell), that the 2LoT applies to heat, not energy. Therefore they replace “energy” in the above description with “heat” giving: Descriptions of the greenhouse effect involve radiation transferring heat from the cooler atmosphere to the warmer surface – a process that does violate the 2LoT. It doesn’t help when some add that this transfer “heats” or “warms” the surface.
Do G&T really misunderstand what climate scientists actually mean? Or are they, as you say, “Having a laugh”? Is there any chance they don’t recognize the greater radiative energy transfer from the warmer earth to the cooler stratosphere? Who knows, since they also misinterpret energy balance diagrams like K&T’s. Furthermore, net radiation isn’t a proper measure of how much heat flows from the surface to the atmosphere. (See below.) Until someone provides a “proper” explanation, G&T and friends can continue to pretend or assert that one doesn’t exist.
I’m wondering if an alternative to the KT energy balance diagram should be constructed to explicitly deal with heat. Anyone using the KT diagram (energy fluxes) to discuss the 2LoT is asking for trouble. A heat transfer diagram would have only five arrows: Arrows from the sun to the surface and to the atmosphere would show 161 W/m2 and 78 W/m2 of heat (energy transfer) respectively. Then we would have 40 W/m2 of heat from the earth to space and 199 W/m2 from the atmosphere to space. In all four of these cases, the radiative energy flux in the opposite direction is negligible, so the one-way radiative energy flux and heat are equivalent. Then we have 17 (sensible heat) + 80 (latent heat) + (396-40) (LWR from surface to atmosphere) – 333 (LWR from atmosphere from surface) = 120 W/m2 of heat from the surface to the atmosphere. (Am I correct saying that all energy fluxes between the surface and the atmosphere must be included in “heat”, even latent heat?) Usually we just talk about net radiation and the 2LoT). 1 W/m2 is changing the temperature of the surface and deep ocean, but this is not heat (energy transfer). A heat diagram side-by-side with an energy flux diagram would clearly show how heat travels from hot to cold (2LoT) and energy is conserved (1LoT) when the earth’s greenhouse effect is operating.
Frank,
It’s not helpful to make the diagram or some other description more abstract just to satisfy the axiomatic setting of Classical Thermodynamics and the related definition of the word heat. It’s a dirty trick of playing with the words that K&T take, when they claim that there’s a contradiction between 2LoT and the descriptions of GHE. The trick is to use the same word (heat) in two different meanings and to observe that a contradiction would ensue, if the meaning would be the same. That’s totally irrelevant, and the only way of countering such sophistry is to point out that it’s only sophistry – or strawman logical fallacy.
More or less everyone understands that atmosphere can emit radiation and that this radiation can be absorbed by the surface. The actual numbers are strongly linked to the application of 2LoT as the value of adiabatic lapse rate is exactly, what 2LoT leads to as the limiting case. The whole atmospheric physics is built on thermodynamics. People like K&T or Bryan don’t try to find errors in that, they choose to forget all the realities and to invent their imaginary world, where climate science is based on something totally different and makes errors that the actual science does not make.
When you create a silly enough claim, it’s not difficult to show that the claim is silly, but that tells nothing about anybody else than yourself.
Pekka,
Which is why I agree with Denker that in an ideal world the term heat should be banished from thermodynamics. It has too many meanings even within thermo. He lists six, several of which are mutually exclusive. But it isn’t going to happen, at least any time soon.
Pekka and DeWitt: I’ve simply tried to describe the conventional explanation for the earth’s GHE using terms that G&T would understand while clearly differentiating between energy fluxes and heat transfer. Many schemes to create perpetual motion of the second kind have been proposed. I’ve tried to demonstrate that the KT energy balance diagram is not one of these – while trying to preserved G&T’s distinction between heat and energy with respect to the 2LoT.
DeWitt is right; the multiple meanings of the word “heat” cause confusion. When there is confusion, it helps to use terminology that everyone can accept. The KT diagram for energy fluxes (1LoT) plus my diagram for heat transfer (2LoT) do so. I’m disappointed that you don’t recognize the advantages of this approach (or haven’t noted a technical mistake).
IMO, the best way to discredit G&T is to show that their objections are incorrect even when the GHE is explained in terms of classical thermodynamics – not to reject the terminology of classical thermodynamics. You can’t discuss the 2LoT in classical terms without converting all energy fluxes to heat transfers. It is unfortunate that the published replies to G&T did not do so.
Frank,
You’re forgetting why Bryan and his ilk never use equations, only words. You can’t play word games with equations. Of course, you can still leave stuff out. G&T are really good at that. They don’t do surface integrals to convert vector fields to fluxes, for example. Well, they do sometimes, but only when it suits them. In fact, the concept of a flux through a surface with an arrow showing the orientation of the surface normal unit vector seems to escape them entirely when they look at an energy balance diagram.
All of G&T’s examples in Section 3 The fictitious atmospheric greenhouse effects, for example, could be cleaned up to pass muster with a few changes in terminology. Bryan and G&T, however, practice the Humpty Dumpty Theory of Language. That allows them to misinterpret anything they like to prove their point.
DeWitt: I haven’t forgotten how Bryan behaves, but I didn’t convert the KT energy balance diagram to a heat transfer diagram for Bryan. I wanted a short, unambiguous explanation as to why the KT estimate of the GHE doesn’t violate the 2LoT using the classic definition of heat. Hopefully the lack of applause is due to the lack of respect for those who would benefit from seeing a heat transfer diagram (rather than the poor quality of my comment).
Frank,
The fact that the all the heat, in the classical sense, fluxes, i.e. Q_dot, are in the right direction in KT97 or TFK09 to satisfy the 2LoT is not exactly news. The problem is convincing some people that all radiative energy fluxes are not identical to heat fluxes. The convective flux is a heat flux,however. Yet at least one commenter here has not accepted that in the past. It might be nice if we used Q or ΔH instead of ‘heat’, btw.
It’s somewhat annoying that there isn’t an easy way to generate a capital Q with a dot on top to represent the rate of change of Q. Denker has an HTML macro that is supposed to do it, but you need to use raw HTML code, which probably wouldn’t work in comments and might not work with all browsers either. And that assumes, incorrectly, that I actually knew how to use the macro.
DeWitt,
How about this: .
.
The code is $ latex \dot{Q}$ with the first space after $ removed. It’s not perfectly aligned on the line, but otherwise this should work.
DeWitt wrote: “The fact that the all the heat, in the classical sense, fluxes, i.e. Q_dot, are in the right direction in KT97 or TFK09 to satisfy the 2LoT is not exactly news.”
Frank responds: Exactly. And if those who replied to G&T had simply calculated the classical heat fluxes associated with KT97 or TFK09 and explained that these transfers came output from GCM’s, G&T would have had nowhere left to go. (Instead they wasted time discussing shell models, suggesting that G&T were stupid enough to believe in one-way radiation, arguing about the calculations that produce 33 degK, etc.)
No one person understands everything that is inside a GCM. GCMs probably contain programming errors of unknown significance; not to mention dozens (hundreds?) of adjustable parameters. They are computational black boxes for everyone. This is especially true for those like G&T, who are sticklers about “heat” and object to the idea that DLR heats the surface. Classical thermodynamics places limits on what can happen inside any black box. That is the beauty of thermodynamics; you don’t need to know details of what happens inside the box – the individual fluxes or the reaction mechanism. How does one prove that a black box doesn’t contain a computational perpetual motion machine? Look at the output. Clear up the meaning of the arrows on KT97 and TFK09, calculate the classical heat transfers, and show they obey the 2LoT!
G&T are also either unaware of the concepts of artistic license and cartoon or are purposefully misinterpreting arrows that are not perpendicular to a surface of interest in an energy balance diagram as representing actual orientations rather than just links to sources and sinks. Again, it’s something that could be cleaned up, but if you did, the cartoon would be more difficult to interpret.
I’m only diving into a trivially small point from the comments, not even directly related to the post. But I find Denker to be fairly arrogant but his expositions thought provoking, enlightening, and, to the extent I’m able to grasp, accurate. I get huge value from his sight, not only on thermo but on other areas of physics and mathematics. This is not to even mention his discussions of flight which, as a pilot and airplane owner, I also find valuable.
pa32r:
Funny you should mention that, I was just puzzling over one of his pages. In discussing the issue of airfoil lift, he dismisses the applicability of the Coanda effect at all. Do you understand his point of view?
Neal: A consensus that climate is changing is meaningless; it has always changed. A consensus that man has made a significant contribution to recent change (more than 50%) is almost as meaningless, because that consensus can’t tell the policymakers whether we must leave valuable fossil fuels in the ground. A consensus that ECS or TCR lies within a relatively narrow range (2.5-3.5 degC for ECS, for example) might be meaningful. If a consensus on ECS existed, we would still face the difficulty doing a cost-benefit analysis and making plans that would account for technological progress. (The best reason for leaving fossil fuels in the ground is that cheaper alternatives exist.)
Do a survey asking experts in the field of climate sensitivity if the necessary consensus exists on ECS! Oops. the IPCC already did one (AR5) and the answer is no.
Frank:
a) “A consensus that climate is changing is meaningless; it has always changed.”
Of course: That’s why what was asked was to categorize the abstract as one of:
1) Explicit endorsement with quantification: explicitly stating humans are the primary cause of recent global warming;
2) Explicit endorsement without quantification: explicitly states humans are causing global warming; or referring to anthropogenic global warming as a known fact;
3) Implicit endorsement: implying that humans are causing global warming; e.g. the research assumes GHG emissions cause warming without explicitly stating humans are the cause;
4a) No position: not mentioning or addressing the cause of global warming;
5) Implicit rejection: implying humans have had minimal impact on global warming without saying so explicitly; e.g. proposing that a natural mechanism is the main cause of global warming;
6) Explicit rejection without quantification: explicitly minimizing or rejecting that humans are causing global warming;
7) Explicit rejection with quantification: explicitly stating that humans are causing less than half of global warming.
But you knew that already, Frank, because you read the paper before deciding it was wrong. Didn’t you?
b) A consensus that man has made a significant contribution to recent change (more than 50%) is almost as meaningless, because that consensus can’t tell the policymakers whether we must leave valuable fossil fuels in the ground. A consensus that ECS or TCR lies within a relatively narrow range (2.5-3.5 degC for ECS, for example) might be meaningful. If a consensus on ECS existed, we would still face the difficulty doing a cost-benefit analysis and making plans that would account for technological progress. A consensus that man has made a significant contribution to recent change (more than 50%) is almost as meaningless, because that consensus can’t tell the policymakers whether we must leave valuable fossil fuels in the ground.
The purpose of measuring the consensus is simply to establish that the simple point that a consensus on GHGs exists, and “to quantify and evaluate the level and evolution of consensus over the last two decades.” And the purpose of that is to document this fact for the general population: because as of 2007, public opinion polls have indicated that the US public feels the scientific community is in disagreement over the fundamental cause of GW; 60% believes that the scientific community is still in disagreement about whether GW is happening at all.
But you knew this already, Frank, didn’t you? Because you read the introduction before deciding the paper was pointless. Didn’t you?
c) “A consensus that ECS or TCR lies within a relatively narrow range (2.5-3.5 degC for ECS, for example) might be meaningful. If a consensus on ECS existed, we would still face the difficulty doing a cost-benefit analysis and making plans that would account for technological progress. Do a survey asking experts in the field of climate sensitivity if the necessary consensus exists on ECS! Oops. the IPCC already did one (AR5) and the answer is no.”
But that’s not what this paper was about, Frank. This paper was about the fact that there really is an overwhelming consensus among scientists working on climate about what is going on – a fact that certain parties have found inconvenient. So they have sought to convince people that there is no such consensus – as they did with the issue of cigarettes & lunch cancer, stalling that for 30 years (and it’s some of the same people: switched from smoking-isn’t-bad-for-you experts to GHGs-aren’t-bad-for-you experts without even a change of post-office box; check DeSmogBlog).
And testing has shown that when people are informed that the degree of consensus is not 50% or 55% or 60%, but 97% (as three independent efforts have now shown), they take the entire issue much more seriously.
And we do want people to take the science seriously. Don’t we?
Neal: Forgive me for not re-reading a paper before ranting inaccurately.
I personally believe that man has probably caused more than 50% of the warming since 1950 – but not with greater than 95% confidence. According to Otto et al, a TCR of 1.35 degC agrees with the total observed warming since 1970. One probably needs a TCR of 1.35/2 = 0.7 for one’s central estimate of man’s contribution to warming to be less than 50%. (Even Lindzen and Choi’s central estimate isn’t quite this low.) Given that water vapor feedback must exist in areas where vapor and liquid are in near equilibrium (the boundary layer over oceans, ascending air where clouds are forming), one needs a lot of negative cloud feedback to believe in a TCR this low. Observations from space are not consistent with this possibility.
However, my main point was that the consensus man is responsible for at least 50% of the warming since 1950 doesn’t imply that TCR and ECS must be as high as GCMs predict. When damage probably escalates exponentially with warming, a three-fold range in future warming (1.5-4.5 degC) – the important “consensus” – isn’t good enough.
If your consensus is only about TCR being above 0.7 degC, it doesn’t appear to be about anything useful for policymakers. (If you don’t agree with my estimates of TCR, please explain where mine went wrong.)
Frank:
When damage probably escalates exponentially with warming, a three-fold range in future warming (1.5-4.5 degC) – the important “consensus” – isn’t good enough.
If your consensus is only about TCR being above 0.7 degC, it doesn’t appear to be about anything useful for policymakers.
If you assume that Lady Luck is always going to be on your side, you can afford to take that approach. But there is no particular reason to ignore the higher end of the range in favor of the lower: We just don’t know. What is the probability that your house will burn down in a fire? Reasonably small, I think. But do you buy fire insurance? I suspect you do.
pa31r,
Denker is almost completely wrong about the chemistry and kinetics of lead acid batteries. He completely ignores the fact that protons and bisulfate anion are at effectively unit activity everywhere in the electrolyte, including at the electrode surfaces, at least at any rational discharge rate. The reaction order for protons is therefore unimportant and bisulfate ion does not need to diffuse against a potential gradient.
DeWitt:
Then why do you recommend him?
Neal,
It’s not merely possible for someone to be knowledgeable about and have valid insights on one thing and be completely wrong about something else. It’s pretty much a certainty. I think it’s called being human. Let him who is without sin, cast the first stone.
I know a very good organic chemist, for example, who believes in ‘creation science’. If I have a question about organic chemistry, I would still go to him.
With reference to Kurt Vonnegut’s Cat’s Cradle, it’s not just simple souls who feel comforted by foma.
DeWitt:
There is a significant difference between those two cases:
– I can imagine someone being an expert in organic chemistry, but compartmentalizing his thinking on purely technical matters away from his view of God, life and so on.
– However, I believe the principles of thermodynamics apply to the chemistry of batteries.
– I cannot imagine someone with a full knowledge of a technical field compartmentalizing knowledge of two related areas away from each other. A mistake, yes – but if someone points out a technical error and yet it is not addressed, that is not simply a mistake. At some point, it becomes a matter of scientific integrity.
Neal,
Then you don’t have a very good imagination.
Scientific integrity? Oh, puhleeze. For one thing, I don’t know if anyone has pointed out his mistakes. I haven’t. I have better things to do and there’s no obvious way to comment. For another, he has just posted it on his web page. He hasn’t tried to get it published.
Actually, while he’s wrong about some key things, it’s not a bad attempt for an amateur. Electrochemical kinetics is not something that’s trivially obvious. Of course, if he’s a physicist by training, he thinks he knows everything he needs to know. Luboš Motl being the poster child for this attitude.
Speaking of people who think they know everything important, I read the post We need a better class of climate “skeptic” at …and Then There’s Physics. The irony is so thick in the comments, you would need a chainsaw to cut it, It just calls out for an article titled We need a better class of “warmers,” people who don’t think, for example, that John Cook and Lewandowsky can walk on water.
– I guess I expect more of people than you do; although if no one’s given Denker any feedback, then there’s no blame.
– Personally, I’m not aware of anyone who thinks John Cook or Lewandowsky walk on water. I am aware of certain dismissives who think he dresses up in Nazi gear – which is equally ridiculous.
Neal,
The walk on water thing was hyperbole. That should have been obvious even without a sarc tag.
Cook’s 97% paper is only fit to line birdcages or wrap fish guts, not to mention the possible ethical problems of the conduct of the research. Of course all the usual suspects at …and Then… probably worked on it, so they’re not exactly unbiased. Still….
Lewandowsky’s Recursive Fury was, if anything, worse.
I may have that backwards. The research ethical problems were Lewandowsky’s, not Cook et.al.
DeWitt:
– No doubt you would find it equally humorous if I were to point out how the participants at The Blackboard seem to believe Lucia walks on air.
– The 2013 study by Cook et al. was simply an attempt to sample as large as possible a collection of peer-reviewed scientific papers concerning climate change. It was not an attempt to test the judges (as Tol seems to think), but to arrive at a fair assessment of the papers.
It’s worth pointing out that access and user interface were available from day 1 to allow and encourage anyone to do their own evaluations on the same collection of abstracts. The only effort I’ve heard of on that matter was by Brandon S.; and the last I heard of that, he was finding that his team of evaluators tended to come up with slightly more CC-supportive ratings than Cook’s team.
– wrt fish wrapping: You’re entitled to you own opinion; but that would be a little more impressive if you were to distinguish clearly between different people.
DeWitt wrote: “Speaking of people who think they know everything important, I read the post We need a better class of climate “skeptic” at …and Then There’s Physics. The irony is so thick in the comments, you would need a chainsaw to cut it, It just calls out for an article titled We need a better class of “warmers,””
While non-scientific activists on both sides will believe whatever they want to believe; it takes a lot more thinking, effort and knowledge for scientists to question the scientific consensus than to accept it. This is particularly when people like Bryan and Sky Dragons make scientific criticism of the consensus a joke. So I believe a “better class of climate skeptics” than “consensus supporters” already existsI It’s just that people like ATTP want pretend Bryan is representative of scientific skepticism.
I often wonder if the pressure of dealing with the Bryan’s of the blogosphere have changed our host. One of his first posts was this one:
The first was this:
While his posts still question the accuracy and limitations of climate science, the posts no longer discuss whether the problems he finds pose a real challenge the consensus. For course, it is possible that 5 years of blogging have convinced him no serious challenges exists.
DeWitt Payne and Frank,
Since there appears to be a discussion about one of my blog posts, I’ll leave a brief comment. Firstly, it was just a blog post. I was ticked off by some of the exchanges I’d had recently and wrote a post illustrating my frustration. If you actually read the post you might notice that most/all of the examples were views that were almost certainly wrong. In my view there are certainly people out there who have high profiles but promote views that are very obviously scientifically incorrect. When this is pointed out they rarely (if ever) acknowledge the error, which either indicates that they don’t understand enough to know they’re in error or they are unwilling to acknowledge the error.
As far as I’ve seen, this is a topic where few people understand the meaning of the term irony. This isn’t to suggest that there weren’t ironic comments on the thread, just that it seems so prevalent in this topic that I would be surprised if my comment thread was particularly extreme in this regard.
Not at all. If you’d read the last paragraph you’d have noted that I mention that there are indeed people who are skeptical and worth having discussions with. One problem is that “skeptic” now means someone who is dubious of mainstream climate science. I should probably have used the word “—-er” [moderator’s note, please check the Etiquette] rather than skeptic, but if I do that then people complain about name calling.
What’s possibly more interesting is why you appear to have identified with those who – in my view – are very obviously wrong. I’m not critical in any way of those who question and probe mainstream climate science. That’s an extremely healthy thing to do (I would argue that I do the same myself at times, although some could never bring themselves to acknowledge that). I’m critical of those who have high-profiles and yet promote views that are almost certainly wrong. I would have thought we’d all agree that that is not the correct way to be skeptical, but YMMV.
Neal,
Ask those people that ‘take the issue more seriously’ how much they would personally spend every year for the rest of their life to stabilize atmospheric CO2. It’s not much. The problem is political and economic, not scientific. Just because WG-I gets the science right doesn’t mean that WG-II and WG-III are equally reliable. Far from it, in fact. Public opinion polls have shown for years that the majority of people believe the science, but doing something about it is not a high priority for them in part because there’s no consensus about what to do other than hand waving about reducing fossil fuel use.
If the warmers would spend more time on developing actual plans that make economic sense rather than arguing the science with the fringe that the public largely doesn’t even know exists, they might actually make progress. It’s not the Koch brothers or some shadowy conspiracy that keep action from being taken,
And then there’s the people who insist we need to eat less meat to minimize climate change. My bet is that sort of thing causes more damage to your cause than the Koch brothers ever could.
DeWitt:
– Ask those people that ‘take the issue more seriously’ how much they would personally spend every year for the rest of their life to stabilize atmospheric CO2. It’s not much.
At the time that Social Security was passed, I’m sure that polling would have set a low ceiling on people’s willingness to pay into the system; some idea can be gathered from the fact that the ceiling on income considered was $3,000/year, and the contribution rate was 1%, so that maxes out at $30/year. Nowadays, the income ceiling is $106,800/year and the rate is 5.3%, implying a maximum of $5,660.40/year. Nonetheless, many older people are happy they contributed.
– The problem is political and economic, not scientific. Just because WG-I gets the science right doesn’t mean that WG-II and WG-III are equally reliable. Far from it, in fact. Public opinion polls have shown for years that the majority of people believe the science, …
As long as people believe there is doubt as to how serious and urgent the issue is, the less willingness they have to prioritize policy changes: and that was exactly the point that Frank Luntz identified for George W. Bush in 2002: “The scientific debate is closing [against us] but not yet closed. There is still a window of opportunity to challenge the science…. Voters believe that there is no consensus about global warming within the scientific community. Should the public come to believe that the scientific issues are settled, their views about global warming will change accordingly. Therefore, you need to continue to make the lack of scientific certainty a primary issue in the debate, and defer to scientists and other experts in the field.” The GOP took that message to heart, and none of the plausible leaders in that party will touch climate change with a 10-foot pole.
– … but doing something about it is not a high priority for them in part because there’s no consensus about what to do other than hand waving about reducing fossil fuel use.
In fact, in Australia and Canada, the governments have retracted CO2 trading schemes that were supported by the majority of voters or arranged for administrative approval on fracking transport that was opposed by the majority of voters. These anti-AGW policies and institutions were supported but not prioritized; and that is consistent with what Luntz was promoting: Keep the impacts fuzzy, and people won’t decide a vote based on them. Tests have been done that show that when people hear that there is a 97% consensus among relevant scientists: a) The Democrats are surprised that it is that large; and b) the Republicans start to get worried for the first time.
– If the warmers would spend more time on developing actual plans that make economic sense rather than arguing the science with the fringe that the public largely doesn’t even know exists, they might actually make progress. It’s not the Koch brothers or some shadowy conspiracy that keep action from being taken,
As shown above, when the governments hear the call of oil, they revert the actions that have already been taken. And as one Canadian friend, involved in the oil industry, put it: “Most of the bad guys in Canada are not shady, but brazenly out there in the open, in government and in lobbying groups like CAPP, the Canadian Association of Petroleum Producers. Things are so bad that regulatory capture has advanced to such a degree that the Alberta Government appointed an ex-CAPP lobbyist Gerry Protti, was appointed to head the provincial regulatory board. This was an appointment that left me speechless.” He happens to know Protti well; but more on him can be found at: http://www.desmog.ca/2013/05/04/new-alberta-energy-regulator-gerry-protti-alberta-oil-lobby-golden-goose
– And then there’s the people who insist we need to eat less meat to minimize climate change. My bet is that sort of thing causes more damage to your cause than the Koch brothers ever could.
I don’t see this as anything to worry about. In any case, as agriculture suffers from climate change, the cost of staple grains will increase, and the cost of meat will grow proportionately (or perhaps disproportionately, given the need for water). [As I’m sure you’re aware, studies of the agricultural impact of climate change that go beyond “CO2 is good for plants!” generally find a net negative effect on global agriculture.]
Neal
“1) Explicit endorsement with quantification: explicitly stating humans are the primary cause of recent global warming;
2) Explicit endorsement without quantification: explicitly states humans are causing global warming; or referring to anthropogenic global warming as a known fact;
3) Implicit endorsement: implying that humans are causing global warming; e.g. the research assumes GHG emissions cause warming without explicitly stating humans are the cause;”
If 97% of scientists agree in this. What so? It does not tell that they agree on more specific knowledge. Most sceptics agree that there have been some emission that have caused some warming.
When this is used in propaganda, it is interpreted as there is consensus that humans are causing most of the warming. When you interpret it in that way the consensus actually drop from 97 to 16 % as far as I can remember from the data of Crook.
Nobody’ssnows:
Item 3) “humans are causing” means that humans are held primarily responsible: If an elephant and a mouse cross a bridge together, and the bridge fails, we don’t blame the mouse.
For contrast, consider items 5 – 7), emphasis added:
5) Implicit rejection: implying humans have had minimal impact on global warming without saying so explicitly; e.g. proposing that a natural mechanism is the main cause of global warming;
6) Explicit rejection without quantification: explicitly minimizing or rejecting that humans are causing global warming;
7) Explicit rejection with quantification: explicitly stating that humans are causing less than half of global warming.
The sceptics’ views fit into 5 – 7, because they don’t see humans as the main cause: and they are the 3%.
You mismembered wrong.
Oh sorry. It was Cook.
Excuse me, I meant “Nobodyknows”.
Most scientists actually fell into this category:
4a) No position: not mentioning or addressing the cause of global warming;
This is a New invention in statistics. To kick out everybody who “don`t know”.
Very common in polling data. Sometimes they try and push the don’t knows, but mostly they don’t
Nobodyknows:
– Among the abstracts, this was the majority. But it’s usually not a question of people not knowing: It’s an issue of article “real estate”: Just as the vast majority of peer-reviewed articles on geography and geology no longer bother to mention that the Earth is round, the vast majority of articles on climate change do not waste the precious wordage of the abstract to state the obvious.
– When the authors of these 20,000 papers were sought out for their own opinion of what their entire paper was meant to convey, a very high percentage of them indeed said that these papers should be interpreted as falling into categories 1 – 3); in fact, 98.4%. That again supports the interpretation that the consensus on AGW is about 97%, but not everybody wants to take up space in the abstract with that.
Correction of my posting of September 1, 2014 at 3:29 am | :
– It wasn’t 20,000 papers, but rather 11,944 ~ 12,000; which I misremembered.
Clarification:
– All lead-authors of the 11,944 papers were sought for their own opinion of how the paper should be categorized; of these 8,547 had plausible email addresses.
– Of these, 14% (1,200) actually responded. After some process loss (papers without abstracts, etc.), 2,142 papers received self-ratings from 1,189 authors.
– The result is that 98.4% of papers by responding authors were claimed (by the authors) to fit into one of the categories 1 – 3).
– Why is this more than 97%? 64.6% of the papers that were both a) categorized as “no AGW position in the abstract” and b) represented by responding authors were claimed by them as being supportive of the consensus. Basically, 2/3 of the “abstract neutrals” weren’t “paper neutrals” but rather “paper supportive”.
– The corresponding % of such papers that were represented by responding authors as being against the consensus was 2.4%.
– This justifies the point that non-statement in the abstract cannot be taken as an indication for, or against, or even actually neutral regarding support AGW: It just means that you have to read the paper.
Here is a little thought experiment.
Take infinite plates and place them opposite each other.
Both emit radiation. The amount and spectral distribution of the radiation matches the blackbody spectrum. They are at T2 > T1
Is the radiation from both of them heat? or only that from the hotter one? Is the radiation simply radiation and heat is only appropriate to describe the net radiative transfer of energy? .
Now here is a twist. Let the colder body absorb in the IR and be perfectly reflecting in the visible. Let the hotter body be perfectly absorptive across the spectrum. Let T2 be ~5000K so essentially all of its emission is in the visible, none of which is absorbed by the colder body. In this case the flow of heat will be from the colder to the warmer body.
Sorry Eli, but no. If T2 > T1, the blackbody emissions from T2 are greater than T1 at ALL frequencies. So even if only a tiny fraction of the emissions from the blackbody plate at T2 are in the IR where the other plate absorbs and emits, it will transmit more radiative power to the colder plate than the colder plate transmits to it, so the resulting heat transfer is from hot to cold.
The fact that blackbody emissions of a higher temperature are greater than those of a lower temperature at all frequencies, combined with the fact that for any object, absorption equals emissivity at all frequencies (and if you get really technical, at all angles), means that you cannot contrive a scenario as you tried where the net heat transfer is from cold to hot.
The 2nd Law is a tough nut to crack…
Yes, the emissions from T2 will be higher than that from T1, but none of that will be absorbed (for 2 dim plates, it will be reflected back to T2 which will heat even further. . .) but the hard nut here is that the NET radiative energy transfer, and thus the heat transfer will be from T1 to T2
Eli,
Now I don’t understand you at all. What Curt wrote is right. Having followed your writing I have come to suspect that you have something clever hidden in the message, when it appears as erroneous, but this time I have no idea of what that could be.
Perhaps you are playing Bryan, but if you are then, how can those, who read your comments know that?
Eli, take a step back and consider the blackbody emission curve as a function of wavelength for different temperatures. The curve is higher for the greater temperature body at EACH and EVERY frequency across the entire spectrum.
So even if your second plate has a temperature T2 that puts the overwhelming majority of its emissions in the visible spectrum, its emissions in the infrared will still be greater than that of the other plate at T1.
So in your simple example, in the visible portion of the spectrum, the plate at T2 will emit, the plate at T1 will reflect, and the plate at T2 will absorb the reflected radiation. No heat transfer here. (The plate at T1 emits none of its own.)
In the IR portion of the spectrum, the plate at T2 emits radiation that is absorbed by the plate at T1. The plate at T1 emits a lesser amount of radiation that is absorbed by the plate at T2. Net heat transfer from the higher T2 to the lower T1.
Summing together, the resulting heat transfer is from T2 to T1.
Some time ago, I spent some time trying to find “loopholes” in the 2nd Law with examples like this. Finally I realized that because the blackbody curve for a higher temperature is above that for any lower temperature at EVERY frequency, and that absorption equals emissivity at EVERY frequency for any object, that net energy transfer is from higher to lower temperature at EVERY frequency (or in the limit, zero), so the same must be true as you integrate over all frequencies.
It’s a fun thought experiment you posed. It was an example just like this that forced me to clarify my thinking and come to a deeper understanding of the subject.
Eli,
What are you on?
– It’s true that the T1 (lower temperature) plate will neither absorb nor emit in the visible.
– But in the IR, if it emits, it absorbs; and vv. In particular, if both T1 and T2 bodies are black in the IR, they both absorb all the IR that falls upon them; and they both emit to the full extent of the Planck distribution. But that means that T2 emits more IR power than does T1, and T1 will absorb more IR power than does T2.
– So net transfer will be from T2 to T1.
Eli,
Reflection is not heat transfer. It is insulation. Perfect reflection is perfect insulation, which is one reason why a perfect reflector cannot exist. If the plate at T1 were a perfect reflector at all wavelengths, then no power would flow into the plate at T2 once it reached 5000K, otherwise the temperature of T2 would increase without limit for any finite power input.
In your example of T2 ~5000K and T1 absorbing at wavelengths greater than, say 4 μm,, the power input to T2 would have to be very small because only a small fraction, not none, of the emitted radiation would be absorbed by the plate at T1. That fraction would only be identically zero if T1 = T2. In which case, it would require identically zero power to maintain T2 at 5000K. In no case with T2 > T1, would power need to flow into the plate at T1 and out of the plate at T2.
Frank wrote: If your consensus is only about TCR being above 0.7 degC, it doesn’t appear to be about anything useful for policymakers.
Neal replied: If you assume that Lady Luck is always going to be on your side, you can afford to take that approach. But there is no particular reason to ignore the higher end of the range in favor of the lower: We just don’t know. What is the probability that your house will burn down in a fire? Reasonably small, I think. But do you buy fire insurance? I suspect you do.
Frank continues: I think I have a reasonable handle on probability and Lady Luck, but analogies to insurance are of limited utility. Mortgage companies force you to pay for home owners insurance; but I would do so anyway because it doesn’t cost much for a lot of reliable protection. On the other hand, many people do without life insurance – and especially without disability insurance – because fully insuring against these risks is far more expensive. The sensible approach is to look at the risk and the cost/benefit ratio. The spread of possibilities is extremely large for climate change when you don’t know whether ECS is 2, 3 or 4 degC. Damage rises so sharply with ECS that most of the benefits come in the unlikely situation (IMO) that ECS is 4 degC.
Worst of all, governments control all aspects of providing insurance against high ECS and climate change. Their is little point in wallowing in cynicism about this subject at a website devoted to science.
The fact remains that the best experts we have have issued a range: If you know better, you should really set them straight; if you can’t, then your preference is simple bias, and you should recognize that.
Since you don’t want to wallow in the issue of governmental control, I don’t know why you dragged it in. But I agree: The scientific issues are logically independent and separable from the policy issues. It’s a pity people often can’t seem to consider them that way.
Neal
“The scientific issues are logically independent and separable from the policy issues. It’s a pity people often can’t seem to consider them that way.”
So why do you play the consensus card in this discussion?
Frank: “it doesn’t appear to be about anything useful for policymakers.”
I agree on that.
Nobodyknows:
So why do you play the consensus card in this discussion?
Because the fact of the consensus indicates that the scientific discussion on the matter has reached a high degree of stability. It means that the really sharp and ambitious folks – the guys who have or think they have a legitimate shot at a Nobel Prize – have concluded that there’s no promising line of inquiry in that direction – it’s a dead horse.
So when you have an indication that the discussion has reached a solid resting point on a matter that affects the future of the biosphere and, more narrowly, the future of the land-based and sea-based food supply, it takes a “thumbs in the ears” frame of mind to conclude that it has no implications for policy.
Or possibly just the attitude that you have no responsibility for your grandchildren.
Neal,
If you think that nuclear power is too dangerous to be used to reduce fossil fuel consumption, your first priority is not climate change. It’s the prevention of the expansion of nuclear power.
One can believe that the radiative transfer calculations are correct and still believe that the costs of using current, non-nuclear, technologies, i.e. wind and solar, to reduce fossil fuel consumption are too high compared to the benefits.
Then there’s the more fatalistic view that China, as well as India and Brazil, are going to continue to expand their consumption of fossil fuels by more than the rest of the developed world can possibly reduce theirs. So shooting ourselves in the foot by drastically increasing the cost of energy for us for no significant global benefit is rather pointless. The Germans, however, appear to be proceeding down that road. It will be interesting to see what happens when their manufacturing economy collapses as a result.
China, by the way, appears to have vast, untapped reserves of unconventional petroleum. So much for Peak Oil.
DeWitt:
– Nuclear power: I haven’t looked into the details of nuclear fission business in 30-odd years; at that time there was some concern as to whether the lifecycle expenses and energy costs of mining/constructing/operating/de-commissioning nuclear power plants gave a net positive for the plants. Mining subsidies and externalization of environmental damages distort the cost issues, and the Price-Anderson act complicates the insurance situation in the event of an incident.
– Subsidies and externalization also affect the cost comparisons between fossil fuels and renewables, although the trends are improving on the renewables side. However, intermittency of availability is certainly a major factor.
– I haven’t considered Brazil; however, my impression is that at the upper levels, the Chinese government is very aware that they face environmental problems, and in particular water supply; and they are aware that the loss of the Himalayan ice complicates their problems enormously. Unlike the politicians in the West, those in positions of power in China have every reason to expect to be there still, 20 years from now; so kicking the can down the road may not be as popular a resolution in the PRC. Being immediate descendents of revolutionaries, they are also keenly aware that there are issues which have to be dealt with, including water; or else there will be real trouble. There is often a disconnect between the stated priorities at the top and the implementation at the regional levels; if a regional official gets caught up in this, there is a chance he can suffer the punishment of extreme prejudice.
For these reasons, I think it is likely that the PRC will be willing to take steps if & when the US is willing. But if the US is not willing to take steps, China will not.
I think India has the same resource issues as China, and it would be reasonable to take the same strategy. They don’t have the same degree of discipline as China; but again, if the US is not willing to take steps, India will not.
So I think the “fatalistic” point of view you describe is actually suicidal: While the US cannot force other countries to take steps, the US can ensure that other countries will not take steps – simply by refusing to be among the leaders.
– Unconventional oil: China has, in principle, 3.54 * 10^8 kilobarrels of reserves of shale oil; which, at the consumption rate of 9.87 * 10^3 kilobarrels/day, represents 98 years of consumption. There does seem to be a problem: According to this article,
http://r-login.wordpress.com/remote-login.php?action=auth&host=qz.com&id=39587363&back=http%3A%2F%2Fqz.com%2F258776%2Fchina-has-more-shale-gas-than-any-other-country-but-getting-it-out-of-the-ground-could-be-disastrous%2F&h=
most of the shale oil is located in regions which are water-poor; and the operation of shale-oil extraction not merely consumes water but also poisons it for human utilization: drinking, agriculture, etc. So it’s possible that China’s vast untapped reserves of petroleum will stay vastly untapped.
But even if not: If you think the oil will last forever, you’ve forgotten about conservation of mass. In terms of global oil usage, China’s stash would last about 10 years. As long as we’re burning fossil fuels faster than the rate of primary production, Peak Oil is still on its way.
Neal,
Nice straw man. Very appropriate in this thread. More specifically, please cite where I said or I implied that I thought oil would last forever.
As to the lack of water: You don’t need a large, continuous supply of water for hydraulic fracturing. Pipe lines will be necessary to transport the oil produced. Build the pipe line and use it to move water to the drill site. You don’t need fresh water either. Sea water will do.
DeWitt:
If China is so comfortable with their domestic oil supply, why are they buying up all the oil in sight in the rest of the world?
Notify me of new posts via email.
Steven, there is a “Subscribe by Email” button on the right hand side of this blog. You need to click that and you will be notified of new posts.
A new reader has attempted to challenge the answer in this article – in comments on another article. Strictly speaking he has not provided an answer, just half an answer and claimed the equations I provided are nonsense because the only solution (to my equations) is Ta = 0.
Well, for those interested, the answers and my questions start here.
For many other readers who are sure the temperature inside a sphere can’t be higher than its outer surface – don’t you wonder why none of the people in agreement with you are able to write down just 2 simple equations (Ta and Tb)?
And why do people lag pipes if the inner temperature won’t increase?
“For many other readers who are sure the temperature inside a sphere can’t be higher than its outer surface – don’t you wonder why none of the people in agreement with you are able to write down just 2 simple equations (Ta and Tb)? And why do people lag pipes if the inner temperature won’t increase?”
Who is saying Ta can’t be higher than Tb? That’s a straw man argument. What people object to is your claim that Tb can warm Ta when Tb has the same temperature or lower temperature than Ta.
“And why do people lag pipes if the inner temperature won’t increase?”
This won’t work if people start out with 100% efficiency as your equations. An uninsulated pipe isn’t at its maximum temperature like your Ta. It is at some lesser temperature due to heat loss. The insulation brings the temperature closer to its maximum. To make Ta hotter than its maximum temperature, you need to add more power. Insulation does not add more power.
In fact, what insulation does is it prevents heat from escaping, so there is less power out. As result, there is also less power in. When Tb increases, P goes down in a pipe. A perfect insulator will cause the power to drop to zero. The water temperature is preserved as a result.
Your main assumption is wrong. Shell B can not warm shell A unless shell B is warmer. A basic law of thermodynamics. Here are the equations that don’t violate heat transfer rules:
Ta = 193.4K; Tb = 192.4K; Ts = 0.00K;
Aa =12.56m^2; Ab = 12.81m^2
P=1000W; P = Pab + Pbs (law of conservation)
This is the total power between Ta and Tb:
Pab = o((12.56m^2)193.3^4 – (12.81m^2)192.4^4) = 0.00W.
This is the total power between Tb and Ts:
Pbs = oAb(192.4^4 – 0) = 1000W.
Notice you have 1000W going in and out and no violations of heat transfer rules. So what has happened when shell B was added? It caused the area that was formerly occupied by space to warm up to 192.4K. When there was only shell A, the mean temperature was 193.4/2 = 96.7K. When shell B is added, it increased to (193.3 + 192.4)/2 = 192.85.
What happens in a greenhouse? Same thing. The ground warms the air when there is a glass shell around it, but the air does not warm the ground that is warmer than the air. Understand?
A minor point: Shell B is always cooler than shell A because shell B has a greater area. So shell B is not the same temperature as shell A.
I posted the same response in the other thread:
mikejacksonauthor,
Shell B radiates how much energy into space? Pbs (shell b to space)
Shell B radiates how much energy inwards? Pba (shell b to a)
I think you are claiming Pbs = 1000W.
Pba = ?
Total energy radiated by B, Pb = Pbs + Pba ?
I expect you will now invent a new law for emission of thermal radiation.
I wait. If you do respond and your answer is not Pba = σTb4 as found in all heat transfer textbooks you will need to provide evidence of your new law of radiation that doesn’t work from the argument from your personal incredulity.
If the answer is something like this:
“The two surfaces of a shell would be expected by crazy people (like Science of Doom) who don’t understand conservation of energy to have the same formula for emission of radiation. But, magically one shell knows it is facing inside and that another shell is radiating towards it, and then the easter bunny appears and then.. ” – it will make my day.
This is actually most people’s answer when they share your convictions. They just miss out the bit about the bunny and add some technical words. Same result.
After you have given your formula for emission of thermal radiation I might explain efficiency.
But given that you got the right answer originally (eventually) by using conservation of energy and the Stefan-Boltzmann law and then changed your answer 2.5 hours after your calculation revealed that the inner sphere got hotter there might just be no point.
If you are prepared to drive a wrecking ball through the Stefan-Boltzmann law then probably isn’t much hope of a satisfactory resolution.
I look forward to the answer on:
– Pb (total emission of radiation by shell B)
– Pba (emission of radiation from shell B inwards towards shell A)
[…] A Challenge for Bryan & A Challenge for Bryan – The Solution […]
Anyway, just to satisfy myself I worked out the real-world equilibrium temperatures of Ta and Tb. To do this, however, I had to give your magic power source a surface area of 12.31M^2.
When it’s the power source alone, 1000W (194.3K) is lost to space Ts (0.00K).
When you add shell A, it’s equilibrium temperature is 162.6K. Only 500W is lost to space and 500W is used to maintain Ta. 500W + 500W = 1000W.
When you add shell B, Ta increases to 174.7K. Tb = 146.14K. 333.3W is used to maintain Ta; 333.3W is used to maintain Tb; 333.4W is lost to Ts.
333.3 + 333.3 +333.4 = 1000W.
Note that Ta does increase like you said, but also note that its temperature never exceeds its power source, nor does the power ever exceed 1000W.
To solve this problem I simply divided the total power by the number of shells and Ts (space). That provides the value of each power drop. The sum of the power drops should equal 1000W. The power drops should be equal to each other so each shell receives the same power in as power out to maintain equilibrium.
For other readers interested, considerable pointless discussion took place in the comments on another thread, finishing here:
mikejacksonauthor
SoD is quite correct in saying that two way radiative exchange between two objects (facilitated by photons) is the current best understanding of heat transfer by radiation.
This involved the simple emission and absorption of the photons without any special exclusion (thought erroneously due to the second law OT).
However the orthodox physics interpretation of photon two way flow is quite consistent with your main point which I think is correct and where SoD is entirely wrong.
Your main point is (as I understand it) that cold objects do not spontaneously ‘heat up’ or increase the temperature of a warmer body as SoD implies.
You ask for an experiment to settle the matter and here is one that should prove the point once and for all.
As SoD says draw a boundary round the two objects to prevent heat from entering or escaping from the two objects under study.
This boundary is called an adiabatic enclosure.
A polystyrene box (such as used to transport bulk chemicals) with the internal face lined with highly reflective aluminium is a practical solution
So two metal blocks A and B sit separated inside the vacuum filled near adiabatic enclosure.
This will be adequate for the purposes of the experiment.
Temperature probes will further slightly reduce the perfect adiabatic condition
(A perfect Adiabatic enclosure would consist of a perfect reflector face surrounded by a perfect insulator)
Initially both blocks are at the same temperature. The zeroth law of thermodynamics applies.
Both emit and absorb equal amounts of radiation.
Neither one is said to heat the other.
One block (A) has a power supply which is now switched on causing the temperature of the block to rise.
This in turn means that it will emit more radiation.
A will now heat B causing its temperature to rise also.
B will in turn emit more radiation but this back radiation is caused by A.
Now comes the clincher
If B were not there at all, the temperature of A would be even higher.
So B cannot be said in any meaning of the word as a cause of heating A
Bryan,
Your description and conclusions are correct for the setup that you describe. Everything SoD has written here is consistent with that.
There’s another setup that leads to a different conclusion, just because the setup is different. In this second setup no enclosure is present, only the two bodies and open space. In this setup A and B cool indefinitely in absence of the power supply. Thus we must have the power supply on all the time to have a stationary state at nonzero temperature. As only the body A has a power supply, B is heated only by the radiation from A, but cooled by radiation to all directions. Only a small fraction of the radiation emitted by A hits B and vice versa. Now the body B will be colder than A, but not at zero temperature and some radiation from B will get absorbed by A. A is heated by both the power source and the little radiation from B that it absorbs. As A is heated more, it’s equilibrium temperature is slightly higher than in absence of B. That addition in the temperature of A is, what is meant, when it’s said that A is warmed by the presence of the colder body B.
The case of a spherical body A surrounded by the shell B is a modification of this second case. In this modification all radiation emitted by A is absorbed by B, but less than half of the radiation emitted by B is absorbed by A. Half of the radiation emitted by B goes into the open space, the other half is either absorbed by A or by B itself at another point. If the shell is close to the surface of a large sphere, the share absorbed by A is very close to half of the total emission of B.
Bryan,
One more point about your setup.
In that case both bodies would keep on warming as long as the power source is on. A would be warmer all the time, but B would heat up as well. No stationary temperatures will be reached before the power supply is turned off. After that the two bodies would move towards a new common temperature between the temperatures of the bodies at the time the power is turned off.
Pekka
Its important to get the fundamentals right
If the two objects are isolated from the rest of the universe (as in my example above) then the direction of heat flow is unambiguous.
Any heating or warming of A is as a result of the power source in A and the surrounding insulating conditions and nothing else.
At school we were to told that if the outside door is left open its not correct to say that this causes the cold to get in.
Rather we should say that you well let the heat out.
Climate science tends to stretch language beyond the point of reason by suggesting that an insulator is an active heat source.
This leaves the poor reader with the complete loss of orientation and results in bewilderment.
The presence of some other material body may act as an insulator.
For instance radiatively active gases such as CO2 will back radiate towards the source and other directions.
Extra CO2 will increase this radiative insulating effect.
That is it decreases the heat flow and increases the temperature of a powered source
Its a reasonable question to ask if this effect will be significant.
Perhaps it will turn out to be negligible as G&T indicate.
Given the many complex methods of heat flow and climatic variables the only way to determine this is by physical measurement of the near surface air and sea and above the troposphere etc for an extended period of time.
If this is done honestly and with competence then conclusions can be drawn.
Its also reasonable to say that the present climate models (arrived at by complex calculations) may be so inaccurate as to be useless in predicting future climatic temperature trends.
Its all down to measurement without prejudging the outcome.
The present pause in near surface climate temperature rise must surely raise some doubts in any open minded observer
“If B were not there at all, the temperature of A would be even higher.
So B cannot be said in any meaning of the word as a cause of heating A”
That’s interesting. And it makes sense, since B is not acting as an insulator but an absorber of A’s energy.
Mike ‘hell yes,
…… and to raise the actual temperature of the Earth by one degree Celsius is no trivial thing.
One aspect of Climate ‘Science’ almost entirely ignored is the heat capacity of materials and the energy involved in its change of temperature.
The steel greenhouse model is supposed to have some relevance to the greenhouse effect on Earth.
SoDs analysis here is a straight ‘lift’ from Willis’s Steel Greenhouse so they both can be considered together.
Factor in real temperature rise after heat absorption and you get an entirely different result
Most people on seeing it for the first time think it defies common sense and hence there must be something wrong with it.
To get it to ‘fly’ you must abandon common sense and make a number of unphysical assumptions.
We will do this but also point out the gross departure from reality on occasion.
The core or planet is heated by a constant heat source of 240W/m2.
A constant heat source in reality is very hard to supply .
That will for example work away through all changes in temperature.
The planet (core) and the shell are superconducting so that each has its own isothermal temperature.
This is to satisfy the requirement for central core heating to reach the surface.
In reality a central heating core through rock would take millions of years to reach the surface.
One model suggests millions of tiny nuclear reactors scattered evenly through the core but we will stick with whichever methods achieves an isothermal core.
The shell also needs to be isothermal for simplicity so that both inner and outer radiate equally.
A metal core and shell both have emissivity values of 1 despite metals having values nearer zero in the infrared but it simplifies the maths and helps the alarmist narrative.
The shell is almost touching the surface to maximize the radiative power/area from the shell.
The input energy = P x t where P = 240w/m2 and t is time in seconds
All other units are from the MKS system and have largely been omitted for clarity
At all the interfaces net energy flow(heat) is from higher to lower temperature.
Output energy = dU1 + dU2 + Radiative losses to space from outer shell surface
U1 = gain in internal energy of core = Cc.Mc.dTc
U2 = gain in internal energy of shell = Cs.Ms.dTs
Cc = specific heat capacity of planet (core) = 2000 (value for rock)
Cs = specific heat capacity of planet (core) = 2000 (value for rock)
Mc = mass of Earth = 6 x 10^24
Ms = mass of shell ( lets make it one thousandth the Earth mass )
dT = delta T is temperature change.
Radiative losses will occur as temperature rises from zero to final temperature .
The instantaneous value is proportional to the temperature raised to the fourth power.
Readers can supply their own values of specific heat ( for example water = 4200 ) but I don’t think it will make a lot of difference.
For temperature change in the first instance we will choose one degree Kelvin.
This modest value allows us to leave the radiative value of outer core as minimum to be calculated later if required.
This gets the model into a rough scale of magnitude.
Input energy for whole planet surface = 240.A.t
A = area of Earth Surface = 5.1 x 10^14
t = time in seconds = bit we are trying to find out
U1 = gain in internal energy of core = Cc.Mc.dTc = 2000 x 6 x10^24
U2 = gain in internal energy of shell = Cs.Ms.dTs = 2000 x 6 x 10^21
Time this all takes in seconds = energy output/power input = 12.002 x 10 ^27/ 1.224 x 10 ^17
Time in seconds = 9.8 x 10 ^10
Time years = 3107 years for a one Kelvin temperature rise.
Keen readers might want to go on and add radiative losses from the outer shell surface but that will only increase the time beyond 3107 years.
This is well beyond anything that can be determined by experiment.
A bit like saying if you empty a bucket of hot water in the Ocean then the Ocean temperature will rise
There is a wise guideline in science that tells us that theories that cannot be supported by experiment cannot be justified.
Its enough to say that if a constant power source is acting then further insulation around the source is added then a temperature rise will occur.
Why would this model be pushed to support a conjecture that a tiny rise in the atmospheric CO2 fraction will push the Earth into dangerous climate territory?
Now of late the average air temperature has failed to move significantly for almost 20 years.
Climate alarmists now want to abandon air temperature as unreliable(AKA unhelpful) and now want to focus on sea temperature where 0.01K either way makes a huge difference.
Now sea temperature measurements of that accuracy do not exist however alarmists can speculate endlessly as usual
Mike Part 2
My initial idea was to split the problem into two parts.
With the core(planet)_ and shell in place from the start;
The first was with the heater to start at zero Kelvin and find out how long it takes to increase by one degree Kelvin the radiation from the outer shell face could be ignored for simplification
Answer 3107 years see post above
Once the temperature rises the radiative losses to space rise very considerably
My next part would be to repeat the calculation at 250K for shell i.e near its new Equilibrium
Input energy for one second = P . A. t = 1.224 x 10^17 Joules (specified at start)
Near equilibrium, core temperature = 1.19 shell temperature.
Output energy = dU1 + dU2 + Radiative losses to space from outer shell surface
U1 = gain in internal energy of core = Cc.Mc.(298 – x)
U2 = gain in internal energy of shell = Cs.Ms.(250 – y)
Cc = specific heat capacity of planet (core) = 2000 (value for rock)
Cs = specific heat capacity of planet (core) = 2000 (value for rock)
Mc = mass of Earth = 6 x 10^24
Ms = mass of shell ( lets make it one thousandth the Earth mass )
dT = delta T is temperature change.
Radiative losses to space from outer shell surface = A . # . T ^4
A = surface area of Earth
# = SB constant
Radiative losses = 1.13 x10^17 Joules/s
x = 1.19y
Solve for x and y
Result. My 12 figure calculator could not find any MEASURABLE increase in temperature of Shell and Core
Conclusion
If specific heat capacities are included the Willis Steel Greenhouse model never reaches anything like the temperatures claimed even if you could wait around for the age of the universe
Why did SoD, Willis and other presenters of similar models fail to include heat capacity?
Bryan, you ask:
“Why did SoD, Willis and other presenters of similar models fail to include heat capacity?”
The fundamental reason is that thermal capacitance is irrelevant to steady-state analysis. In these types of analyses, you virtually always start with the steady-state (static) case, because the dynamic case will always tend toward the static.
You try to throw some “real-world” numbers at the problem, but you get one parameter horribly wrong, leading to ridiculous results.
That ridiculously wrong parameter is a thermal resistance of zero (superconductivity), which means that the entire mass of the earth would be affected. With real-world thermal resistance values, only a very thin outer layer of the earth would be affected by these changes.
Curt
It would appear that a finite heat capacity and superconductivity are mutually incompatible.
Yet in SoDs graphs above a heat capacity is included but little in the way of equations to explain its introduction and consequences.
One point (as an aside) about the graphs, shows that near equilibrium a small change in input power will take a very long time to produce an observable temperature effect.
However SoDs & Willis models are not planet models at all
They are model Suns.
A central heated core supplies the energy.
No diurnal or equator to pole temperature difference.
If there is heat capacity there must be a drop in temperature from the core centre out
So Chandrasekhar’s equations can be used with confidence as they are based on the thermodynamics of Solar emissions or so we are informed on page 50 of Gerhard Gerlich and Ralf D. Tscheuschner.
arxiv.org/pdf/0707.1161
However G&T question whether these equations can be used at low temperatures
Bryan has his own page and this is why.
For new readers who somehow arrived here.
1. Many basic heat transfer problems ask for steady state solutions (and often in the early part of a heat transfer course these class of problems dominate) even though the “steady state solution” always takes infinite time to be reached.
Why? The steady state solution is where the final result will be. The practical question of how long to reach, say 95% or 99% or 99.99% of the final temperatures, depends on many factors, mainly heat capacity.
2. Problems like this that are very unlike the real world are often used to demonstrate a simple outcome.
In this case, the teaching value of this problem should be clear – taking the very simplest example of a principle makes it easier to demonstrate the key point than in a problem which is more “real world”.
Very many readers are convinced that the answer presented here is wrong, but cannot come up with the right answer. Bryan himself could not, or would not, present the correct solution when challenged in the previous article to do so.
The more “real world” factors we add, the harder it is to demonstrate the key point.
3. In this problem I added some heat capacity values just to show the dynamic solution. This was done for educational purposes. It allowed me to show why and where the energy was stored during the early stages. The stored energy increased the temperature of the inner shell, leading to all the outcomes we see.
It is a teaching model – that is all.
4. The real climate has many more complexities. It was necessary to reduce the problem to this massive over-simplification example for people like Bryan.
Luckily for Bryan, he can point out the massive over-simplification and thereby demonstrate his mastery of the subject.
Only an idiot would reduce a climate problem to the point where is no atmosphere and no convection. [Or someone trying to prove a simple point to..]
5. The real climate has something important in common with this model.
Solar radiation heats the surface of the planet, through the mostly transparent atmosphere (solar radiation is 0-4μm). But the atmosphere is not transparent to terrestrial radiation (terrestrial radiation is 4-100+μ), so the radiation out to space from the climate system comes mostly from the atmosphere. The atmosphere is colder than the surface.
The important difference in atmospheric transparency/opacity is what makes the climate a little like this problem – the climate is heated “from inside” by the sun, but does not radiate to space from the same point.
More on this in The “Greenhouse” Effect Explained in Simple Terms.
SoD says
“3. In this problem I added some heat capacity values just to show the dynamic solution. This was done for educational purposes. It allowed me to show why and where the energy was stored during the early stages. The stored energy increased the temperature of the inner shell, leading to all the outcomes we see.”
However heat capacity was not mentioned in your ‘problem’ addressed to me as anyone can check.
In fact I am the one who has included it in my reply as unavoidable, we have yet to see any equation from you with a heat capacity included.
DeWitt Payne gave a link to the Willis model and I agreed with the conclusions based on the assumptions and said so early on.
For some unknown reason you wanted me to copy out the Willis solution here.
Now the fact that you have done so and are not embarrassed to claim this as your own work I will leave to your own conscience .
I would on the other hand regard this as plagiarism and instead give a link to the article in the same way as DeWitt Payne.
Why didn’t you simply give a link to the Willis article and ask if I agreed with it?
This whole episode started when I proved that your analysis of the Gerlich &Tscheuschner. paper was little more than ascribing them false opinions and then shooting them down before moving on swiftly to deal with Miskolczi.
What a fascinating discussion! In my physics class we did an experiment similar to yours where a 100 Watt light bulb was enclosed in glass container and the glass container was enclosed in a larger glass container covered with foil. The temperature of each of these items was measured and recorded. The light bulb did warm up when enclosed in the first container and warmed up some more when the second container was added. Its temperature stayed within its 100 Watt capacity and was warmer than the first container. The first container was was warmer than the second.
Is it possible for one or both of the containers to be hotter than the light bulb? Particularly the inner container, since it was insulated by the outer one.
I also noticed in your article above that you have energy in = energy out. How is this accomplished if the outer surface is colder than the source?
Thanks in advance.
PhysicsStudent asked: “Is it possible for one or both of the containers to be hotter than the light bulb? Particularly the inner container, since it was insulated by the outer one.”
Not as you have described your experiment. If energy is transferred (heat) from the light bulb to the first container and the first container to the second container and the second container to the room; the 2LoT demands that each transfer must be from hotter to colder. (We know that such energy transfer must have occurred, because 100 W of electric power was flowing into the tungsten filament of the light bulb and through each container. They would melt if they couldn’t radiate away the energy they received.) Unfortunately, your classroom receives energy from a variety of sources (light bulbs, the school’s furnace, the sun, your bodies) and the walls of your classroom are radiating about 450 W/m2 towards everything in the room. So our host simplified the problem in this post by eliminating the classroom and imagined doing the experiment in space at 0 K.
PhysicsStudent asked: “I also noticed in your article above that you have energy in = energy out. How is this accomplished if the outer surface is colder than the source?”
Our host’s problem specified that the components of the experiment had reached “equilibrium”, a term that means different things in different fields. Wikipedia lists several dozen types of equilibrium. Here it means radiative equilibrium, a “steady state” where none of the components is changing temperature because their energy in is equal to their energy out. You may be thinking of “thermal equilibrium”, where heat transfer ceases because all components are at the same temperature.
Energy in = energy out isn’t “accomplished”. Objects that emit more energy than they receive gradually cool – until they emit just as much as they receive.
Objects that emit less energy than they receive gradually warm – until they emit just as much as they receive. The amount of energy an object emits depends on its temperature and surface area (inside and out). The amount of energy an object receives from other objects depends on their temperature and geometric relationship. (A surface parallel to the sun’s rays receives no energy, while a surface at 30 degC receives half as much as one perpendicular. Unlike your classroom experiment, our host designed his problem to avoid these geometric complications.)
One last comment
The Willis metal greenhouse model and similar models like SoDs here start, like most tricks, with a simple almost throwaway remark
“The energy supply to the planet core does not mater ‘lets say its nuclear’.”
Sounds innocent enough doesn’t it ?
Hell no!
By this remark they say the second law does not matter.
Energy has quality (2nd law) as well as quantity (1st law)
A simple AAA 1.5 volt battery contains much less energy,(or internal energy) than say a litre of water at 90 degrees C
Yet by attaching the battery to a circuit with a tungsten MES lamp temperatures of 3000C are easily achieved
Try getting the litre of water to spontaneously transform itself any higher than 90C – its impossible
Electrical energy in the battery is highest quality and has no problem in being transferred into other energy forms.
Thermal energy like the litre of hot water is low quality and the transfer into other more useful forms is strictly regulated by the second LOT.
So start off your model with a more thermodynamically possible energy supply like a controllable massive electrical source.
If instead the supply to the core is an unlimited thermal quantity at 194K the Willis steel greenhouse will not get any warmer.
Its notable that the chemists who post here like DeWitt, Frank and Josh Halpern have a difficulty with the second law.
Some go as far as wanting the word ‘heat’ abolished.
I put it down to their thermodynamics curriculum being over ‘simplified’ for them.
Pekka and nealjking being physicists on the other hand do not seem to have any problem with the technical language of thermodynamics
Another pathetic comment from Bryan – no wonder SoD banned you!
When a problem states that there is a constant 1000W supply (or some such), the automatic implication is that the supply is capable of providing this power to the system. It would make no sense otherwise.
When someone says that it doesn’t matter what is providing this power, it simply means that there are many possible sources capable of doing this. (It is folks like you that make simple contracts 20 pages long, with paragraph after paragraph required to prevent any possible misinterpretation of an obvious point.
When has anyone here even suggested that a lower temperature source could provide this kind of power to a system?
And it is really rich that you, who has demonstrated that he cannot solve even the simplest problems from an introductory thermodynamics class, has the unmitigated gall to state that folks like DeWitt “have a difficulty with the second law”.
On the avoidance of the word “heat”, you should consider the words of Oxford professor Peter Atkins, in his excellent book “Four Laws That Drive the Universe”:
“In everyday language, heat is both a noun and a verb. Heat flows; we heat. In thermodynamics heat is not an entity or even a form of energy: heat is a mode of transfer of energy. It is not a form of energy, or a fluid of some kind, or anything of any kind. Heat is the transfer of energy by virtue of a temperature difference. Heat is the name of a process, not the name of an entity.
Everyday discourse would be stultified if we were to insist on the precise use of the word heat, for it is enormously convenient to speak of heat flowing from here to there, and to speak of heating an object. The first of these everyday usages was motivated by the view that heat is an actual fluid that flows between objects at different temperatures, and this powerful imagery is embedded indelibly in our language. Indeed, there are many aspects of the migration of energy down temperature gradients that are fruitfully treated mathematically by regarding heat as the flow of a massless (“imponderable”) fluid. But that is essentially a coincidence, it is not an indicator that heat is actually a fluid any more than the spread of consumer choice in a population, which can also be treated by similar equations, is a tangible fluid.”
Well Curt it seems you have had problems in the past with contracts.
I’m not surprised
SoD says of his power source type “but it doesn’t matter)”
This thread is full of comments by folk like yourself who either don’t have clue about the second law or find it inconvenient W.R.T the fictitious greenhouse effect.
So a little more exactitude does not go amiss.
You quote extensively from Peter Atkins for some reason.
I agree with him and indeed have used his books in the past!
What point were you trying to make?
Peter was not talking about abandoning the word ‘heat’ in competent thermodynamics discourse (which I think is the aim of this site).
He was talking about the general public use of the word.
Many people think a whale is a fish but if a biology lecturer said the same I would think it rather odd.
Further your reply above verged on personal abuse from which I have always steered clear.
Many of your comments are grossly offensive.
So this thread is about a challenge to me so perhaps I can return the complement
I challenge you to take a basic physics exam something like’A Level Physics’ jointly with me at a public place.
I think I can arrange that
You can suggest an alternative arrangement if you like.
The loser will pay £1000 to a charity of his opponents choice .
Bryan – You accuse others of technical incompetence, then scream when someone accuses you of the same. Unbelievable!
Lots of commenters on these blogs tie themselves in knots because they think of “heat” as a real physical substance. But when more careful commenters avoid the term, you object. When I point out why they would avoid the term, you object more.
SoD gave you a whole thread inviting you to post your analysis of a trivially basic problem in thermodynamics and heat transfer. You steadfastly refused to do so. Why should anyone take you seriously?
Curt says
“SoD gave you a whole thread inviting you to post your analysis of a trivially basic problem in thermodynamics and heat transfer. You steadfastly refused to do so. Why should anyone take you seriously?”
Again,……. as I said above
” DeWitt Payne gave a link to the Willis model and I agreed with the conclusions based on the assumptions and said so early on”
Copying out the Willis solution again would be rather pointless…don’t you think? – duh!
I would regard this as plagiarism and instead give a link to the article in the same way as DeWitt Payne.”
I have avoided all further comment on this and other SoD threads and was happy enough it would stay that way.
Its SoDs site and he has complete freedom to ban or not ban and his wishes must be respected.
However mikejacksonauthor directly commented to me above a few days ago.
I replied to him with a different approach (above) with an emphasis on internal energy gained in the core and shell and time scales.
My comment on the possible oversimplification of the thermodynamics curriculum of chemists is not a comment about the chemists themselves.
Perhaps I was wrong and maybe the chemists shared the same classes for physics and thermodynamics as physics students for the Ist and 2nd years.
This was was the case at my university.
Perhaps I’m wrong, but the atmosphere of discussions between skeptics and believers has got sharply more poisonous of late.
Look at your own contribution.
Perhaps this is down to the ‘pause’…who knows.
Bryan wrote: “Its notable that the chemists who post here like DeWitt, Frank and Josh Halpern have a difficulty with the second law. Some go as far as wanting the word ‘heat’ abolished.”
Bryan is stuck in the 19-century with Clausius, but without molecules, photons, QM or statistical mechanics. Unlike Bryan, I recognize that the 2LoT is a CONSEQUENCE of the innate behavior of individual molecules, not a law the CONTROLS the behavior of individual molecules. Clausius’s version of 2LoT requires heat transfer from “hot” to “cold” – terms that refer to temperature. We now know that temperature is a bulk property proportional to the mean kinetic energy of a group of colliding molecules that tells us nothing about the kinetic energy (or internal energy) of any one molecule in that group. The 2LoT therefore places no restrictions on the behavior of individual molecules or photons. When heat is transferred by radiation, the 2LoT applies only to the net transfer of energy between two large groups of colliding molecules.
On the other hand, I have listened with interest to technical discussions about heat and difference between heat and energy – the first half-dozen times Bryan raised the subject. Since then, I’ve tried to avoid personally misusing the term “heat”, especially when referring to the GHE.
Frank I can see why many professional scientists can go through their entire working lives without using the word ‘heat’.
Its origins indeed date back to Carnot and Clausius.
How to transform the maximum amount of thermal energy into useful energy in steam engines which was a major practical question.
The germ of the idea behind energy transfer between two temperatures (source and sink) still survives and is useful and indeed essential in physics.
I am starting to think in several branches of science like climate science where the word is not often required it is dropped.
An even bigger simplification is to start calling EMradiation ‘heat’ in any direction.
Again a useful simplification for many and will mostly cause no practical problems.
This usage then leads unfortunately to diagrams showing clouds with a big bold letter HEAT going from clouds to a warmer Earth surface .
The someone from a physics tradiation sees this and thinks fraud or incompetence is in evidence.
Others wonder what all the fuss is about.
As far as I know from hot object to colder object there is a two way flow of photons with the greater intensity being hot to cold.
Unfortunately no experimental evidence to date has been provided to separate the streams and deal individually with them.
If this was possible it would be very easy to prove that no work could be done with the cold to hot stream
Hence it cannot be heat. QED.
There is no debate in physics circles about dropping the word ‘heat’.
Rest assured I am fully aware of photons and nuclear physics.
Bryan wrote: “As far as I know from hot object to colder object there is a two way flow of photons with the greater intensity being hot to cold. Unfortunately no experimental evidence to date has been provided to separate the streams and deal individually with them. If this was possible it would be very easy to prove that no work could be done with the cold to hot stream
Hence it cannot be heat. QED.”
Get and infrared thermometer. Point it at the ground – that is OLR. Then point it at the sky – that is DLR. Two streams of photons moving in opposite directions; measured individually.
If you want simultaneous measurement, buy two infrared thermometers and point them in opposite directions along the same line.
Any system that intercepts and measures the flux from cold to hot will also interfere with the flux from hot to cold. Both travel along the same straight line. Photons either travel both directions or neither. Heat is the net flux or there is no flux.
Frank,
You’re wasting your time. For one thing, Bryan doesn’t believe that IR thermometers or pyrgeometers work like you and I think they work. Also, a one way radiation model would give you the same temperature measurement on an IR thermometer as two way. One way, however, requires a complete revamp of physics a la C.J. and discussion of that sort of thing is strongly discouraged here.
DeWitt: Even if the instruments don’t work as commonly believed, I assume the different readings from different directions provides experimental evidence of unequal radiative fluxes traveling in oppose directions. Bryan says there is no such evidence.
And if he want to challenge the accepted physics of IR thermometers at this site …
(Criticize me for “feeding the troll” when he brought up the “difficulty” chemists have with the 2LoT!)
Frank and DeWitt
IR thermometers measure the surface temperature of the object illuminated (usually with a red dot from a laser as a pointing device) although several different types of technology are employed so there is not a unique common device.
The pyrgeometer purports to measure the actual flux.
This is the device that we are all should be interested in .
If it could indeed measure and in some way indicate that the cold flux can be separated from that hot flux I would be delighted!
Then I could challenge you to demonstrate that the cold flux can produce work for example power a sterling engine or increase the temperature of a hotter object in an adiabatic enclosure.
This you would fail to do.
Then all would shout ‘eureka’, the colder flux is indeed not heat !.
The best discussion between believers and skeptics on this topic can be found in Tallblokes blog.
Use google to find the link.
Claes Johnson plows a lonely furrow
He thinks QM is an unnecessary departure from 19th century physics .
Good luck to him on his endeavors.
I would point to the fact that history indicates that the current paradigm will not be changed until the paradigm fails to explain some critical physical fact.
Since QM has answered all current problems I will stick with the physics found in current textbooks.
Bryan wrote: “The pyrgeometer purports to measure the actual flux.
This is the device that we are all should be interested in. If it could indeed measure and in some way indicate that the cold flux can be separated from that hot flux I would be delighted!”
Some IR thermometers are reasonably directional – with a distance to spot diameter ratio of 12:1. Draw a line from a warmer location to a colder location. Point the IR thermometer down the line towards the colder location and then toward the warmer location. Observe the difference. (Correct for emissivity, if necessary.) You HAVE now separated a “cold flux” from a “hot flux”.
A solar panel generates electricity by collecting photons from a 5000 degK star. I can use the electricity to heat an object to 10,000 degK. Have I violated the 2LoT?
Frank,
A pyrgeometer, or precision infrared radiometer is much like an IR thermometer except that the field of view is 2π steradians, a hemisphere. The dome covering the detector has a bandpass of ~5-50μm and the dome temperature is measured to compensate for emission from the dome. The field of view of an inexpensive IR thermometer with a distance to size ratio of 10:1 is 0.05 steradians.
By the way, I’ve been here before more than once with Bryan. His claim of lack of knowledge is disingenuous. Usually the next step is to claim that the instruments are biased and insufficiently accurate. Don’t let him suck you in.
There is a lot of fascination in the “climate interested but confused about the science basics” part of the blogosphere in:
– measurements of longwave radiation (especially downwards from the atmosphere)
– emissivity of the earth’s surface, and even
– the Stefan-Boltzmann law
Here’s the reason for the fascination, best as I can tell. After some (often lengthy) period of claiming that the greenhouse effect doesn’t exist or can’t happen because “energy is being created” or “the (imaginary) second law of thermodynamics doesn’t allow it”, etc, etc – the person finds out that the climate system emits approx 240 W/m2 to space (globally annually averaged) while the surface appears to emit approx 390 W/m2.
This is unpleasant information for someone who has spent a lot of time, effort and passion putting forward such a strong case against “the greenhouse effect”.
What to do? Clearly the measurements must be wrong!
The climate interested didn’t start out doubting these measurements / equations. Just the dawning realization that their claims would be complete rubbish leaves no alternative (other than the completely unpalatable alternative that they had absolutely no idea about heat transfer basics and need to retract 100s of comments on blogs and, in some cases, blog articles).
I found the same when presenting extracts from undergraduate heat transfer textbooks on 2-way transfer of thermal radiation. First, it’s a climate “science” textbook, give me a real textbook. Second, silence. Third, “still ignoring that reference and really don’t want to admit it says the opposite of what I have been claiming and is really a standard heat transfer textbook”..
Leon Festinger would be very happy.
Frank
The second law gives the maximum useful energy that can be obtain from a diffuse thermal source and sink with a temperature difference.
A good example of such diffuse thermal energy is the vast amount of thermal energy stored in the sea.
For all practical purposes it is unavailable for use for conversion to a higher quality energy form although some marginal heat pump examples do exist if a suitable sink can be found and a heat pump employed.
A rectilinear radiation source such as the Sun is not diffuse and can be brought to a focus by a parabolic mirror or lens to achieve the surface temperature of the Sun.
The Carnot cycle gives the second law maximum conversion possible in any particular situation, this is the historical basis of the second law.
Diffuse electromagnetic radiation such as Earth surface upward and LWIRD radiation also fall into this diffuse category.
The second law formula gives an exact theoretical maximum of the conversion obtainable from such a diffuse thermal source .
Once this fraction is obtained it can readily be changed into other energy forms without a theoretical restriction.
For instance to charge an electric cell and then this electrical energy can be used without restriction to obtain temperatures such as 10000K (providing suitable materials are used).
The odd thing is that all examples of analysis of the Carnot Cycle that I have found do so with a classical one direction heat flow model.
SoD was quite right to pose the question of what happens to the cold to hotter radiation in the two flow situation.
I must admit that at first I simply did not know as the physics textbooks did not help and the topic seems to be ignored and oversimplified.
So guesses about filled microstates and other possibilities were offered but now I am pretty much in agreement with yourself on such details.
G&T are unnecessary confrontational but I think that they raise legitimate questions about present IPCC advice to governments.
These questions have never been answered.
Correction
My books are by C J Adkins Cambridge not Peter Atkins Oxford
Thermal Physics
Equilibrium Thermodynamics
DeWitt wrote: “A pyrgeometer, or precision infrared radiometer is much like an IR thermometer except that the field of view is 2π steradians, a hemisphere. The dome covering the detector has a bandpass of ~5-50μm and the dome temperature is measured to compensate for emission from the dome. The field of view of an inexpensive IR thermometer with a distance to size ratio of 10:1 is 0.05 steradians.
By the way, I’ve been here before more than once with Bryan. His claim of lack of knowledge is disingenuous. Usually the next step is to claim that the instruments are biased and insufficiently accurate. Don’t let him suck you in.”
Thanks for the info. I was vaguely aware of some of these differences, but not comfortable commenting on them. In this case, I deliberately chose an IR thermometer, because I wanted a highly directional reading of the flux from each direction along a line from warmer to colder. As long as the IR thermometer gives a different flux from each direction (for whatever reason), I believe I met Bryan’s challenge of separating two different fluxes in opposite directions. Unlike with DLR, I don’t need a precise result, just two different measurements. (Like you, I have seen this act before and wouldn’t have started down this path without this answer in hand. Interestingly, I haven’t needed it so far: “now I am pretty much in agreement with yourself on such details” (Bryan, 5/21, 10:49). And apparently he now agrees with SOD’s answer to “A challenge for Brian”.)
Frank
The IR thermometers reading is based on an ‘inferred’ calculation rather than an actual direct measurement.
So in other words if the theory is correct for a particular application then the calculations from the theory will be correct.
Wiki gives several examples of when IR thermometers will give a false reading
There are several types of IR thermometers but the quote from Wiki is as good as any I have come across.
http://en.wikipedia.org/wiki/Infrared_thermometer
Other sources had oversimplified answers or were just plain wrong
‘IR thermometers work by reflected IR radiation’ – plain wrong
‘IR thermometers can work through the air because the air does not absorb IR radiation’ – oversimplified because IR thermometers work in the radiation window around 8um
As we all know there are IR active gases like CO2 and H2O which absorb actively outside the window
The pyrgeometer has some similarities with the IR thermometer particularly that its readings are inferred not direct.
Net energy flux per second = heat flow = A*σ*(Thot^4– Tcool^4)
As DeWitt says “A pyrgeometer, or precision infrared radiometer is much like an IR thermometer except that the field of view is 2π steradians, a hemisphere. The dome covering the detector has a bandpass of ~5-50μm and the dome temperature is measured to compensate for emission from the dome. The field of view of an inexpensive IR thermometer with a distance to size ratio of 10:1 is 0.05 steradians.”
Several pyrgeometers have passive sensors that they will give an electrical reading without a separate power supply.
A look at the above equation will explain why
When the pyrgeometer points up to measure DLRIR
Thot is usually the pyrgeometers temperature itself and is determined by an onboard thermometer so the calculation value
Thot^4 should be correct
Tcold is usually the air all around the pyrgeometer
So there is usually more flux leaving the pyrgeometer than entering it.
Tcool^4 factor is assuming
1. The air is a black body radiator
This is incorrect the airs IR spectrum has band radiation with gaps such as the atmospheric window.
2. It can be shown that such radiation does not follow a T^4 law
3. Air at say 283K will not always have the same emission/ absorption spectra
The humidly or H2O fraction can very enormously.
So its not a big surprise for me that the history of the pyrgeometer is one of constant disappointment with the results it produces.
The best link I have on the pyrgeometer is
Bryan: Despite all of your reservations about IR thermometers, they can still be used to demonstrate that a two-way radiative flux exists between warmer and cooler objects with a larger flux from the warmer one to the cooler one. If you are worried about differences in emissivity or the fact that air doesn’t emit at all wavelengths, let’s make our two objects with different temperature from the same high emissivity material (say white marble, e = 0.95) or the same material coated with a high emissivity paint. Set two 1 m2 slabs of these materials at different temperatures facing each other 1 m apart and point the IR thermometer perpendicular to the center of each slab from the midpoint between the two slabs. You will get different readings.
Frank,
Actually they can’t. One would get the same reading from the IR thermometer using one way radiative transfer calculation as with two way. There is either a net flow from the IR detector to the object being measured if the temperature is lower than the detector or a flow into the detector if the object is warmer. That creates a temperature gradient across a thin insulating layer at the surface of the detector, which is what is used to measure the net flux. Then knowing the flux and the temperature of the detector, one calculates the effective temperature of the object using the Stefan-Boltzmann equation.
I still say the best argument against one way only flux is that it would need new physics to explain how a surface would vary it’s emission rate depending on the temperature of the surroundings. Continuous emission based only on temperature and emissivity is the simpler explanation and requires nothing new.
But the fact that you can read a temperature of the sky with an IR thermometer does prove the existence of the greenhouse effect. Even with one way transfer, at constant flux the temperature of the higher temperature surface must increase to maintain that constant flux if the temperature of the lower temperature surface increases. Assuming infinite parallel planes:
Flux = σ (ε_1 T_1^4 – ε_2 T_2^4)
Make F constant and T_1 depends on T_2 and vice versa. That equation is also bullet proof. Waving your hands about the 2LoT is irrelevant to that. Words aren’t math.
Actually, in discussing this same concept with Claes Johnson, a reductio ad absurdum occurred to me:
– If an object A at temperature Ta radiates at an object B at temperature Tb only when Ta > Tb, that means that radiation ‘pencils’ leading from A outward either define radiant transport from differential areas on A to differential areas on B when Ta > Tb; or else define transport possibilities that don’t actually have any radiation, when Ta =< Tb. This means that if you have a cool object B observed against the backdrop of the solar surface, medium-temperature object A will radiate towards B but not towards the sun. So if the distance from A to B is R, and the transverse diameter of B is rb, the half-angle of the radiation pencil from A to B must be (rb)/(2*R); and all other nearby pencils, which terminate in the sun, have no radiation. But according to the most basic ideas of diffraction, the minimal half-angle of spread of a beam of radiation from A to B would be (wavelength)/(2*ra). So:
WL/(2*ra) < (rb)/(2*R) ; or
R 2*(ra)*(rb)/WL, I end up with
2 < 1
In other words, this concept that the radiation from A can switch from being "on" when aimed at the cooler B but "off" when aimed at the hotter sun makes demands on how fast a beam of radiation spreads which are not in accord with established concepts of diffraction (nor, thus, with Maxwell's equations).
– It gets worse if we consider millions of cooler Bs observed against the backdrop of the sun: the radiation field looks now like a porcupine of needles pointed inward (because each 'pencil' is wider at the outer edge than at the surface of A); and as before, each individual pencil violates the minimal spreading behavior of diffraction.
So, for sufficiently large distances R between A and B, the proposal that A would radiate to B but not to the sun is untenable.
One last point: I ask anyone who believes that the usual description of the transfer of radiant power, as the net result of two-way transmission, violates the 2nd law of thermodynamics (2LoT) to prove it – by explicitly showing how reliance upon the usual understanding can lead to the construction of a perpetual motion machine of the 2nd type. If that cannot be done, then such a toothless "violation of the 2LoT" is nothing to be concerned about.
DeWitt: As you note, one-way flux due to some sort of interference is the alternative hypothesis (Hypothesis B, for Bryan). In that case, the measurement from the “cold direction” would be the same – whatever reading zero flux produces on the IR thermometer.
Consider the experiment I proposed with two parallel 1 m2 slabs of limestone 1 m apart, with the slabs having different temperatures and with the iR thermometer reading perpendicular to each face from half way between. If we gradually cool the colder slab, the signal from it won’t change according to Hypothesis B, since there is never any flux from the cooler direction. The signal from the warmer slab will appear to get stronger use to less “interference”. In reality, the reading from the warmer slab stays the same and the reading from the colder slab changes/weakens.
By varying the temperature of either slab, you can demonstrate the existence of two different fluxes without cancellation of the flux from cooler to warmer.
Frank
If you cool one slab but keep the hotter ones temperature constant you will increase the flux between them
The maximum Carnot efficient transfer = (Th -Tc)/Th
Gives the maximum fraction that can be turned into an electrical signal for example
Lets say one slab Th = 500K and the other is at 400K
Then using SB transfer equation with emissivity 1 and unit area for both
Heat energy transferred = 2092J
Energy available for work (electrical or mechanical)
= E(Th -Tc)/Th = 2092(500-400)/500 = 418J
If the colder slab is further cooled to say 350K then a greater quantity of heat is transferred = 2693J
Energy available for work (electrical or mechanical)
2693(500-350)/500 =808J
Now since only the net energy flow is observed or measurable (say on an electric meter) you can see the greater electrical signal will come from the biggest temperature difference between source and sink
Frank
Flux is energy(J) per second(S) or watts which may be a better unit to use
Frank,
If we set the slab temperatures such that the IR thermometer detector temperature is between the slab temperatures, the flux is from the IR thermometer detector element to the colder slab, so it will change as you cool the colder slab. But I think you may run into Neal’s diffraction problem as the spot you’re observing with the IR thermometer can also be seen by the warmer slab if the area of the slabs and the distance between them is large compared to the area of the IR thermometer, the field of view of the IR thermometer increases towards 2π steradians perpendicular to the slab, i.e. it can see the entire slab, and the area of the slabs increases without limit.
Suppose instead of slabs, we have a closed box with the walls at constant temperature, i.e. a hohlraum. Constant emission requires that the interior of the box contains a photon gas with a Planck wavelength spectrum determined by the wall temperature. One way emission says that there are no photons inside the box as the walls are all the same temperature. Now we have a very fast shutter covering a small hole in the side of the box with the shutter at the same temperature as the walls. If we have detector behind the shutter and the distance between the walls is large, one way radiation would seem to predict that there would be no radiation detected when the shutter was first opened, as it takes a finite amount of time for radiation to be emitted from the walls and travel to the detector and that the time would increase as the distance between the walls increased. With a photon gas present, the time would be determined only by the distance from the detector to the shutter.
I have some fundamental problems with all this discussion.
Photons are a concept of Quantum Mechanics (or Quantum Electrodynamics). If we accept QM, then we know, what’s going on, if not nothing written on one- or two-way radiative transfers is well enough defined to make sense.
Maxwell’s equations are not sufficient for this discussion, because they lead to the incorrect results without the introduction of QM.
Pekka,
You are, of course, correct. If all parties accepted QM rather than being stuck in the 19th century, we wouldn’t be having this discussion. I still think it’s not entirely futile, however, to create a thought experiment.
Pekka wrote: “Photons are a concept of Quantum Mechanics (or Quantum Electrodynamics). If we accept QM, then we know, what’s going on, if not nothing written on one- or two-way radiative transfers is well enough defined to make sense.”
I’m not sure that photons (particles) eliminate all complications, since particles moving in opposite directions can collide. A particle and its anti-particle collide and disappear, producing two photons moving in opposite directions. What happens when two photons collide? Scattering?
(FWIW. This discussion started when Bryan said their was no experimental evidence for a flux from hot to cold and a separate lesser flux from cold to hot – even though he now accepts conventional wisdom on this subject. My position is that separate and different fluxes could be convincingly detected with an IR thermometer despite the fact that Bryan and others don’t accept the conventional explanation for how they work.)
Frank,
That’s an essential part of my point. QED (Quantum Electrodynamics) provides the theory that tells, how important the interaction between photons is. It gives very exact quantitative answers that tell that the corrections are negligible in the situations relevant for the atmospheric physics (it has often been said that QED is the theory that has been verified with greatest precision of all physical theories, thus the answer is really accurate and reliable).
Based on QM and QED we know that the only fundamental forms of interaction that need to be considered to get accurate results are
– spontaneous emission of photons by matter (molecules or condensed matter),
– absorption of photons by matter,
– stimulated emission of photons by matter,
– scattering of photons by matter (this is not of equal significance as the three other).
The three coefficients of absorption and emission are listed in the HITRAN database for relevant gas molecules. These coefficients are not independent, but all three can be easily calculated, when one is known.
All more complex forms of interaction like photon-photon interaction have a negligible effect.
The above implies also that events of spontaneous emission and absorption are independent. The spontaneous emission rate is dependent only on the properties of the emitting matter, not on the further destiny of the photons.
Pekka:
The macroscopic distribution of radiation is a topic well within the scope of macroscopic physics, including Maxwell’s equations. Quantum mechanics was developed with a close eye on the implications for thermodynamics; and within the scope of thermodynamics, there are no contradictions with quantum physics.
If it were no longer valid to do careful reasoning on macroscopic phenomena using macroscopic physics, there would indeed be little value to most of the physics curriculum.
Nealjking,
Without QM the theory fails due to the “ultraviolet catastrophe”. Planck presented an ad hoc solution that had no valid theoretical basis before QM was applied to solve the problem. QM is needed for that reason and also for other reasons to get a consistent explanation of the situation.
As long as QM is not introduced only partially incorrect heuristic arguments can be presented. As all such arguments are partially incorrect, it’s possible to argue forever, which of them is less seriously wrong than the other similar arguments.
It is precisely when we know the limitations of a theory that we know how far we can trust it.
Right, and in this case the limitations of exiting theories that exclude QM make them fail totally. The quantum nature of radiation is absolutely essential for the correct outcome.
Pekka,
No, I don’t think the quantum nature of light enables you to create arbitrarily small widths of radiation pencils: Another way of looking at the same issue is known as the uncertainty principle: Cancel out the factors of Planck’s constant and it’s the same result.
IR is not emitted to a particular direction or “narrow pencil” but typically allowing all directions or, in case of a surface, half of all directions. Every photon is, however, emitted essentially at one point and absorbed at another.
Nonetheless, although the “apparent trajectory” from the starting point to the end point of a photon is individual, within the context of an experimental set up these rays can be confined to angular regions: Hence we get maxima and minima of diffraction patterns.
The double-slit diffraction measurement was not invented for quantum physicists; nonetheless it plays a prominent role in discussions of quantum theory.
So if, as seems to be the case, a fervent believer in one-way only thermal radiation transfer has to believe that radiation peaks are actually confined to angular regions smaller than permitted by classical diffraction theory, he gets no protection from QM: All I have to do is multiply both sides by h, and I get an application of the uncertainty principle.
And where did the uncertainty principle come from? Originally, not from the non-commutativity of X and P_x, although that is its mathematical starting point. But the cogitation regarding the physical meaning of the UP began as early as 1923 and did not really end until 1930.
In the atmosphere, there’s nothing that confines the trajectory. To get the two slit diffraction pattern two slits are needed, to get some other diffraction pattern, some other structure with suitable properties is required. Nothing of that kind exists in the atmosphere. In practice nothing of that kind exists even on solid surfaces.
Diffraction theory has no role at all in the error people make in their erroneous application of the 2nd law. They have it wrong, but not for that reason.
Pekka:
The point is that people like Claes Johnson and Bryan claim that a blackbody can radiate towards cooler bodies, but not towards warmer bodies, than themselves. So now imagine three temperatures:
T1 > T2 > T3
where T1 is the temperature of a large screen,
T2 is the temperature of a blackbody of radius R2 at distance D2 from the screen,
and T3 is the temperature of blackbody of radius R3 and at distance D3 from the screen, between the T2 body and the screen, with D3 R2 + (D2 – D3)*wavelength/(2*R2)
But since, according to the “1-way only” postulate, width width > R2 + (D2 – D3)* wavelength/(2*R2)
(R3/R2) > 1 + [(D2 – D3)/R2]*[wavelength/(2*R2)]
R3 > R2 + (D2 – D3)*[wavelength/(2*R2)]
(R3 – R2)/(D2 – D3) > [wavelength/(2*R2)]
(R3 – R2)/[wavelength/(2*R2)] > (D2 – D3)
Fixing R2, R3, D3 and the wavelength, I can easily stretch the distance D2 until this inequality is violated. At that point, the “1-way only” concept that bb#2 can radiate towards only bb#3 but not towards the screen at temperature T1 is in violation with what is possible with classical diffraction limits. Indeed, there are no metallic slits: that makes it even more impossible to impose such behavior.
The “1-way only” concept of radiant heat transfer makes impossible demands. Therefore it is incorrect.
QED.
Neal,
You are missing a very important point – of all the many people claiming the second law violation or the first law violation – none of them can write down an equation.
Equations are a mysterious – and unnecessary – language.
The people writing down equations are just trying to confuse them, as they have confused themselves, given that the WORDS make it crystal clear that:
– “you have invented energy!!!” or
– “the second law says this can’t happen!!!”
The accepted way – in the old days – of passing a physics, chemistry or engineering degree included proving or disproving certain propositions and deriving the equations for a particular phenomenon described in the exam.
It is the only way I know. Very old school I’m sure. But once this recourse has been taken away I have nothing.
SoD:
It’s been quite apparent for some time that Bryan is not going to write down any useful equations. He has a comrade-in-arms, Claes Johnson, who does write down many equations, though to little effect.
So I’m missing your point about what point I’m missing.
Frank (May 27, 2015 at 3:47 am)
Within classical radiation theory, two opposed beams of radiation in a vacuum pass through each other without effect, since Maxwell’s equations are linear.
Within quantum radiation theory, the lowest-order Feynman diagram for the interaction of two photons that effects scattering has each of them splitting into an electron-positron pair briefly; the two pairs “switch partners” and then reform into two photons that are each going off in a different direction than the two original. This diagram has 4 vertices, so it contributes a very tiny amount towards the calculation’s end result – another way of saying that the effect is negligible under nearly all conditions.
By comparison, photon-electron scattering, which is the interaction between electrons and the EM field, has lowest-order scattering diagrams of one vertex.
Recently it has been suggested that there might be as many as 20 photon-photon events at CERN’s Large Hadron Collider detectible each year. http://physics.aps.org/synopsis-for/10.1103/PhysRevLett.111.080405
SoD and Neal,
In this case we have seen also people, who write equations, but wrong equations. That originates from the complexity of the theory that includes partially similar equations at two levels:
– equations that describe electromagnetic fields (most importantly Maxwell’s equations)
– equations of Quantum Mechanics like Schrödinger’s equation.
Both equations involve phases. When phases are interlinked we have coherence and either the field strengths of wave functions must be added in calculation of overall physical quantities. Only after that can the overall observable quantities be determined by calculating the absolute square of the sum.
When phases are not interlinked but randomly related to each other the absolute square of the field strength or wave function must be calculated first for each component process and the results of those calculations added up as the last step.
In the case if IR radiation in the atmosphere each photon is emitted independently and has a QM phase that’s not linked to the phases of other photons (except for the stimulated emission, which does not change the overall picture and is handled correctly as well). For this reason each photon must be handled as if there were no other photons around. CJ makes his error at this point as he insists that emission from the target body affects, how photons reach its surface and get absorbed.
Other people have presented in earlier discussion on this site arguments based on the same error. They have claimed that we should consider coherently Poynting vectors that are related to all photons. They have tried to apply classical electromagnetism to a problem that it cannot handle, because the problem is so inherently one of QM and QED. I do think that it’s impossible to argue properly against such erroneous arguments in any other way than by referring to QM (and in some cases also to QED).
Neal,
You are correct, Claes writes equations. As did Gerlich & Tscheuschner. A couple of exceptions. One attempting to rewrite physics of the last 100 years. The others, having a laugh.
But I meant that of the people showing up at this blog and saying “that isn’t right”, they cannot formulate or defend an equation.
So proving them wrong by writing an equation doesn’t help.
My comment was (of course) tongue in cheek. It’s my English humor. I can’t help it.
SoD:
But the point of the equation is to get to a point that might be understood: That the behavior being required of the radiation field in order to satisfy some funny interpretation of 2LoT is unreasonably porcupine-like.
Interestingly, of the many and various criticisms of CJ’s theory on one of his recent promulgations, that is the only one he hasn’t either explicitly dismissed or ‘disappeared’. Maybe it bothers him?
nealjking says
“The point is that people like Claes Johnson and Bryan claim that a blackbody can radiate towards cooler bodies, but not towards warmer bodies, than themselves.”
Neal , with all due respect where have I said anything like that?
If you cannot find any evidence for your statement then I think an apology is in order
For WIW my view is colder object to hotter object spontaneous emission and subsequent absorption happens.
My point is that this flow is not a heat transfer.
For the benefit of yourself and SoD I will even put it in the form of an equation
Hot to colder radiative energy flow = Eh
Colder to hotter radiative energy flow = Ec
Heat transferred = E = Eh – Ec
Heat has the property of being changed into thermodynamic work with the maximum efficeint convesion given by second law
= E(Th -Tc)/Th
Pekka,
The point is not to consider heat radiation as a coherent wave. The point is that CJ’s concept that the cooler bodies emit no radiation towards the warmer makes a demand on incoherent radiation, with regard to angular confinement, that EVEN coherent radiation cannot achieve. But it is well-known that the angular confinement of a laser beam cannot be matched by that of thermal emission. So CJ’s concept is relying on the impossible.
To my mind, it seems to be an impoverished attitude that one would only rely on “the most fundamental” theory available: these fundamental theories have been created in careful awareness of what has worked before, and where. Certainly I have never seen Feynman display such an attitude: whenever I saw him deal with a new question, he would tackle it six ways from Sunday, and with whatever came to hand.
Bryan wrote above: “The odd thing is that all examples of analysis of the Carnot Cycle that I have found do so with a classical one direction heat flow model.”
No one can see individual collisions transferring energy from slower-moving molecules to faster-moving ones, because they take place on a molecular scale. You can see detect photons going from cold to hot because they can travel vastly further. Of course, far more collisions and photons transfer energy from hot to cold. If you believe in the Boltzmann distribution of molecular speed, you know some collisions must be transferring kinetic energy from slower-moving molecules to faster-moving ones and that individual molecules don’t have a temperature.
Bryan wrote: “The Carnot cycle gives the second law maximum conversion possible in any particular situation, this is the historical basis of the second law.
Diffuse electromagnetic radiation such as Earth surface upward and LWIRD radiation also fall into this diffuse category. The second law formula gives an exact theoretical maximum of the conversion obtainable from such a diffuse thermal source.
I believe the Carnot cycle tells us the maximum conversion that can be obtained transferring heat from hot to cold using a heat engine. Our economy may still be dominated by heat engines, but we can convert liquid hydrocarbons to electricity without such limitation in a fuel cell, visible light to electricity with photovoltaics, and a temperature difference to electricity with the Seebach effect. Lasers and microwave ovens APPEAR to transfer heat from cold to hot.
Suppose I develop a semiconductor that creates electron/hole pairs from infrared light rather than visible light. Can I use such “DLR panels” to generate electricity from an atmosphere that is colder than the panel? Or do I have to cool my “DLR panels” until they are colder than the atmosphere? (Radiation is a form of heat.) Will their maximum efficiency depend on how cold they are ((T2-T1)/T1)? (:)) These are far more interesting questions than whether GHE is incompatible with the 2LoT.
Frank:
The efficiency of photovoltaics will not approach that of a Carnot cycle, because Carnot cycles are reversible, but photovoltaic conversion of EM to electrical energy is not.
Neal,
No real heat engine can reach the Carnot limit either because it won’t be reversible either. Reversibility requires infinite time and perfect insulation for the heat transfer, compression and expansion cycles. Any finite rate for these processes increases entropy and reduces efficiency.
DeWitt,
The Carnot efficiency can in principle be approached infinitesimally closely by operating the Carnot cycle slowly and carefully enough that all temperature gradients are infinitesimal.
But the parameters of interest for a photovoltaic cannot be adjusted, so there will be gross levels of entropy production that cannot be smoothed over. An absorbed photon is always going to make a splash.
For each individual case of absorption the efficiency depends on the ratio of the photon energy and the excitation energy that corresponds to the voltage of the photovoltaic conversion. Thus the efficiency is low, when the photon energy is much higher than the threshold. A multilayer photovoltaic cell can absorb the most energetic photons in the topmost layer and lower energy photons in the lower layers. In the ideal case (which is not attainable in practice) the efficiently is close to the Carnot efficiency calculated from the (radiative) temperature of the sun and the temperature of the PV cell.
The Carnot efficiency is so close to 1.0 (about 0.95) that the actual efficiency is almost totally determined by the other factors.
SOD: If you follow your Wikipedia link on Leon Festinger to a link to cognitive dissonance (at the top of the page) and then to the education subheading, you will learn that “constructivists” believe that true learning doesn’t occur without cognitive dissonance. (Constructivism is at the heart of “reformed math curricula” in the US.)
Maybe you should have provided a link to Lewandowsky.
*shudder*
Someone please save us from the educationist’s application, generally without adequate testing, of new theories of learning. Of course, one could also argue the morality of using children as test subjects.
NealKing asked above: “One last point: I ask anyone who believes that the usual description of the transfer of radiant power, as the net result of two-way transmission, violates the 2nd law of thermodynamics (2LoT) to prove it – by explicitly showing how reliance upon the usual understanding can lead to the construction of a perpetual motion machine of the 2nd type. If that cannot be done, then such a toothless “violation of the 2LoT” is nothing to be concerned about.”
Immediately below, I had already proposed the following dilemma, to which no one responded: “Suppose I develop a semiconductor that creates electron/hole pairs from infrared light rather than visible light. Can I use such “DLR panels” – analogous to solar panels – to generate electricity from an atmosphere that is colder than the panel? Or do I have to cool my “DLR panels” until they are colder than the atmosphere? (Radiation is a form of heat.) Will their maximum efficiency depend on how cold they are ((T2-T1)/T1)?
I think I partially understand the answer, but I’m limited by my knowledge of solid-state physics.
What are “DLR” panels?
(The introduction of a new item to the discussion is a bad time to indulge in undefined acronyms.)
Sorry Neal. Hypothetic “DLR panels” are a made out of a semi-conductor that produces electron/hole pairs when exposed to thermal IR such as DLR using the same mechanism as a solar panel does with visible light. Bryan has assured me that the 2LoT will prevent me from getting any work out of DLR.
Frank,
Your “DLR panel” has the serious fault that it not only absorbs IR photons, but also emits them making it incapable of producing electricity from photons of wavelength similar to the emission at the temperature of the panel.
Photovoltaic panels work, because the incoming photons have a much higher energy than typical for the IR emission at the temperature of the panel (of kT at the temperature of the panel).
Here you do really hit the limitations from the 2nd law.
Frank,
I don’t think so. The holes and electrons would already be there for radiation from a thermal source at a temperature lower than the semiconductor. Radiation from a nonthermal source like an IR laser would be a different matter. I also think it would be fairly easy to show perpetual motion of the second kind, but I’m not going to bother to try, as I’m convinced it won’t work already.
DeWItt: I’ll give you part credit for your answer to Part A. If thermal IR can create electron/hole pairs in my semiconductor “DLR panels”, so can thermal collisions. With all those thermally generated holes and electrons floating around, the material won’t be a semi-conductor at normal temperature. If I lower the temperature and reduce thermal excitation into the conduction band, it could become a semiconductor and might function like a conventional photovoltaic device. (T2-T1)/T1 might even turn out to be the theoretical limit for efficiency.
Now for Part B (Extra credit). Forget the DLR. We have a material with thermally generated holes and electrons. When we have such pairs generated from visible light in a photovoltaic device, electricity flows. Why won’t electricity flow from a semi-conductor with thermally generated electron/hole pairs?
If electricity did flow, the semi-conductor would certainly be cooled by this process. We can calculate the entropy loss from cooling. Does electric current have entropy? (Correctly analyzing a conventional photovoltaic device in terms of entropy would be a good place to start. I had an answer when I asked about the “DLR panels”, but I don’t have answers to these questions.)
Frank,
For electricity to flow on exposure to EM radiation, the electrons have to get into the conduction band from the valence band and the conduction band cannot overlap the valence band ( http://hyperphysics.phy-astr.gsu.edu/hbase/solids/band.html ). If thermal IR photons can put electrons into the conduction band, then internal thermal excitation already has them there and the material is a conductor. Basically, the band gap has to be large enough that the energy of a photon in the thermal IR is not going to be sufficient to put an electron into the conduction band for a material at ambient temperature.
Spontaneous cooling would be a violation of the 2LoT.
There are pyroelectric materials that react to changes in IR radiation by changing temperature and increasing or decreasing surface charge, but you can’t get power from them. They’re used in devices like passive motion detectors and FT-IR spectrophotometers.
It’s quite easy for me to accept that IR photons cannot be used effectively to generate electricity at temperatures at which IR photons are a common part of the electromagnetic background.
What I am trying to pry out is why some people pretend to fear a violation the 2LoT from two-way radiation transfer, when there is no danger of such a violation.
In fact, donuts gets you dollars that a one-way only transfer will result in a 2LoT violation.
The proofs should be findable by building a perpetual motion machine to operate in either case.
Neal: Thanks for the info on photon-photon interactions. A naive interpretation of EM waves suggests that they can interfere, producing a one-way flux. Pekka suggest that thinking of light as photons could eliminate this possibility. Naively, photons can collide interfering with a two-way flux. A full understanding of QM or traditional EM waves eliminates these possibilities, but Feynman diagrams and coherence require some sophistication.
The original challenge was to prove experimentally that radiative fluxes travel from hot to cold and cold to hot, with the former being stronger – despite questions of how to interpret the readings from an IR thermometer into W/m2.
Frank,
There are limits on, what can be deduced from observations. The way you propose that the empirical observations are done does not solve the problem fully as adding the detector between the two original bodies A and B means that a third body (D for detector) is added.
Originally the question was whether there’s separately energy transfer from A to B and B to A or only one transfer, the net transfer from A to B. With the detector we have two pairs: (1) A and D, (2) D and B. Now the same questions can be asked about these two pairs. The original question gets answered only if you dismiss the fact that you have pairs again.
As long as we observe really only net energy flows between pairs of bodies classical thermodynamics covers everything. At that level we may, indeed, conclude that everything of interest are the net flows, separate flows in the two directions have been defined as issues that cannot be considered at all.
The two-way flow of photons remains a theoretical construct. In QM a separation is made between the description of the state of the system based on the wave function and results of actual measurements. In this philosophical approach unique answers are obtained for the measured quantities, but significant freedom is allowed for the way the state of the system is described, when it’s not measured. That freedom allows for several mathematical formulations that are different, but physically equivalent. That freedom allows also for different ways of describing in words the physical processes that take place between observations. That allows also, in principle, for some of the formulations that refer to one-way flow of energy. There’s some freedom, but only as long as the ultimate results that concern actual observations are unchanged. What CJ and many others have done is to misuse that freedom to extend also into questions where result in wrong physics.
Considering the two flows separately makes the mathematical analysis of the situation easier even in the case of two bodies of given temperatures, but the difference is not great. The difference does, however, become really great, when we consider a physical system like the atmosphere, where the temperature is different at every height. Then it would be really difficult to add up all cases that define both the point of emission and the point of absorption. It gets hugely easier to calculate first the emission, then the photon fluxes in all directions and then the absorption as a separate event again.
SoD
My previous post is held up in moderation, any reason why?
If not shall I repost it?
This is the repost addressed to Neal
nealjking says
“The point is that people like Claes Johnson and Bryan claim that a blackbody can radiate towards cooler bodies, but not towards warmer bodies, than themselves.”
Neal , with all due respect where have I said anything like that?
If you cannot find any evidence for your statement then I think an apology is in order
For WIW my view is colder object to hotter object spontaneous emission and subsequent absorption happens.
My point is that this flow is not a heat transfer.
For the benefit of yourself and SoD I will even put it in the form of an equation
Hot to colder radiative energy flow = Eh
Colder to hotter radiative energy flow = Ec
Heat transferred = E = Eh – Ec
Heat has the property of being changed into thermodynamic work with the maximum efficeint convesion given by second law
= E(Th -Tc)/Th
Bryan,
Given your lack of helpfulness with regard to answering simple questions in a straightforward way, no apology is proffered. If you force people to guess what you’re thinking, sometimes they’ll guess wrong.
It doesn’t work any better in discussion than on the freeway.
nealjking
Above on May 11, 2015 at 7:53 am I said
“SoD is quite correct in saying that two way radiative exchange between two objects (facilitated by photons) is the current best understanding of heat transfer by radiation.
This involved the simple emission and absorption of the photons without any special exclusion (thought erroneously due to the second law OT).”
Several other of my remarks on this thread are of a similar type so you don’t have to, as you say “guess”
All that’s required is an ability to read.
There is no point is attributing to other posters false positions and then analyzing them, is there?
I would like to think that if I had made your mistake I would respond by saying
” sorry, stand corrected” or something similar
Bryan:
You said many things, most of which were not to any point under discussion at the time. I make no apology for being unable to guess the velocity of a random walk.
bryan:
A couple of points here. First, we are not talking at all about thermodynamic work here, but simply heat transfer. The “efficiency” equation you give is irrelevant to this discussion.
None of us here would disagree with your equation:
E = Eh – Ec
with Eh always greater than Ec (as you have defined the terms), or with your characterization that this heat transfer E is just the difference between the two radiative energy flows.
But lets add a little something to the puzzle. First, lets talk about power (rate of energy/heat transfer). So by differentiation your equation becomes:
P = Ph – Pc
Now lets add a power input Pin to the warmer body. If Pin = P and there are no other transfers, the warmer body is in steady state, at constant temperature. So far so good.
Next, lets replace the cooler body “c” with one that is a little warmer “c*”, but still colder than the hotter body “h”. So now we have a reduced radiative heat transfer:
P* = Ph – Pc*
This means that the warmer body has a higher power input (Pin has not changed) than power output (P* is lower than P), so its internal energy, and hence its temperature, will increase.
This temperature increase will continue until it is hot enough that the radiative power output:
P** = Ph* – Pc*
once again matches the constant power input Pin.
Curt
E = Eh – Ec is a Ist law equation (conservation of energy).
Heat has the property of being partly changed into thermodynamic work and so is a useful test of whether some thermal energy transfer can be identified as a heat transfer.
The changing of diffuse thermal energy into higher quality electrical energy is governed by the second law with a maximum limit (or
maximum efficient conversion) given by second law
= E(Th -Tc)/Th
Of course in the real world the conversion would be much less efficient than that as this is not a reversible Carnot engine.
The topic is relevant as we were discussing the pyrgeometer
A large number of them are passive in design.
That is they are designed to produce an electrical signal from the flux passing through them rather than needing an electrical power supply.
For a while my impression has been that the remaining disagreements between Bryan and many others commenting here are mostly semantic. That hasn’t always been the case, but that appears to be true now.
Pekka wrote: “There are limits on, what can be deduced from observations. The way you propose that the empirical observations are done does not solve the problem fully as adding the detector between the two original bodies A and B means that a third body (D for detector) is added.
Originally the question was whether there’s separately energy transfer from A to B and B to A or only one transfer, the net transfer from A to B. With the detector we have two pairs: (1) A and D, (2) D and B. Now the same questions can be asked about these two pairs. The original question gets answered only if you dismiss the fact that you have pairs again.
As long as we observe really only net energy flows between pairs of bodies classical thermodynamics covers everything. At that level we may, indeed, conclude that everything of interest are the net flows, separate flows in the two directions have been defined as issues that cannot be considered at all.”
Thanks for taking the time to write a thorough response. Let’s see how it applies to the situation I proposed: An IR detector with temperature Td located halfway between two limestone slabs (emissivity 1) facing each other 1 m apart with different temperatures, Th and Tc. The conventional hypothesis (Hypothesis C) is that the flux from the hotter slab to the colder will be oTh^4 and from colder to hotter will be oTc^4. The alternative hypothesis (Hypothesis A) is that the flux from hotter to colder will be o(Th^4 – Tc^4) and the flux from colder to hotter will be zero.
Suppose the IR detector samples these fluxes without perturbing them in any way. When the temperature of the colder slab is changed, Hypothesis C says that the change will be recorded when the detector is pointed at the colder slab, but Hypothesis A says that the change will be recorded when the detector is pointed at the hotter slab. This should be an unambiguous demonstration of two-way flux.
Now suppose the IR detector is colder than both slabs but can interfere with the reported flux from the slab. Nothing changes with Hypothesis C. Under this version of Hypothesis A (Hypothesis A’), the detector receives o(Th^4 – Td^4) from the hotter slab and o(Tc^4 – Td^4) and the smaller signal will come from the colder slab. So we will observe two fluxes. Two fluxes is compatible with Hypothesis A’ or C.
What happens if we gradually cool the colder slab and it becomes colder than the detector (Td) under Hypothesis A? The signal from the colder slab reaches zero and stays there. This would demonstrate that Hypothesis A’ is correct. If two fluxes are always present no matter what the temperature relationship is between the slabs and the detector, Hypothesis C is correct.
Now let’s consider Hypothesis A” – the detector interferes with the incoming flux and reports continuously lower values as the source becomes colder than the detector. As the source is cooled, there is no plateau in the output at zero flux once the source is cooler than the detector. If detection works by sensing the difference between the source temperature and the detector temperature and negative differences are allowed, the result will always be two fluxes and we can’t distinguish between Hypotheses A” and C. I know that pyrometers work by sensing differences. A device that worked by photo-excitation or counting photons might be different. Such a device would have to operate at very low temperature to avoid thermal excitation.
Hopefully, I now understand your point, even though I’m not totally convinced by it.
Frank,
The fundamental argument is that as long as everything you observe results from some net fluxes, you cannot tell, whether those net fluxes are formed as differences of one-way fluxes or are the only fluxes that exist. At this level everything observed can be expressed using variables of classical thermodynamics. That means also that heat is observed only as a net energy flow in the language of classical thermodynamics.
IR thermometers and pyrgeometers are devices based on observing net energy fluxes, and are therefore limited in the way I describe above.
Someone brought earlier up the idea of detecting the pressure from emitting and absorbing photons as a proof of the two-way photon fluxes, but it’s possible that using that approach for proof is also based on formulating erroneously the alternative hypothesis (i.e. some alternatives can be disproved, but not all alternatives).
We know that the simplest way of describing correctly radiative energy transfer is based on the two-way process. The correct result is the difference of two simple terms. Thus it’s natural to say that each of the two terms has an independent physical meaning, and that the full process consists of them. We have also comprehensive theories like QED that follow the same paradigm. From a philosophical point of view that does not prove that there’s anything fundamentally wrong in the alternative approach, where only the net flux is real.
Those who insist that the standard two-way description is wrong or worse than the net one-way description are definitely wrong. The two.way description is correct in the same sense any good physical theories are correct. What is not as clear is, whether the one-way description can be also developed to an equally complete theory. That would be cumbersome and impractical, but that may be possible. The same physics may often be formulated in different ways that are equally correct in spite of apparent major differences in the way they are formulated.
An experiment can be done that distinguishes these two interpretations:
Slab H is on the left, slab C is on the right; they are parallel.
A perfectly reflecting pipe conducts radiation from slab H to slab C in the normal direction; as well as the reverse. However, there is a break in the center of the pipe, halfway between H and C: This allows small particles, which have atomic transitions in the IR, to be dropped vertically through the gap in the pipe. As they fall through, photons from H and from C will interact with some of them, and the average landing position in the horizontal plane will be deflected; since T_h > T_c, the deflection will be towards C.
– If we assume that there is an intensity of radiation proportional to (T_h)^4 from the left and an intensity proportional to (T_c)^4 coming from the right, there will be a total deflecting force proportional to F = (T_h)^4 – (T_c)^4 to the right.
– If we assume there is ONLY radiation coming from the left, but of intensity proportional to (T_h)^4 – (T_c)^4, again the total deflecting force will be proportional to F.
– So looking at the deflection alone doesn’t help. However, if you further count the number of scattered/emitted IR photons coming from the gap in the pipe, there is a difference: Under the assumption that photons are coming ONLY from the right, the rate of counted photons should be proportional to (T_h)^4 – (T_c)^4
but if photons are coming from both, it should be proportional to:
(T_h)^4 + (T_c)^4
The ratio of these counts: R_c/R_h = (T_h)^4 – (T_c)^4/[(T_h)^4 + (T_c)^4]
~ 4 * dT * (T_c)^3/2(T_c)^4
~ 2*dT/T_c
So it should not be difficult in principle to tell which of these situations apply.
Under the assumption that photons are coming ONLY from the right, the rate of counted photons should be proportional to
(T_h)^4 – (T_c)^4
but if photons are coming from both, it should be proportional to:
(T_h)^4 + (T_c)^4
The ratio of these counts:
R_c/R_h = (T_h)^4 – (T_c)^4/[(T_h)^4 + (T_c)^4]
~ 4 * dT * (T_c)^3/[2(T_c)^4]
~ 2*dT/T_c
Frank:
The above is a proposal to answer the question you stated before:
“The original challenge was to prove experimentally that radiative fluxes travel from hot to cold and cold to hot, with the former being stronger – despite questions of how to interpret the readings from an IR thermometer into W/m2.”
It doesn’t use an IR thermometer, however.
Neal,
I suspect that when you get into the details, it still won’t work. You’re leaving out quite a lot. Photons are not emitted strictly perpendicular to the surface and a perfectly reflecting pipe is not a collimator. It’s identical, in terms of emission and absorption of radiation, to a disk with the area of the pipe at the end of the pipe. Your detecting molecules can emit as well as absorb. A perfectly reflecting pipe perfectly reflects molecules as well as photons. And that’s just off the top of my head.
“I suspect that when you get into the details, it still won’t work. You’re leaving out quite a lot.” OK, let’s consider:
– “Photons are not emitted strictly perpendicular to the surface and a perfectly reflecting pipe is not a collimator. It’s identical, in terms of emission and absorption of radiation, to a disk with the area of the pipe at the end of the pipe.” Good point: We only need the caps (not the whole slabs), which are held at T = T-left = T_h and T-right = T_c. The pipe is cooled to T_0 << T_c < T_h, so it does not emit at the IR frequency of interest. The purpose of the pipe is to keep T-left-sourced photons coming generally from the left and T-right-sourced photons coming generally from the right, so that they impart opposing forces on the dropping molecules or clusters. The deflection will be towards T_c: switching T-left with T-right will switch the direction of deflection, but the total rate of IR photon counts will not change.
– "Your detecting molecules can emit as well as absorb." Detection is by IR-frequency photocell banks cooled to T_0.
– "A perfectly reflecting pipe perfectly reflects molecules as well as photons." So what?
– "And that’s just off the top of my head." Keep scratching.
Neal,
Molecules deflected into the pipes don’t disappear. They’ll eventually absorb another photon and be deflected back into the gap or emit a photon with the same effect.
But this is all idle speculation as no one is ever going to get funding to build a real device, not to mention that perfect reflectors don’t exist. I’m convinced that it won’t work, you’re convinced that it will. Further discussion is pointless.
DeWitt:
– If the molecules/particles are falling at the right speed when they pass the pipe, they won’t be deflected into the pipe, they’ll just be deflected from where they land below the pipe break.
– If you think these sorts of arrangements can’t be done, check out the Nobel Prize award for 1997. The details were at least as delicate – and Bell Labs funded it in the 1980s.
This discussion is of little relevance for the actual question, i.e. for finding ways to prove that photons move in both directions. The only existing consistent and comprehensive theory is standard physics. All alternatives are so poorly defined that little generally valid can be said on, what differentiates them from the standard physics.
Concerning the thought experiment with the pipe you must notice that photons move with comparable intensity in all possible directions in a perfectly reflective pipe, not only in the direction along the pipe but also at all angles relative to its direction. Some of them get reflected very often, some only rarely depending on the angle. Therefore many photons will escape through any gap in the pipe independently of the presence of some molecules in the pipe. Doing such a real experiment seems to by impossible anyway. Therefore this is just a thought experiment.
I come back to what I have written before. We have theories of physics that describe extremely well everything related to electromagnetic radiation. Any theory that disagrees significantly in any of its predictions from the present physical understanding in these areas is virtually certain to be wrong. It’s, however, possible that the same physical theory can be expressed using different concepts in a way that appears very different, while it makes exactly the same predictions for every observable effect. For this reason it’s difficult to be sure about the possibility of extending a partial different theory to a correct comprehensive theory. Proving that the partial theory is a wrong partial theory may be impossible, and also unnecessary as long as the alternative represents only loose ideas rather than full theory.
Pekka:
– The purpose of the pipe is not to align the velocities of the photons to be along the axis, but to confine the region of photon/molecule or photon/cluster interaction.
– Doing such an experiment is not only possible, but is exceeded in difficulty by particle physics experiments at CERN on a daily basis.
Pekka, Neal, DeWitt: Do all of the instruments available for measuring IR work by measuring a difference between to fluxes?
In the case of visible radiation, photomultipliers that are capable of counting individual photons don’t appear to measure flux by taking a difference. So if we are dealing with temperatures hot enough to emit visible light, I suspect one could demonstrate a two-way flux. If an analogous device existed for measuring IR (with a detector near absolute zero to suppress thermal excitation), it would do he job.
Frank:
– Photomultipliers detect incident photons, not differences between forward/backward fluxes. However, a photomultiplier is a macroscopic piece of equipment, and works in only one direction. If you stick a photomultiplier pair, back-to-back, between T_c and T_h, you are no longer measuring the same situation.
– This is why I have set up my photomultipliers to detect the photons that have scattered or been emitted from the region of interaction, not between T_c and T_h.
Frank,
To me the natural approach is to test theories wherever they produce predictions that differentiate them from alternatives. The photovoltaic effect at short enough wavelengths is a good example of such an opportunity.
All measurements of properties listed in HITRAN database confirm as well that the theory is correct, because all lines listed have been also calculated from the theory (many weaker lines have been only calculated, but a very large number of lines has been both measured and calculated).
The success of the quantum theory of electromagnetic interaction does not end with the above, and nothing has been observed that would contradict it. (We would know, if something had been observed and the observation confirmed.)
All in all there’s no space for doubting the theory. There isn’t any reason to avoid using the concepts and language of the theory. On this basis the description of the radiative energy transfer as a two-way process is valid. It’s also the only existing valid description. That’s enough, there’s no reason to worry about the possibility of an alternative formulation that uses different concepts, but it’s good to remember that alternative equivalent formulations are rather common in physics.
“There isn’t any reason to avoid using the concepts and language of the theory.”
On this point I will agree with Pekka. The concept that both T_c and T_h emit photons at each other makes sense and fits in perfectly with all aspects of normal physics. CJ’s idea, that emission from T_c to T_h is prevented and emission from T_h to T_c is reduced, is incoherent, relies on spooky action-at-a-distance, and does not fit into normal physics.
There is therefore no need to experimentally test which theory works better; however, it is worth knowing that such an experiment could be done, and that there is essentially no doubt as to which would be supported.
Neal,
The purpose of the pipe is not to align photons, but that’s fully consistent with my point.
The problem is that photons do exit the pipe through any gap, whether you add some molecules in the pipe or not, and that takes place in all directions. Thus there’s no way of knowing whether a photon has scattered from a molecule. Adding molecules may make some photons to scatter towards the detector, but the molecules may also prevent some other photons from hitting the detector. Thus it’s not obvious that any detectable signal results from adding molecules.
Particle beams at CERN have very different properties from those of the photons in your thought experiment. Polished metal surfaces may be very good reflectors for IR photons, but they are not perfect. Any surface will also absorb and emit IR. That’s likely to make the experiment impossible in practice.
SoD:
Is there a way to incorporate an image (*.gif or *.png) into a reply? I think the discussion would benefit from some pictures. Or maybe I can send you a picture, and you can paste it in.
Pekka & DeWitt:
There is a clear and unsubtle difference between a two-directional flow of photons and a one-directional flow with the same net magnitude. That you think it difficult to demonstrate I find surprising; that you think it impossible, I find astonishing.
Neal,
Pekka has restated in a more elegant form exactly what I said above. It can’t work. As long as the detector can see any part of the disk directly, there will be no difference between the observed intensity calculated by a one way or a two way formulation. It is, in fact, no different than putting the detector between the disks, other than the observed intensity will be smaller due to the smaller view factor.
A collimator works because radiation at high angles is reflected so many times that it’s attenuated beyond detection. Therefore, you can’t make a collimator from a perfect reflector. An ideal collimator, in fact, would have perfectly absorbing walls and infinitely long. But a perfect collimator would also have zero intensity, as the units of the Planck equation for emission intensity from a surface are Watts/(Area * solid angle). As the solid angle goes to zero, so does the intensity.
DeWitt:
– The detector will not see any part of the disk.
– The pipe serves to define a small region for interaction, not to collimate the IR photons.
Pekka:
– Molecules/clusters can be dropped individually to avoid mutual interference problems.
– I realized that a reflecting pipe is much less useful than an absorbing pipe, so the set-up has been modified accordingly.
SoD has kindly agreed to insert my diagram with this posting, so here is where it goes:
The diagram shows the vertical cross section of a chamber for detecting photons going from left to right and from right to left.
On the left is the radiating disk at temperature T_L, shown as red; on the right is the disk at T_R, shown as blue. The disks are embedded as end-caps of cylinders of radius R_1, of blackbody material held at temperature T0 << T_c ≤ T_L, T_R; therefore IR photons emitted from the disks will, to large extent, be absorbed by the walls of the cylinder of radius R_1 they inhabit; those photons that miss the walls will enter the "photon interaction zone", through which the molecules/clusters will pass; and photons that do not interact with the m/c will enter the annular region of radius R_2 of the opposite cylinder, where they will largely be absorbed.
Thus, if there are no interactions between m/c and IR photons, one expects that all photons from the radiating disks will be absorbed by the very cool blackbody cylinders on one side or the other. However, if there are interactions between the IR photons and the m/c, the scattered or emitted photons can be emitted in all directions. In particular, some will be emitted roughly perpendicular to the cylinders and will thus escape both the interaction zone and the cylinders. Thus, if we aim a bank of IR photomultipliers at the green zone, they will pick up a signal if m/c – photon interaction is going on; otherwise not.
So what do we expect? It depends on the cases:
– If T_L = T_R = T0 < T_c, no m/c: Ideally, there would be no signal, because every photon should be absorbed by a blackbody at temperature T0. In fact, characterizing what actually occurs in this case will be the most difficult and most crucial aspect of the measurement.
– If T_L > T_R, no m/c: Similar to above. As a check: the signal for the case T_L – T_R = dT should be the same as for the case
T_R – T_L = dT. If it’s not, something is wrong.
Now add the m/c. They can be dropped individually, so that their interactions are not confused.
– If T_L = T_R = T0, there should be no signal.
– If T_L = T_R > T_c, there should be a signal; but the average deflection from a straight vertical drop will be zero, by symmetry.
– If T_L > T_R, there should be a photomultiplier signal, and the average deflection will be towards the right.
In general, I would expect the average deflection to be proportional to (T_L^4 – T_R^4), whereas the photomultiplier signal would look like (T_L^4 + T_R^4). However, if CJ’s concept is right, we would expect the photomultiplier signal to also look like:
(T_L^4 – T_R^4).
Neal,
I find it very easy to justify that the energy transfer is two-directional, but I find it easy only through accepting that standard physical theories and paradigm are applied.
That does not imply only that it’s accepted that the concrete results from those theories are correct, but also that the concepts and language of those theories are to be used.
When the above is accepted, there’s nothing more left to show. If the paradigm, concepts and language are rejected, then we lack the prerequisites for discussing the whole issue.
Pekka:
What remains is Frank’s query: “The original challenge was to prove experimentally that radiative fluxes travel from hot to cold and cold to hot, with the former being stronger – despite questions of how to interpret the readings from an IR thermometer into W/m2.”
The fact that something need not be done does not imply that it cannot be done.
Neal,
What I have been saying that the whole question may become badly defined, if we give up present physical theories. (What is “radiative flux”, when we cannot apply existing standard physics?)
When we accept present physics, we know the answer, when we give up the present theories, even the question may become poorly defined or at least we lose the theoretical background that’s needed for doing any measurements, because all empirical methods are built on the present physical theories.
By your reasoning, a high-school measurement of the acceleration of gravity constitutes a challenge to Newton’s laws.
Neal,
As long as QM plays no essential role, similar problems do not come up.
In QM many things are in a sense undefined until they have been observed. That allows for alternative formulations of the theory and different interpretations for that part of the physics that’s not directly observed.
Here QM is essential, because Planck’s law can be understood only using QM. (Planck presented it before QM was developed, but could not really justify it theoretically without QM.)
Pekka:
Frank is not asking about Planck’s laws. He’s asking if it’s possible to tell whether there is a 1-directional flow of photons or if there is a 2-directional flow.
The answer is, Yes, it is.
Pekka:
As I pointed out above, a collimated beam of radiation from a thermal source will have very low intensity. The detector geometry is an additional several orders of magnitude reduction in detection efficiency. You’re going to have to have a lot of molecules in the gap at any time to have any absorption, and even more to be able to detect emission.
I’m assuming that the molecules in question have very low kinetic energy so they’ll stay in the gap long enough to absorb and emit. That has the consequence of drastically narrowing the absorption line width, making absorption much less likely, requiring an even higher number density in the gap.
Another problem is momentum. With a collimated beam, absorption of a photon will tend to push the absorbing molecule out of the gap, or make it collide with another molecule and possibly lose energy.
I don’t know if stimulated emission will be significant under the conditions in question, but if it is, then stimulated emission photons will not be emitted in a direction that allows detection.
And that’s still just scratching the surface.
DeWitt:
As I pointed out above, a collimated beam of radiation from a thermal source will have very low intensity.
– The solid angle subtended at the center of a radiating disk is
2π (1 – cos(θ)), with cos(θ) = L/sqrt(L^2 + R1^2) ; although taking into consideration the Lambert intensity shape, the reduction factor is not
2π (1 – cos(θ))/ 2π = (1 – cos(θ), but
π *sin^2(θ)/π = sin^2(θ)
In the diagram, L ~ 8 R1, so
sin^2(θ) = (R1)^2/[R1^2+ L^2] = 1/65 = 0.0154
Wow, that’s bad enough to make one give up physics, right? NOT.
The detector geometry is an additional several orders of magnitude reduction in detection efficiency. You’re going to have to have a lot of molecules in the gap at any time to have any absorption, and even more to be able to detect emission.
– That is a function of the background temperature T0, and how much time one wants to spend: I would run thin on the m/c rate. A Japanese group ran an experiment over 9 years, with thousands of full days, in hopes of detecting proton decay. They managed to set a lower bound.
I’m assuming that the molecules in question have very low kinetic energy so they’ll stay in the gap long enough to absorb and emit. That has the consequence of drastically narrowing the absorption line width, making absorption much less likely, requiring an even higher number density in the gap.
– Again, that is an issue of time.
Another problem is momentum. With a collimated beam, absorption of a photon will tend to push the absorbing molecule out of the gap, or make it collide with another molecule and possibly lose energy.
– Since I haven’t specified whether we’re talking molecules or clusters, the mass of the target is a free parameter that can be picked for experimental convenience. Suffice it to say that it should be possible to arrange matters so that the m/c falls to the bottom, as expected. I am not concerned about multiphoton impact on a single m/c.
I don’t know if stimulated emission will be significant under the conditions in question, but if it is, then stimulated emission photons will not be emitted in a direction that allows detection.
– The m/c would have to already be in the excited state for stimulated emission to occur. I don’t think that excluding such states would be difficult, in the context of the manipulations utilized for the cold m/c.
And that’s still just scratching the surface.
– Seems more like scraping the barrel than scratching the surface.
Can’t be done. That implies that photons are classical particles. They’re not. But even particles with a rest mass, like electrons, cannot be detected without perturbing them in some way. That’s where the Uncertainty Principle comes in. If you set up a two slit electron diffraction experiment with a method of determining which slit each electron passes through, you don’t get an interference pattern.
Pekka wrote: “To me the natural approach is to test theories wherever they produce predictions that differentiate them from alternatives.” “All in all there’s no space for doubting the theory.”
I’m don’t doubt QM. It has been thoroughly test and has no challenges. I was merely interested in a SIMPLE experiment or thought experiment that would demonstrate one of the consequence of QM, two-way fluxes. You and others have successfully demonstrated that I haven’t properly considered the problems. IMO, the absence of a simple way to detect a two-way flux (classically or “quantumly”) does not constitute a challenge to QM or its consequences.
Frank:
Please consider:
SoD should be adding the diagram soon.
[Sorry for the delay]
DeWitt: I left this old conversation with the impression (possibly wrong) that no experiment could distinguish between a two-way flux of photons (oTa^4 and oTb^4) and a one-way flux o(Ta^4 – Tb^4). Does this conclusion change if we are able to count individual photons?
Frank:
My points directly above argue that it is not impossible to tell the difference between one-way and two-way photon flux; in fact, it’s not even difficult.
Neal:
Although DeWitt convinced me that my experiments proposed above would not produce results inconsistent with one-way flux, I didn’t fully understand the experiment you proposed and counter-arguments made above. I recently showed a family member the video of Feynman’s first QED lecture and was reminded of the reasons that light is a particle. When we talk about one-way vs two-way flux, we assume that o*(Ta^4 – Tb^4) can take on any value, that it is continuous. So my poorly thought out question was: Does the discrete nature of light provide a simple way to prove that the flux must be in both directions. When we think of light as waves, they can cancel. If we think of them as countable particles with amplitudes that constructively and destructively interfere, there are additional possibilities.
Re-reading what you wrote above, I see that you HAVE taken advantage of the particulate discrete nature of light in your proposed experiment. I’m sorry I didn’t take the time to earlier to understand the information you generously shared with readers of SOD, but I’m sure you recognize the difficulty of separating the wheat from the chaff at blogs.
Frank:
l believe I have produced a reasonable method by which the 1-way flux theory can be distinguished from the generally accepted 2-way flux theory. DeWitt does not agree, but refuses to present a meaningful argument. This is one of only two occasions that I have concluded that he has been flat-out wrong on a matter of scientific fact.
The other point I’ve addressed is that the 1-wft leads to a violation of Heisenberg’s uncertainty principle, so it’s dead in the water anyway.
Yes; particularly when one person is arguing against folks who are rarely wrong.
Neal,
I could be wrong and your experiment could work, but I think the Uncertainty Principle won’t allow it to work. It’s too bad that Pekka isn’t around any more. I would yield to his expertise.
DeWitt:
The points you have raised recently mention only issues related to how long the experiment must be run. You have not raised any questions related to the Uncertainty Principle.
There was an issue with regard to “perfectly reflecting pipes”; but when I recognised that in fact the setup made more sense with blackbody pipes (see “vantablack”), I believe that eliminates the concerns Pekka raised: emissions from the T0 areas will be minimal, and 2nd-order emissions from m/c’s will be 2nd-order in the density..
So what exactly is your objection?
Neal,
I didn’t go back and check exactly which experiment was being discussed. If you’re talking about perfectly collimated, perfect detectors which absorb but don’t emit, whether behind looking through a tiny hole or at right angles to the parallel planes at different temperatures with tiny mirrors, it won’t prove to the one-wayers that there is two way absorption and emission.
Any non-emitting area that is larger than the wavelength of interest will detect photons by either the one-way or two-way theory. Remember that in the one-way construction, there is extra physics that doesn’t allow photons to be emitted if they can’t be absorbed. Conversely, if they can be absorbed, they will be emitted.
I thought I had made the Uncertainty Principle argument as well, but maybe I didn’t. It has to do with the size of the mirrors and how they are suspended and oriented. Since I’m not going to bother going back and re-reading the thread, I’m not going to go into more detail than that.
DeWitt:
There are no tiny mirrors. I don’t know where you are getting this from. I’m sorry, but your claims amount to “I don’t have an argument but I know you’re wrong; so I’ll make stuff up.” Very disappointing, DeWitt: not up to your usual standard.
If you want to look for a UP problem, consider the case of an hot sphere (radius r) sending photons to cold objects but not to cold objects (1-way flux theory). Place it at the centre of a large spherical shell (radius R) constructed of small sectors that are mutually isolated from teach other, thermally; each is of size d and subtends the small half-angle theta. Take a cold sector and surround it by hot sectors. According to the1WFT, the photons emitted towards that cold sector will not land at any of the hot sectors around it. Therefore, the momentum in the transverse direction must be less than (p * theta), where p is the photon momentum. The location of the photons at their starting point is confined to transverse distance 2*r.
Therefore:
uncertainty in original transverse location < r
uncertainty in transverse momentum within the beam-let < p * theta
But 2 * theta = d/R
Therefore the product of the uncertainties is:
(delta-x)*(delta-p_x) (pi) * [r*d/wavelength] , then
(delta-x)*(delta-p_x) (pi) (1 (m)) ~ 3 meters
So according to the 1WFT, I can violate the Heisenberg Uncertainty Principle by proper construction of a spherical shell of radius slightly larger than 3 meters. What a deal!
Sorry, the math didn’t get presented right. Try again:
a) Uncertainty in transverse location = r
b) Uncertainty in transverse momentum within the beam is = p * theta
But. theta = d/(2R).
c) Therefore the product of the uncertainties is:
(delta-x)*(delta-p_x) = r * p * theta = p * rd/(2R)
d) Since p = Planck’s constant/wavelength
= h/WL
(dx)*(dp) = h * rd/(2R*WL)
= hbar * (pi)rd/(R*WL)
If we choose:
r = 1 mm = 1e-3 (m)
d = 1 mm = 1e-3 (m)
WL = 1 micron = 1e-6 (m) for infrared
(dx)*(dp) = hbar * (pi)/(R/meter)
This will be 2(pi) ~ 6.3 meters
So when applied to an easily constructible spherical shell, the 1WFT violates the Heisenberg Uncertainty Principle.
But my main point remains: No supportable argument has been presented that 2WFT cannot be distinguished easily from 1WFT. Indeed, my experience talking with friends who know more about experimental physics than I do is that, when I can imagine a way to do a measurement, they can design one that is much more sensitive; and the people who end up actually doing the real experiments have been cleverer still.
DeWitt: You may want to review Neal’s diagram above dated June 2, 2015 at 11:43 am, which is located just above where I restarted this topic. I didn’t re-read everything above either, but the diagram is intriguing. The beam of molecules crossing perpendicular to the two light sources separates two or three beams due to the momentum of the absorbed photon when the light source(s) are turned on.
FWIW, I’m thinking in terms of a beam of He+ ions. The uncertainty principle certainly places theoretical limits our ability to focus that beam on a certain location. I’m not sure how this is calculated. In practice, HR-MS apparently can resolve a difference of 0.003 amu in ions with mass of 1000 amu. For hydrogen, the ground to first excited state line is at 121 nm, so I’m guessing this is 60 nm for He+. p = h/lambda = 1E-26 kg-m/s. 4 amu is 6.6E-27 kg, so the perpendicular velocity change per helium ion absorbing a photon would be about 1 m/s? Are you sure we can’t detect a change in velocity this large?
I too would like to express my regrets in hearing that Pekka is no longer with us.
I found his comments to be insightful and well worth reading.
Sadly missed.
Frank wrote: “You may want to review Neal’s diagram above dated June 2, 2015 at 11:43 am,”
But the diagram is pointless without a description of the experiment and an explanation of what it is meant to show. I suspect those are given, scattered through the over 300 posts here, but that is not much help. Any chance of a summary of where the discussion stands?
Mike M.
I’m totally bored with this subject. There’s literally nothing you can do to convince, say, C. Johnson to admit that he’s wrong about one way only radiative energy transfer. He’s always going to find a way to explain results to fit his theory.
DeWitt: Claes is a mathematician, not a scientist. Euclidean and non-Euclidean geometries are equally valid from a postulates+deduction point of view – no matter which one agrees with our observations of the real world. Both are logically consistent. Likewise, his theory of radiation and quantum mechanics are equally valid. The idea of identifying situations where two different theories make different predictions and running an experiment to decide which is correct doesn’t seem to be part of his way of thinking.
On the other hand, experiments are the way scientists discard unsound theories. I find one-way flux absurdly more complicated to rationalize than two-way flux and I hate its origins in a misunderstanding of the 2LoT. That doesn’t mean I’m not interested in an experiment that proves it wrong.
Mike: I don’t know what kind of experimental set up Neal envisioned. If one imagines an atomic system, He+ is about as light as one can get with as tightly held an electron as possible. This makes the largest possible change in velocity (1 m/s) when a photon is absorbed. Chemists have lots of practical experience working with ion beams. He atoms do have an average molecular speed of about 1000 m/s at 300 K and 7 m/s at 0.01 K, but we can select, focus, or “trap” the ones we want. The uncertainty principle presumably limits our ability to focus. So it may be worth contemplating an experiment done with He+.
Nuclear physics involves gamma rays with much larger amounts of momentum. I don’t know much about working with them. Advocates of one way flux may capriously reject gamma rays as models for thermal infrared. The quantum/particulate nature of light generally becomes more apparent at higher frequency. What processes involve absorption of photons of 1 nm or less?
Frank,
The absorption or emission of a photon causes a recoil. That can surely be measured. But what is the point of the experiment? To prove something to certain skeptics, it seems, but what is to be proved? Neal J. King makes reference to one way or two way transfers, but I have no idea what that means.
Mike: Imagine the beam of He+ ions passing through a chamber with UV light sources of equal intensity perpendicular on either side. In the dark, the He+ ions are detected in one place. Turn of the left light source, some of the He+ ions are deflected to the right due to absorption and most pass through unchanged. Does the uncertainty principle prevent us from observing He+ arrive at two different locations?
If no, turn on the right light source and the opposite should happen. Then turn on both: Do you see He+ ions deflected to three different positions (two-way flux), or mostly arriving the dark position (one-way flux canceling)?
If I were smarter, I might be able to express this in terms of amplitudes instead of semi-classically.
The advocates of one-way flux might assert that the He+ atoms constitute some sort of detector with a temperature that emits radiation that interferes with the incoming radiation. However, a negligible amount of “thermal” UV radiation capable of exciting He+ above its ground electronic state will be present at ordinary lab temperature and He+ doesn’t appear to have any excitable rotational or vibrational states. The excited state of He+ ions can emit a photon. We probably need to know something about the life-time of the excited state so we can be sure that most excited He+ ions reach the detector before emitting a photon (relaxing to ground state). If I remember correctly, the delta_E*delta_t version of the uncertainty principle is responsible for the width of emission lines when nothing larger (like Doppler shift) interferes.
Mike M.
Some people don’t think that all objects always emit EM radiation according to their emissivity and temperature. In the specific example of two parallel plates of infinite extent at different temperatures, they think that only the warmer plate radiates energy to the colder plate, i.e. one way transfer. You would think that having two plates that radiate in the visible, but at different temperatures would be a counter example. But you only know if both plates radiate if you look at them. As I pointed out above, the counter-argument is that radiation only occurs if there is an absorber at a lower temperature. The observer provides that absorber so it’s not proof. It also requires unknown extra physics, but who cares about that.
They also think that one way transfer invalidates the greenhouse effect. But it doesn’t. Even if there is only one way transfer, the temperature of the absorber affects the temperature of the emitter. P = ε(σ1T1^4-σ2T2^4). In other words, the sky still has an effective temperature well above absolute zero.
I think I managed to reverse ε and σ. σ should be the Stefan-Boltzmann constant and ε the emissivity, but the equation is written the other way around.
I think I have to agree with DeWitt. Anyone with the knowledge base and reasoning skills to be convinced by the experiment will be convinced by fundamental theoretical arguments. And the sort who believe that the temperature of the absorber influences emission from a source, will always find something to object to, so as to avoid having to change their minds.
The purpose of the experiment was not to convince CJ of the falseness of 1-way flux (1-wf), but to show that if thermal radiation actually were to operate by means of 1-wf, that an experiment could be done to detect it.
Basically, the idea the idea is that if everything is at lowest available temperature T0, we define that as a “no signal”; if T_Left (or T_Right) is higher than T0, but there are no atoms/molecules/clusters dropped into the interaction chamber, there is still essentially no signal. Why no signal? Because the T0 surfaces absorb everything that hits them and emits at a very low blackbody rate. (Remember, this is not at thermal equilibrium.)
If you drop “a/m/c”s, which have absorption lines in the stronger parts of the blackbody spectra for T_Left and T_Right, into the chamber, there will be absorption of photons at the lines, and the amount will be proportional to the average density of “a/m/c”s in the chamber. If the particles are hit from the left, they’ll recoil to the right; if from the right, they’ll recoil left.
So if 1wf theory is believed, you would expect that if T_Left > T_Right, no photons will be going from right to left; therefore all deflections will be to the right. Under conventional theory, photons will be coming from left and right; there will be more from the left. As Frank said, you could look for a 3-spot pattern or a 2-spot pattern.
I did not make a selection of particle, because I wanted to be able to choose a value of mass and absorption line that would make the numbers work out. The first concern would be to reduce the random horizontal velocity of the particles, so that there are well-defined spots (either 3 or 2). The bigger the mass, the less the random horizontal velocity; but also the smaller the deflection from absorbing and later emitting the photon.
But I don’t feel particularly concerned about these constraints:
– We have yet to specify the drop distance below the interaction chamber: the longer it is, the higher the velocity bump resolution, so the larger the mass (and lower the random component) we can use.
– Transverse velocity for atoms can be greatly reduced with special techniques. The teaching assistant for the freshman physics class I took later did an experiment at Bell Labs that reduced the speed of atoms to such an extent that, when they were released from his trap, they would fall to the ground.
And in addition to the bump pattern on the ground, there will be a difference in the photon counts from the interaction chamber: According to conventional theory, the photon counts should be proportional to the sum of the Planck distributions for T_Left and T_Right, evaluated at the frequency of the absorption line; whereas by the 1wf theory, it should be proportional to the difference.
Neal,
As I said above, it doesn’t matter in terms of the atmospheric greenhouse effect if the energy transfer is one or two way. The result is exactly the same. The fluxes still approximately balance whether gross or net. An imbalance will still result in heating or cooling depending on the sign of the imbalance.
And an IR thermometer pointed at the sky will register an effective temperature provided it’s not so cold as to be below the lower range of the device. You can even get a fairly good measure of the total column water vapor using IR thermometer readings. http://journals.ametsoc.org/doi/pdf/10.1175/2011BAMS3215.1
Yes, but there are other questions related to radiation than the issue of the greenhouse effect. Frank had raised one of them, and that is what we had been discussing.
Neal, Mike, DeWItt: I think the value of an experiment of the type that Neal proposed (and I detailed for He+ ions) goes far beyond Claes or the GHE. There are very few simple experiments that demonstrate the quantum and atomic properties of light and matter. I did a quick search for experiments proving that light has momentum. Apparently you can shine a laser at a mirror mounted on a quartz fiber torsion balance and measure the deflection. A demonstration of the deflection of individual atoms when they absorb a photon of a specific wavelength would be a much more dramatic demonstration of the quantum nature of matter.
The problem is that generations of physicists seeking such demonstrations don’t appear to have performed one like this, so DeWItt’s concerns about the difficulties posed by the uncertainty principle may be well founded. The Manhattan Project tried to separate U-235 and U-238 by mass spec, so the technology to do this experiment has been available for almost a century.
In any case, I’d like to also consider a version of the experiment where the light coming from the left and right comes from a laser with a beam splitter and therefore is coherent. Will we still see the stream of He+ ions still split into three paths when the light comes from both sides?
Frank wrote: ” There are very few simple experiments that demonstrate the quantum and atomic properties of light and matter. I did a quick search for experiments proving that light has momentum. Apparently you can shine a laser at a mirror mounted on a quartz fiber torsion balance and measure the deflection. A demonstration of the deflection of individual atoms when they absorb a photon of a specific wavelength would be a much more dramatic demonstration of the quantum nature of matter.”
The momentum of light is a classical result. The experimental demonstration using a quartz fiber torsion balance is called a Nichols radiometer, first done in 1901 (so says Wikipedia). No need for lasers or quantum mechanics. You can buy toys that are sometimes claimed to demonstrate thE effect, but they are actually driven by differential heating. Another demonstration of the classical effect is the fact that NASA can send probes to other planets. In at least some cases, failure to account for radiation pressure would cause them to miss badly.
The Compton Effect demonstrates the momentum of individual photons, via scattering of X-rays off electrons. I guess it was dramatic: it was a key experiment in establishing quantum mechanics and got Compton the Nobel Prize. Other phenomena that depend on the momentum of individual photons are the Mossbauer Effect and laser cooling. The former uses gamma rays, the latter using visible light, but at extremely low temperatures. I think that says something about how the energy of the photon needs to compare to the thermal energy of the interacting particle if you want to observe a result.
I don’t think any of these experiments, or Neal’s, could be called simple. But I suppose simple is in the eye of the beholder.
You missed the Stern-Gerlach experiment, which featured prominently in Feynman’s Physics Lectures. There’s also the Davisson-Germer experiment that demonstrated electron diffraction. Electron diffraction can also be used to demonstrate the Uncertainty Principle. If you put detectors on the slits that can tell which slit the electron goes through, you don’t get a diffraction pattern. Detectors with lower detection certainty will give a mixed pattern.
The interaction of lasers with atomic beams is well studied.
http://iopscience.iop.org/article/10.1088/0022-3700/17/23/009/meta;jsessionid=61D2F89D4156D725AD65A688C2694E77.ip-10-40-2-73
DeWitt Payne wrote: “You missed the Stern-Gerlach experiment, which featured prominently in Feynman’s Physics Lectures. There’s also the Davisson-Germer experiment that demonstrated electron diffraction.”
Well, I was not trying to be comprehensive. And although those were among the key experiments establishing quantum mechanics, I don’t think either had to do with the momentum of individual photons.
Momentum associated with a radiation field is a classical result. I think that in the “old quantum mechanics” (prior to the contributions of Heisenberg and Schrodinger) momentum was a statistical property of an ensemble of photons but not a property of individual photons. The Compton experiment killed off the old quantum mechanics and was promptly followed by what we now know as quantum mechanics. I am not sure, but I think the full significance of the Stern-Gerlach and Davisson-Germer experiments was not realized until after the new quantum mechanics was introduced.
Frank wrote: “There are very few simple experiments that demonstrate the quantum and atomic properties of light and matter.”
When I wrote this dubious statement, I was thinking of experiments simple enough to be demonstrated in college physics lecture or laboratory. If it is possible to observe the transfer of momentum to ions in a beam when they absorbs photons, I’m not smart enough to have been the first to have thought of the idea. I haven’t even been able to figure out what limitations the uncertainty principle places on such an experiment. So I searched for experiments demonstrating photon momentum. OTOH, I may be the first one dumb enough to think that such an experiment would mean anything to advocates of one-way flux.
If one did use a laser and beam splitter to illuminate a beam of ions from both sides, I think interference would be observed: However, interference would vary with location – changing from no absorption (beam deflection) along some parts of the beam path to doubling of absorption (beam deflection) in other locations. I’m not sure if the advocates of one-way flux believe interference is responsible, but interference doesn’t make photons “disappear”. When they disappear from some locations and are found elsewhere.
This article derives the “momentum density” for classical EM waves (pc = EXB) and asserts that p = energy/phase velocity for all waves. So the QM revolution didn’t add momentum to EM waves, it merely quantized momentum as well as energy of light.
Click to access peskin.pdf
Frank,
We did the Mossbauer effect experiment in undergraduate physics lab in the mid 1960’s. I forget whether it was freshman or sophomore physics lab. If you search on ‘mossbauer effect undergraduate physics experiments’, you’ll get a lot of hits.
Frank:
A split coherent beam coming at the dropped particles from two sides will not show interference, for an interesting reason: If the photon came in from the left, the particle gets kicked to the right; but if the photon came in from the right, the particle gets kicked to the left. So by looking at the end state we can tell which way the photon came; therefore, you cannot add the amplitudes, and so there will be no interference.
However, in the case of macroscopically large particles, which won’t be effectively jolted by the photon, there would be interference effects. This would be a situation constrained by the UP.
Neal: You are certainly correct, if I can tell which way an absorbed photon came from, so the probabilities are added not the amplitudes. What happens to all of the photons that are not absorbed? They experience interference? What if the uncertainty principle prevents me from telling which side the photon came from?
I like to point out the absurdity of an emitting molecule sensing the mean kinetic energy of it neighbors, encoding that information in a photon it emits, and then a potential absorbing molecule sensing the mean kinetic energy of all of its neighbors before deciding whether to absorb an incoming photon.
Which scenario is more absurd? (:))
I have checked my thought experiment with an experimentalist friend from grad school. He likewise sees no specific uncertainty-principle issue. There is a large range of parameter values which can be used to fine-tune the experiment. A claim that NONE of them would permit the experiment to be carried out would seem to require justification.
I think this is another question, but it is about energy transfer from cold to warm. There are many cold objects in space that are photographed by IR cameras. The rings of Saturn which are minus 150 to 200 deg C, can give images to earthgrounded observatories. (impossible according to Claes Johnson and others).
I wonder if it is possible to gather IR radiation through IR parabolic reflector if it is directed out to space at night. And i wonder if you can get some warming from Saturn rings. If you could filter out all IR radiation from the atmosphere. Is this tried out in Experiments?
IR parabolic reflectors are very effective in receiving heat energy from distance.
NK: It also helps to distinguish between energy transfer (photons) and heat flow. Photons travel from the cold icy rings of Saturn and are focused by a parabolic reflector on warmer detectors or film on Earth. However, more photons travel from the warmer detectors or film on Earth through the same reflector to the rings of Saturn. Heat flux is the NET result of both processes and it is always flows from hot to cold. Individual molecules and photons don’t have a temperature (which is proportional to the mean kinetic energy of a group of colliding molecules). The 2LoT doesn’t restrict what individual molecules and photons can do – the 2LoT is the result when large numbers of colliding molecules follow the rules of QM.
Unless you are trying to melt the rings of Saturn, you don’t need to collect IR photons from Earth and direct them to a special location in space with a parabolic reflector. The surface of the Earth emits almost like a blackbody, meaning that no ordinary materials emit much more than the Earth, given its surface temperature. (Fluorescent lights, LEDs, and lasers are exceptional materials). The problem is that atmosphere is fairly opaque to the thermal IR emitted by the surface or focused by a reflector.
The atmosphere is fairly transparent to SWR. We can cool the planet by reflecting more SWR to space (increasing albedo). Ice caps, seasonal snow cover, clouds and sulfate aerosols are the biggest reflectors, while increasing vegetation and urbanization tend to reduce albedo. The other way to get heat above the opaque lower atmosphere is by convection, but convection requires an unstable lapse rate. To some extent, increasing convection requires warming of the surface.
nobodysknowledge:
The reference http://www.ipac.caltech.edu/outreach/Edu/Windows/irwindows.html
describes the IR windows in the Earth’s atmosphere: these are the frequency regions where there is visibility for astronomical purposes.
Whether you can measure interference or not depends on the measurement set-up. It’s hard to think about a UP issue until you have a definite arrangement you’re thinking about.
Thank you for answers.
I was not thinking of the problem with trapping heat at he focal point of a reflector, and that this point reflects back. A couple of years ago there was som writing about harvestinging blackbody earth IR energy. Should it be a problem to turn the device the other way, and harvest IR energy from the atmosphere? I know that it would cost more than you get.
nobodysknowledge wrote: “A couple of years ago there was som writing about harvestinging blackbody earth IR energy. ”
To convert thermal energy into work, you need to be able to dump heat to something that is colder than the thermal source. I think the idea that you refer to was that a device could be built that would convert ambient thermal energy (perhaps in the form of IR, I don’t recall) partly to work and dump the waste heat to space (very cold) using IR emission in the atmospheric window regions. That could work, at least in theory, but since the emission has to be in the IR windows, than so does any absorption. So the energy source could not be IR emission from the atmosphere.
The general rule is that you can only harvest effectively FROM something hotter than what you are harvesting FOR.
Neal: It would be very interesting if we could build devices that break your general rule. The general rule that nothing can emit more that a blackbody constrains the efficiency of incandescent lights and other devices, but is avoided by lasers, fluorescent lights and LEDs – which don’t have a “thermodynamic temperature”.
Can we have photovoltaic devices that harvest DLR? Average DLR is twice average SWR and arrives with only modest variability 24/7/52. Clearly such a device would emit more thermal radiation than it absorbed. If the incoming photons produced electron-hole pairs, they could be collected as electricity. Certainly such a device would quickly get cold, which could be useful since energy could flow from the cold atmosphere to a colder device. The strangest thing about following this line of thought is that any material capable of producing an electron-hole pair by absorbing a DLR photon will also be excited by thermal collisions. In other words, you won’t need to point the device at the sky to collect energy. We do have IR thermometers and thermopiles that convert thermal energy to electricity, but I don’t know if creation of electron/hole pairs is part of their physics. The 2LoT certainly should place some limits on what is possible, but electricity is one form of heat flux that I don’t understand how to analyze in terms of entropy and which is not usually discussed in terms of flowing from hot to cold. It would be interesting to know more about this subject.
Frank wrote: “Neal: It would be very interesting if we could build devices that break your general rule.”
Interesting does not begin to say it. The general rule is just the Second Law of Thermodynamics.
Frank wrote: “Can we have photovoltaic devices that harvest DLR? Average DLR is twice average SWR and arrives with only modest variability 24/7/52. Clearly such a device would emit more thermal radiation than it absorbed.”
Which says concisely why you can’t get work from such a device.
Electricity is a form of work, not heat.
“Frank wrote: “Can we have photovoltaic devices that harvest DLR? Average DLR is twice average SWR and arrives with only modest variability 24/7/52. Clearly such a device would emit more thermal radiation than it absorbed.”
We don’t communicate often enough that the meaning of your acronyms remains in my mind. Would you restate the issue w/o acronyms?
Neal and Mike: DLR = downward long wavelength radiation (LWR). 99+% of the roughly 333 W/m2 of DLR arriving at the surface of the Earth was emitted by the atmosphere. SWR = short wavelength radiation. 99+% of the SWR averaging about 168 W/m2 arriving at the surface of the Earth was emitted by the sun. Since the emitting sun is much warmer than photovoltaic devices creating electricity from SWR photons, the 2LoT places no thermodynamic constraints on generating electricity from SWR. Since DLR is emitted by the colder atmosphere, it is hard to picture being able to obtain useful work from DLR photons (unless the absorbing devices are colder than the atmosphere). Nevertheless, both types of photons (LWR/DLR and SWR) presumably could create electron/hole pairs and therefore electricity. I would be more confident applying the 2LoT to the problem of getting electricity from DLR if I understood how the 2LoT applies to electricity – which I assume is a form of heat transfer. Electricity doesn’t flow from hot to cold.
From some perspectives, the 2LoT is not a fundamental law. Statistical mechanics explains how large numbers of molecules that aren’t constrained by the 2LoT create a macroscopic world where there is negligible probability that the 2LoT will be violated. Planck’s Law appears to set a maximum limit for the amount of power emitted at a particular wavelength based on the emitter’s temperature. However, the derivation of Planck’s Law depends on the existence of a Boltzmann distribution of excited and ground states. We have been able to circumvent Planck’s Law by creating devices (lasers, fluorescent lights, LEDs) where a Boltzmann distribution of excited and ground states doesn’t exist – where temperature is undefined. Once temperature is undefined, the 2LoT doesn’t apply. That how we can have lasers, microwave ovens, LED’s and fluorescent lights. Boltzmann and his peers might have believed such devices were impossible, so I’m reluctant accept blanket statements about what isn’t possible. However, revolutionary changes are unpredictable and relatively infrequent.
So I find it interesting to speculate about a material in which an absorbed LWR photon can create an electron/hole pair and therefore electricity. What could we say about such a device?
1) As long as absorptivity = emissivity (which depends on LTE according to Grant Petty), if any electricity flows out, conservation of energy demands that the device get cold.
2) If electron/hole pairs are created by absorption of an LWR photon, thermal collisions should also be able to create electron/hole pairs in the material – unless it is much colder than things that emit LWR (thermal IR).
3) Probably need a system where a Boltzmann distribution of excited states is not present. That would avoid LTE and the 2LoT.
Others have speculated about the possible existence of various forms of Maxwell’s Demons for decades, so I hope others won’t judge my comments too harshly. At saner moments, I agree with Mike: “Which says concisely why you can’t get work from such a device.”
https://en.wikipedia.org/wiki/Maxwell%27s_demon
Frank,
It’s not just about creating electron-hole pairs. You have to be able to separate them. That involves concepts like the Fermi level and band gaps.
http://hyperphysics.phy-astr.gsu.edu/hbase/solids/band.html
A thermal IR photovoltaic device would have to be cooled significantly to generate power from atmospheric thermal IR. I’m pretty sure that it would take more power to keep the device cool than it could generate. Otherwise, you have a perpetual motion machine of the second kind, I think.
It seems possible to build both IR cooling devices and IR warming devices, dependent on the wavelength of the radiation. If you have one device picking up near surface IR radiation, it could harvest some energy according to that temperature. If you have another device filtering out IR from the atmosphere, it could become a passive cooling machine. I would think that the combination of these two could make some work, creating energy. And it could become a kind of perpetual motion machine, exept that the energy always will come from the sun.
As reference to IR cooling there are some interesting articles. (abstract)
“The recent progress on radiative cooling reveals its potential for applications
in highly effi cient passive cooling. This approach utilizes the maximized
emission of infrared thermal radiation through the atmospheric window for
releasing heat and minimized absorption of incoming atmospheric radiation.
These simultaneous processes can lead to a device temperature substantially
below the ambient temperature. Although the application of radiative cooling
for nighttime cooling was demonstrated a few decades ago, signifi cant
cooling under direct sunlight has been achieved only recently, indicating
its potential as a practical passive cooler during the day. In this article, the
basic principles of radiative cooling and its performance characteristics for
nonradiative contributions, solar radiation, and atmospheric conditions
are discussed. The recent advancements over the traditional approaches
and their material and structural characteristics are outlined. The key
characteristics of the thermal radiators and solar refl ectors of the current
state-of-the-art radiative coolers are evaluated and their benchmarks are
remarked for the peak cooling ability. The scopes for further improvements
on radiative cooling effi ciency for optimized device characteristics are also
theoretically estimated.” Radiative Cooling: Principles, Progress, and Potentials. Md. Muntasir Hossain and Min Gu*
Or as some scientists Raman AP1, Anoma MA2, Zhu L3, Rephaeli E1, Fan S1. write in Nature:” Further, the cold darkness of the Universe can be used as a renewable thermodynamic resource, ”
As Frank says, I am not sure if these things violate physical laws
nobodysknowledge,
If you’re enhancing cooling by radiating to space through the atmospheric window, you’re not violating the Second Law. Deep space has an effective temperature of 2.7K, so you have plenty of thermal efficiency to work with. Neither does low-E glass and high visible absorptivity, low thermal IR emissivity coatings for solar water heaters violate fundamental thermodynamics.
However, a device at ambient temperature that creates electricity from the ambient thermal radiation does violate the Second Law as well as solid-state physics.
A semiconductor cannot have a clean band gap of the magnitude the thermal energy kT, because the continual background thermal radiation will effectively erase the gap.
Frank,
I think there are some misconceptions in your post.
“how the 2LoT applies to electricity – which I assume is a form of heat transfer. Electricity doesn’t flow from hot to cold.”
Since electricity does not, in general, flow from hot to cold it is not a form of heat transfer. That makes it a form of work. By definition, heat transfer is a flow of energy that results solely from a difference in temperature.
“From some perspectives, the 2LoT is not a fundamental law.”
I think that all physicists would agree with me when I say that the second law is fundamental.
“Planck’s Law appears to set a maximum limit for the amount of power emitted at a particular wavelength based on the emitter’s temperature. However, the derivation of Planck’s Law depends on the existence of a Boltzmann distribution of excited and ground states. We have been able to circumvent Planck’s Law by creating devices (lasers, fluorescent lights, LEDs) where a Boltzmann distribution of excited and ground states doesn’t exist – where temperature is undefined.”
Planck’s Law applies specifically to systems at equilibrium. Constructing a non-equilibrium system does not “circumvent” the law, it just creates a situation for which the law does not apply.
“Once temperature is undefined, the 2LoT doesn’t apply.”
No. The second law still applies, although it might be hard to calculations.
“That how we can have lasers, microwave ovens, LED’s and fluorescent lights. Boltzmann and his peers might have believed such devices were impossible”
No, such devices simply convert work (typically provided in the form of electricity) directly into light; as opposed to an incandescent bulb that turns work into random thermal energy, some of which is emitted as light. I doubt that Boltzmann would have had any problem with the general concept of turning work into light, although he might well have had no idea how to do it.
“so I’m reluctant accept blanket statements about what isn’t possible. However, revolutionary changes are unpredictable and relatively infrequent.”
I am with Einstein on this one: “classical thermodynamics … is the only physical theory of universal content concerning which I am convinced that within the framework of the applicability of its basic concepts, it will never be overthrown.”
“So I find it interesting to speculate about a material in which an absorbed LWR photon can create an electron/hole pair and therefore electricity. What could we say about such a device?”
Dewitt has answered this: It is not enough to create the pairs, you also have to be able to separate them. In *any* semiconductor, electron hole pairs are constantly being created by thermal energy. That is the only reason the semiconductor can conduct electricity at all. But that does not generate an electric current.
Correction: After Hertz’s work on antennas in the 1880’s, Boltzmann and his peers did know how to convert electricity into electromagnetic radiation.
Mike and DeWitt: I certainly have misconceptions, and I appreciate your trying to illuminate the most blatant ones.
Arguments from authority don’t provide much illumination. Einstein also notoriously didn’t like QM and said God doesn’t dice with the universe. His opinion of that subject – so far – hasn’t proven valuable. Given the fact that the behavior of molecules, atoms and subatomic particles produces the behavior of the macroscopic world, I don’t think it is unreasonable to suggest that the 2LoT isn’t fundamental. On a microscopic scale, entropy has been “explained” as disorder in some systems. There are other problems:
The issue is that we have theories of the macroscopic world – gravity/relativity and thermodynamics – that are not completely consistent with (or understood in terms of) the behavior of the microscopic world – QM. Non-equilibrium thermodynamics is a controversial area that is new to me.
It’s my understanding that a p/n junction in a semiconductor creates the means to harvest electron/hole pairs as electricity. Why can’t the same thing work for lower energy electron/hole pairs created by DLR photons? One possible objection is that a semiconductor in which electron/hole pairs are created by DLR – and likely also thermal collisions – is no longer a semi-conductor – unless you cool it. That, of course, gets rid of the 2LoT problem too.
I’ll have to think more about Mike’s comments about electricity being work, but not heat. We can burn fuels in a conventional electricity plant and be limited in efficiency by a temperature differential, but not in a fuel cell.
Frank,
If the second law can be violated, all sorts on non-sensical things become possible; such as water pumping itself up hill or a cup of water spontaneously heating itself to the boiling point.
Imaging putting your hypothetical device inside a black box at uniform temperature. Do you think it would absorb IR from the box and produce an electric current? If so, then submerge the box in a pot of water and run the current through a resistor immersed in the same pot of water. The water heats itself.
You wrote: “I’ll have to think more about Mike’s comments about electricity being work, but not heat. We can burn fuels in a conventional electricity plant and be limited in efficiency by a temperature differential, but not in a fuel cell.”
Right. Chemical energy can, in principle, be converted completely to work. An ideal battery or fuel cell would do that; real ones don’t do as well. But heat can only be partially converted to work, with the fraction depending on the temperature difference available. When you burn a fuel, you convert the chemical energy to heat (an increase the entropy of the universe); then only a portion of that energy can be converted to work.
Frank,
According to statistical mechanics, the Second Law is not an absolute requirement. Violations, however, are extremely improbable and get less probable the larger the system. Think about shuffling cards and having them come up in order by suit. Now shuffle ten decks at once. Another classic example is having all the gas molecules in a room end up in one half of the room. The probability isn’t identically zero, but it’s really, really small. Nonetheless, there is speculation that in an infinite universe, there could be volumes where entropy is decreasing.
I’m guessing that you didn’t read the Hyperphysics link I posted above, or you didn’t understand it. If you have a semiconductor with a band gap such that only a small fraction of electrons are in the conduction band at ambient temperature, subjecting that semiconductor to ambient thermal IR will not increase the number of electrons in the conduction band.
DeWitt wrote: “According to statistical mechanics, the Second Law is not an absolute requirement.”
I don’t think that is quite right. The Second Law still applies to average behavior. What statistical mechanics adds is that there can be fluctuations about that average behavior. Such fluctuations are both small and brief on a macroscopic scale.
Given a large enough system and enough time, any given magnitude of a fluctuation can happen. But one can question if that is meaningful. Monkeys, typewriters, Shakespeare.
Mike,
Consider the ‘all the gas molecules move to one side of a box’ problem. If there is only one molecule, it will be on one or the other side, so if we pick one side, then the probability is 0.5. With two molecules, the probability that they will both be on the chosen side drops to 0.25, three molecules, 0.125. The general formula is 1/(2^n). If we take a 22.4 liter box at STP, the probability is 1/(2^6.022E23). As I said, not identically zero but very small. The classical formulation of the Second Law would have the probability be identically zero.
DeWitt,
“not identically zero but very small. The classical formulation of the Second Law would have the probability be identically zero.”
A distinction without a meaningful difference. Since the molecules are moving, such a situation would not only be extremely unlikely, it would be extremely transient. It would take a molecule about one millisecond to travel across a 22 L cubic box. I stand by what I wrote: The Second Law still applies to average behavior. What statistical mechanics adds is that there can be small, brief fluctuations about that average behavior.
Frank wrote: “I’ll have to think more about Mike’s comments about electricity being work, but not heat. We can burn fuels in a conventional electricity plant and be limited in efficiency by a temperature differential, but not in a fuel cell.”
Mike agrees: “Right. Chemical energy can, in principle, be converted completely to work. An ideal battery or fuel cell would do that; real ones don’t do as well. But heat can only be partially converted to work, with the fraction depending on the temperature difference available. When you burn a fuel, you convert the chemical energy to heat (an increase the entropy of the universe); then only a portion of that energy can be converted to work.”
Frank continues: So we agree that thermo places limitations on the efficiency of heat engines and not on fuel cells. What about light? Visible light from the 6000 K sun can be converted to electricity by a semiconductor near 300 K, but thermal infrared from the atmosphere at 250 K supposedly can not. Why? According to you (assuming I understand correctly), light is a form of heat and we must analyze this system (macroscopically) using the same rules as heat engines, not fuel cells. If we analyze the spectrum of thousands of photons from the sun or the atmosphere – but not a single photon – we can determine the temperature of their source. Therefore the absorption of a single photon and production of an electron/hole pair occurs without any information about the relative temperature of the emitter and absorber. We can’t apply the 2LoT to this situation. We can only apply the 2LoT to the net flux of large numbers of photons between the ground and the atmosphere. Assuming absorptivity equals emissivity (which is true if LTE exists), any collector of DLR photons must emit more photons towards the atmosphere than it absorbs. As best I can tell – unless we treat the electricity generated as heat and calculate its entropy – electron/hole pairs created by DLR might generate electricity. If electricity did flow from such a device, conservation of energy demands that its temperature decrease. There is nothing unprecedented about converting heat to electricity.
https://en.wikipedia.org/wiki/Thermoelectric_effect
The role of entropy (and band gap?) in the thermoelectric effect is discussed at the link below, but I failed to understand this material.
https://en.wikipedia.org/wiki/Heat_transfer_physics#Electron
The ultimate way of showing the a process violates the 2LoT is so show that delta_S for the whole process is negative. I’m not sure delta_S is negative unless the “entropy of electricity” is included.
Lasers, microwave ovens and other devices can appear to transfer light/heat from cold to hot because they don’t have a thermodynamic temperature – they have more molecules in an excited state than in the ground state. So, if I wave my magic wand and say that my DLR collector – like these devises – doesn’t have a thermodynamic temperature and is not in LTE, can we escape the 2LoT? More importantly, can we build a practical device that does so?
More than a century of failures to create devices equivalent to a Maxwell’s demon strongly suggests that Nature will “conspire” to defeat all such efforts. However, Nature is comprised of a microscopic world of particles that do not individually obey the 2LoT. The 2LoT is a consequence of the laws governing the behavior of these particles. As best I can tell, physics hasn’t completely mastered the interface between theories that apply to microscopic and macroscopic phenomena (quantum gravity, for example) nor non-equilibrium thermodynamics. See link below: On paper, the existence of macroscopic systems without a thermodynamic temperature and LTE appear to provide an opportunity for escaping the 2LoT. If electricity isn’t a form of heat flux involving entropy (dq/T), a second opportunity for circumventing the 2LoT may exist.
Frank wrote: “I’ll have to think more about Mike’s comments about electricity being work, but not heat. We can burn fuels in a conventional electricity plant and be limited in efficiency by a temperature differential, but not in a fuel cell.”
Mike agrees: “Right. Chemical energy can, in principle, be converted completely to work. An ideal battery or fuel cell would do that; real ones don’t do as well. But heat can only be partially converted to work, with the fraction depending on the temperature difference available. When you burn a fuel, you convert the chemical energy to heat (an increase the entropy of the universe); then only a portion of that energy can be converted to work.”
Frank continues: So we agree that thermo places limitations on the efficiency of heat engines and not on fuel cells. What about light? Visible light from the 6000 K sun can be converted to electricity by a semiconductor near 300 K, but thermal infrared from the atmosphere at 250 K supposedly can not. Why? According to you (assuming I understand correctly), light is a form of heat and we must analyze this system (macroscopically) using the same rules as heat engines, not fuel cells. If we analyze the spectrum of thousands of photons from the sun or the atmosphere – but not a single photon – we can determine the temperature of their source. Therefore the absorption of a single photon and production of an electron/hole pair occurs without any information about the relative temperature of the emitter and absorber. We can’t apply the 2LoT to this situation. We can only apply the 2LoT to the net flux of large numbers of photons between the ground and the atmosphere. Assuming absorptivity equals emissivity (which is true if LTE exists), any collector of DLR photons must emit more photons towards the atmosphere than it absorbs. As best I can tell – unless we treat the electricity generated as heat and calculate its entropy – electron/hole pairs created by DLR might generate electricity. If electricity did flow from such a device, conservation of energy demands that its temperature decrease. There is nothing unprecedented about converting heat to electricity. (You can complain that the mechanism of my device is now changing from photoelectric to thermoelectric and you may be right.)
https://en.wikipedia.org/wiki/Thermoelectric_effect
The role of entropy (and band gap?) in the thermoelectric effect is discussed at the link below, but I failed to understand this material.
https://en.wikipedia.org/wiki/Heat_transfer_physics#Electron
The ultimate way of showing the a process violates the 2LoT is so show that delta_S for the whole process is negative.
Changing directions slightly, lasers, microwave ovens and other devices can appear to transfer light/heat from cold to hot because they don’t have a thermodynamic temperature – they have more molecules in an excited state than in the ground state. So, if I wave my magic wand and say that my DLR collector – like these devises – doesn’t have a thermodynamic temperature and is not in LTE, can we escape the 2LoT? More importantly, can we build a practical device that does so?
More than a century of failures to create devices equivalent to a Maxwell’s demon strongly suggests that Nature will “conspire” to defeat all such efforts. However, Nature is comprised of a microscopic world of particles that do not individually obey the 2LoT. The 2LoT is a consequence of the laws governing the behavior of these particles. As best I can tell, physics hasn’t completely mastered the interface between theories that apply to microscopic and macroscopic phenomena (quantum gravity, for example), non-equilibrium thermodynamics or some areas in the link below. The existence of macroscopic systems without a thermodynamic temperature and LTE appear to provide a loophole for escaping the 2LoT. If electricity isn’t a form of heat flux involving entropy (dq/T) in phenomena like the thermoelectric effect, a second loophole may exist.
Click to access 12.pdf
Frank wrote: “So we agree that thermo places limitations on the efficiency of heat engines and not on fuel cells.”
Mike replies: At the risk of being pedantic, there are limits in both cases, but they are different. For heat engines, the limiting efficiency is usually much less than 100%, for other conversions it is usually around 100%, but can be somewhat smaller or larger depending on what constraints apply. For example, the electrical work done by a battery can be different from the change in internal energy if the battery changes volume or exchanges heat with its surroundings.
Frank wrote: “What about light? … light is a form of heat and we must analyze this system (macroscopically) using the same rules as heat engines, not fuel cells.”
Mike replies: Yes, but it gets complicated unless you have an equilibrium radiation field inside a black body. Otherwise you can not really assign a temperature to radiation. To get a rigorous result, you must work with entropy rather than temperature. To calculate the entropy of radiation you have to account for the spectrum, the flux, and the divergence of the beam (a well collimated bean has less entropy than a rapidly diverging beam). Imagine letting sunlight ht a diffuser than scatters light but does not absorb light. That does not change the spectrum, but it increases the entropy of the light. As a result, you will no longer be able to focus the sunlight to achieve a high temperature.
Frank wrote: “If we analyze the spectrum of thousands of photons from the sun or the atmosphere – but not a single photon – we can determine the temperature of their source.”
Mike replies: Yes, as stated above, but the temperature of the sources is not the only thing that determines the amount of work that can be obtained. The theoretical limit to efficiency for a solar cell using concentrated sunlight is greater than for a cell using direct sunlight.
Frank wrote: “Therefore the absorption of a single photon … We can’t apply the 2LoT to this situation.”
Mike replies: But we can say something about the probabilities of what happens next. When averaged over a large ensemble, those probabilities must give a result consistent with the 2nd Law.
Frank wrote: “Assuming absorptivity equals emissivity (which is true if LTE exists), any collector of DLR photons must emit more photons towards the atmosphere than it absorbs. As best I can tell – unless we treat the electricity generated as heat and calculate its entropy – electron/hole pairs created by DLR might generate electricity. If electricity did flow from such a device, conservation of energy demands that its temperature decrease. There is nothing unprecedented about converting heat to electricity.”
Mike replies: Such a device would be absorbing thermal energy from its surroundings, converting some of that thermal energy to work, and dumping the rest as waste heat via IR emitted to the atmosphere. If there were less DLR, the device would work better, so it is not DLR that would be converted to work. To convert heat to work requires a difference in temperature. Since the atmosphere is cooler than the surface, such a difference exists and could, in principle, be exploited. Doing so would reduce the spontaneous flow of heat from the surface to the atmosphere by converting some of it to work.
Frank wrote: “The ultimate way of showing the a process violates the 2LoT is so show that delta_S for the whole process is negative. I’m not sure delta_S is negative unless the “entropy of electricity” is included. ”
Mike replies: An electric current per se does not have entropy. The electrons do have random thermal motion in addition to the directed motion of the current. There is entropy associated with that random motion, but there is no general reason why it should be greater when there is a current than when the electrons are on average at rest. Of course, if there is a current electrical resistance will convert some of the work to heat and increase the entropy of the universe (superconductors excepted). Water molecules have thermal energy; but the turbine uses the directed motion of flowing water to do work.
Frank wrote: “Lasers, microwave ovens and other devices can appear to transfer light/heat from cold to hot because they don’t have a thermodynamic temperature – they have more molecules in an excited state than in the ground state. So, if I wave my magic wand and say that my DLR collector – like these devises – doesn’t have a thermodynamic temperature and is not in LTE, can we escape the 2LoT? More importantly, can we build a practical device that does so?
Mike replies: I can use a battery at room temperature to create an electric current that I pass through a resistor immersed in hot water. That transfers energy from cold to hot. But the transfer is in the form of work, which is then converted to heat at the high temperature. The devices that you mention use work to produce radiation that can have very low entropy. When absorbed, even by a hot body, there can be a large increase in entropy thus making the absorption irreversible.
Frank wrote: “More than a century of failures to create devices equivalent to a Maxwell’s demon strongly suggests that Nature will “conspire” to defeat all such efforts. However, Nature is comprised of a microscopic world of particles that do not individually obey the 2LoT. The 2LoT is a consequence of the laws governing the behavior of these particles.”
Mike replies: Correct.
Frank wrote: “As best I can tell, physics hasn’t completely mastered the interface between theories that apply to microscopic and macroscopic phenomena (quantum gravity, for example) nor non-equilibrium thermodynamics.”
Mike replies: OK, but the Second Law is based on the observed behavior of macroscopic systems, so difficulties with the microscopic interpretation should not call the Second Law into question. Back when scientists were arguing about the existence of molecules, the difficult of making the connection was regarded as evidence against molecular theory.
Frank wrote: “See link below: On paper, the existence of macroscopic systems without a thermodynamic temperature and LTE appear to provide an opportunity for escaping the 2LoT. If electricity isn’t a form of heat flux involving entropy (dq/T), a second opportunity for circumventing the 2LoT may exist.”
Mike replies: The first abstract from the link states: ” … different versions of Maxwell’s demon have been presented. Most versions have been answered with reasonable physical arguments, with each of these answers (apparently) keeping the Second Law intact. Though the laws of physics did not change in this process of questioning and answering, we have learned a lot along the way about statistical mechanics and thermodynamics.”
I think that gets it right. The Second Law will never be circumvented. But thinking about ways to circumvent it can improve our understanding.
DeWitt wrote above: “I’m guessing that you didn’t read the Hyperphysics link I posted above, or you didn’t understand it. If you have a semiconductor with a band gap such that only a small fraction of electrons are in the conduction band at ambient temperature, subjecting that semiconductor to ambient thermal IR will not increase the number of electrons in the conduction band.”
When you are generous enough to share a link, I read it (unless I’m rushed or careless). I read that one. However, I was already fairly sure that any material capable for producing electron/hole pairs from DLR photons would also create such pairs by thermal collisions and therefore will be a conductor, not a semi-conductor. So the device may need to be cooled until it becomes a semi-conductor – and coincidentally is colder than the atmosphere and obeys the 2LoT. Its efficiency could be limited by the same formula as heat engines. This would make Mike happy. It is an interesting possible example of how phenomena on the atomic scale might “conspire” to preserve the 2LoT. Logically, I should always expect this to happen, but it doesn’t happen in the case of lasers etc.
Frank,
Lasers don’t violate the Second Law. Neither do fluorescent light bulbs, heat pumps or microwave ovens. The entropy of the system increases because work is being done by electricity, not thermal energy transfer. You’re beginning to sound like Bryan.
Reversible systems are, by definition, infinitely slow. In a real fuel cell, for example, the electrode potential goes down as the current density increases, the cell has internal resistance to electricity flow so heat is generated, and the electrodes may not be reversible to start with, like the oxygen electrode in a hydrogen/oxygen fuel cell, so the potential decreases even faster with increasing load.
I was listening on NPR about the Indian Prime Minister’s visit to the US. He made the usual bows to climate change and renewable energy and the news readers were speculating that we could help India with that by transferring energy storage technology. It’s news to me that anything that would make energy storage economically viable, other than pumped water storage, exists here.
DeWitt wrote: “You’re beginning to sound like Bryan.”
That is unfair to Frank. He wants to learn and to correct his errors. Very different from people like Bryan or RW.
“That is unfair to Frank. He wants to learn and to correct his errors. Very different from people like Bryan or RW.”
Hey, I resent that. I have acknowledged errors of mine pointed out many times here.
Mike M. says
“That is unfair to Frank. He wants to learn and to correct his errors. Very different from people like Bryan or RW.”
Name one error that I have made!
That should be easy (according to you)…….but I bet you cannot.
Bryan,
What you write is so confused that it is hard to point out specifically what is wrong. But it seems you think that greenhouse gases in the atmosphere can not make the surface warmer. Very wrong.
But the real problem is that you won’t listen to attempts to explain things.
Mike M.
“But it seems you think that greenhouse gases in the atmosphere can not make the surface warmer. Very wrong.”
Its a matter of significance.
How much warmer?
Even G&T accept that the radiative properties of CO2 have a significant role at the temperature of furnaces but play an insignificant part in atmospheric heat transfer.
There are folk who post here who think that a shoebox sized enclosure can produce a significant greenhouse effect.
Indeed full sized greenhouses show no significant radiative greenhouse effect.
My take on the greenhouse effect is not far off the writers of this paper
Click to access Wagoner%20AJP%202010.pdf
Where I differ from them is that I regard the small radiative effect that they calculate as having real world significance
When a small radiative effect competes with other forms of heat transfer in the atmosphere it is truly insignificant.
The experiment carried out by R W Woods convinced him that a radiative explanation for the Earths enhanced temperature is unjustified.
Reading your posts on this thread indicates that you have a good grasp of physics.
Read the G&T paper and make a critique.
I would be interested to read any errors that you can find
I am about to go on a sailing trip to the Hebrides (fairly remote Scottish Islands) with little or no internet access so I will not be able to reply for some weeks
DeWitt and Mike: My fellow alumnus, DeWitt, is usually generous sharing his knowledge and experience. His impatience suggests I am fairly far off base applying the 2LoT to devices that are not in LTE when they emit radiation. Grant Petty discusses this situation in one of his two sections on LTE. First some common ground.
According to wikipedia, “heat is energy that spontaneously passes between a system and its surroundings in some way other than through work or the transfer of matter”. This definition doesn’t clearly exclude the possibility that electricity is a form of heat. Assuming electricity is not heat, I have questions about how the 2LoT applies thermoelectric devices that convert heat into electricity.
In a heat engine, heat flows from hot to cold. If I put power (electricity) into a heat engine (refrigerator), I can move heat from cold to hot. If I burn methane in a heat engine, the maximum efficiency is (T2-T1)/T2, but I can “burn” that same methane in a fuel cell without this limitation.
By the above definition, heat is also transferred by emission of radiation (spontaneous or stimulated). When we think about BB or S-B radiation, heat (the net flux) always flows from the hotter light source to the cooler one. However, we can put power (electricity) into devices like lasers, LEDs and fluorescent lights and get out more radiation than expected from BB or S-B considerations. The net flux of radiation in this situation can appear to be from cold to hot in this situation. This apparent difficulty disappears if we recognize that these devices are not in LTE – and therefore don’t “thermodynamic temperature” useful for distinguishing between hot and cold. I learned this rational somewhere (“rational 1”), but I can not find a reference. So rational #1 may be the source of my confusion. In any case, we can put electricity into these devices and produce excited states without relying on temperature-dependent collisional excitation. Does the external source of power allow radiation (heat) to be transferred from cold to hot in a manner analogous to a refrigerator? This analogy avoids using rational #1. If we convert electrical power into heat by resistance, we are now constrained by the 2LoT and (T2-T1)/T2.
Other definitions for heat emphasize its connection to molecular motion and temperature, and don’t simply refer to a flux of energy (as wikipedia does).
“In physics, a form of energy associated with the movement of atoms and molecules in any material. The higher the temperature of a material, the faster the atoms are moving, and hence the greater the amount of energy present as heat.”
This definition of heat seems to exclude electricity, but does not necessarily include radiation as a form of heat. It also makes heat a microscopic property random molecular motion – linking disorder to entropy and the 2LoT. Whenever energy has been converted to random molecular motion, the 2LoT and (T2-T2)/T2 apply. Is the radiation from an incandescent light “heat” and the radiation from a laser “not heat”, because the latter has never been random molecular motion?
(*%#^**!! Re-reading this, DeWitt’s characterization stings a little more.)
Frank,
One problem is that the term ‘heat’ has more than one meaning. Only one of those meanings is the Clausius definition of the energy flow from hotter to colder. It would be better, IMO, if the term were to be banished from thermodynamics. Energy and entropy are all you need.
A fuel cell isn’t necessarily 100% efficient either. The efficiency depends on the change in entropy of the reactants and products and the temperature of the reaction. A hydrogen/oxygen fuel cell at standard conditions has a maximum efficiency of 83% because of this.
I don’t endorse everything he says, but John Denker raises interesting points about the way thermodynamics is currently taught here: http://www.av8n.com/physics/thermo-laws.pdf
See chapter 17 about the problems with the term ‘heat’.
DeWitt,
You wrote “One problem is that the term ‘heat’ has more than one meaning. Only one of those meanings is the Clausius definition of the energy flow from hotter to colder. It would be better, IMO, if the term were to be banished from thermodynamics. Energy and entropy are all you need.”
I strongly disagree. “Energy” also has more than one meaning as do “force” and “momentum”. But in science, those terms each have a specific meaning, as does heat (what you call the Clausius definition). Good texts, and goo instructors, make that clear.
You can not banish “heat” from thermodynamics since you can not macroscopically define entropy except in terms of heat.
I’ll take a look at the Denker article.
Mike M.
Heat has multiple definitions within thermodynamics. See, for example, heat of reaction and heat capacity.
DeWitt wrote: “Heat has multiple definitions within thermodynamics. See, for example, heat of reaction and heat capacity.”
Those quantities are equal to heat (Clausius definition) under appropriate conditions, so they are not different definitions of heat. Since the appropriate conditions may not apply, the use of “heat” in such terms can cause confusion. So I prefer “enthalpy of reaction” to “heat of reaction. I see no real problem with the term heat capacity.
Frank,
Don’t be too hard on yourself. Your questions are forcing me to think about things that I have not carefully thought about before. In my book, that makes them excellent questions.
The statement in Wikipedia is not quite right. Heat is not energy, it is a means of transferring energy; specifically, the transfer of energy that results from a difference in temperature. Radiation is energy, not heat. But the transfer of energy resulting from a difference in temperature can be accomplished by means of radiation. So a transfer of energy by means of radiation might be heat, or it might be work, or it might be some of each. I think that the transfer is only entirely heat if the radiation is due to thermal emission and if the absorbed radiation is fully thermalized.
An electric current transfers energy as work. But the thermal motion of electrons can result in the transfer of energy as heat.
DeWitt complains about the use of the term heat in thermodynamics. I think the real problem is failing to clearly and consistently distinguish between the scientific definition of heat as a process and the colloquial use of heat as something physical. That is aggravated by the use of terms that have their origin in the concept (prevalent 200 years ago) of heat as something physical.
The second Wikipedia definition of heat as thermal energy is flat wrong. It would seem to be a good example of the confusion that results from not being careful about terminology. I suppose I will now have to go find that article and correct it.
Apropos of nothing except that it comes to mind in this context: I read somewhere that Lavoisier’s original list of chemical elements included one called “calorique”.
Mike: Thanks for the kind comment about hard questions. I see so much ignorance in the blogosphere that I am forced to wonder when I am being ignorant. The only way to find out is to stick one’s neck out and see if an axe falls. DeWitt has handed me my head several times and I am more knowledgeable (and cautious) for it. And our host has been very generous in this past, especially when I felt deceived (and therefore intemperate) by some aspects of climate science.
Mike M.:
We seem to be talking past each other.
I am NOT saying that absolute entropy values are not needed.
What I AM saying: For practical reasons, if you want to measure the entropy function for a physical system in a certain region of macroscopic variables appropriate relevant to thermodynamics (say in a region X centered at x), it makes sense to calculate the entropy integral dS(x1, x) = integral dQ/T from x to x1; and then define:
S(x1) = S(x) + dS(x1, x)
S(x) can be found once for all, by undertaking to measure dS from T=0 to the state x. Very likely, x will itself be far from T=0, so there may be other intermediate states that would have been defined and evaluated in order to pin down S(x).
For example, I believe the triple-point of pure water is a unique and recognizable thermodynamic state. So it would make sense for someone to undertake to measure S(tp-H2O) so that it could be used as a reference point for the calculation of entropy values at more typical temperatures.
Mike and DeWitt: Thanks for you replies. Unfortunately, you missed two issues: 1) What is Clausius’s definition for heat, which you both think is most useful? 2) How do we rationalize the behavior of lasers, fluorescent lights, LEDs and microwave ovens given the 2LoT. I proposed two rationals: #1 an absence of LTE makes temperature undefined. #2 an analogy to refrigerators transferring heat from cold to hot.
Earlier in this thread I was criticized for suggesting that an absence of LTE provided an opportunity to “escape’ from the limitations of the 2LoT.
When discussing six different definitions of heat, Chapter 17 of Denker’s online book (linked by DeWItt above) discusses microwave ovens (and presumably other devices where LTE doesn’t exist). Chapter 17 has about ten pages on problems with how “heat” is defined. Denker sometimes finds it useful to define heat as TdS rather than entropy as dq/T. This avoids the problem of defining what heat is and creates (for me) the greater problem of defining what entropy is. Perhaps the rest of the book makes entropy clearer.
“consider the notion that a microwave oven heats a potato. Clearly (1) the food gets hotter. Clearly (2) the entropy of the food changes. However, (5) no entropy was transferred across the boundary of the food. Energy was transferred, but the entropy was created from scratch, within the food. According to any reasonable definition of temperature, the microwave- generating components inside the oven aren’t very hot, so you can’t say the energy was transferred as the result of a difference in temperature”.
This implies that radiation transfers energy, but sometimes not heat. It is consistent with my suggestion that excitation by thermal collision can result in emission that carries heat (entropy), but excitation by other mechanisms does not.
There are two general approaches that I’m aware can be used to define entropy:
a) Thermodynamically, by calculating the integral dQ/T over a process from a standard reference state. The entropy is an extensive variable, so twice the amount of stuff has twice the amount of entropy. By assumption/postulate, the entropy is a state function, so the result must be unique.
b) Within statistical mechanics, the entropy is defined as kB * LN(phase space of all variables in the dynamical system), where the range of integration covers all microscopic values of the dynamical variables that are simultaneously consistent with the macroscopic state (total mass, temperature, total energy, chemical concentration of whatever, volume, magnetic field, and so on). kB is Boltzmann’s constant
Approach a) is empirical and relies on no models for the system under study, just lots of measurements. Approach b) relies on the precise dynamical model one is using to represent the system.
[There is a slight fuzziness regarding the simultaneous definition of the exact temperature and the exact energy. Also, the temperature of an extended system will vary with the local gravitational potential. But these are refinements, generally of little significance.]
Earlier you raised questions about:
a) Clausius’ definition of heat: I haven’t studied the history of thermal physics, but my impression is that his point of view is that heat is the transfer of energy in a way which is not easily seen to be reversed. By contrast, you can transfer energy by means of work; and by doing the work backwards you reverse the gift. So that is NOT heat transfer. Likewise, you could increase the magnetic field: this is also easily reversed by reducing it.
In general, I have found that you can generally avoid the word “heat” or use the fuller term “heat transfer”, and the ideas become a bit clearer.
b) With regard to lasers, etc.: Operating lasers do not have a normal temperature (or if you insist on one, it is negative!). That doesn’t mean that the 2LoT doesn’t apply. But it means that you have to define the entropy and energy within the context of the larger systems, for which the lasers are agents; sort of like paddles for a paddle-wheel.
I’m not sure how to be more specific about this, unless you set up a thought-experiment. That would give a picture we could consider.
Frank,
The Clausius definition is that heat is the transfer of energy that results from a difference in temperature. Heat is not a form of energy, it is a mode of transfer of energy. Radiation is a form of energy, so radiation is not heat. But radiation can provide the mechanism by which energy is transferred as heat. In devices such as lasers and microwave ovens, energy is provided as work, usually in the form of an electric current and converted to radiation. Heat transfer is not part of the picture.
Frank,
Based on my reading of Chapter 17, it seems that Denker’s understanding of thermodynamics leaves much to be desired. In the heating of a potato in a microwave, the energy transfer is in the form of work, not heat. The dissipation of the absorbed energy in the potato is an irreversible process that increases the entropy of the universe. One could produce the same change in state of the potato by transferring energy as heat. In that case, the change of entropy of the universe would likely be different.
In a process like heating something in a microwave oven, we often say that “work has been converted to heat”. That is shorthand for something like “energy was transferred to the potato as work with dissipative processes in the potato causing the change in state of the potato to be the same as if an equivalent amount of energy was transferred to the potato as heat”. For some reason, people always use the shorthand form.
nealjking,
You wrote: “a) Thermodynamically, by calculating the integral dQ/T over a process from a standard reference state. The entropy is an extensive variable, so twice the amount of stuff has twice the amount of entropy. By assumption/postulate, the entropy is a state function, so the result must be unique.”
That is close, but you omitted a critical detail. The integral must be calculated for a *reversible* process. Also there is no need to state from either a standard state or a reference state.
Entropy is not assumed to be a state function, it is mathematically proven to be a state function. But few texts include a rigorous general proof.
Mike M:
– You are correct in asserting the importance of using reversible processes to calculate the dQ/T integral.
– I had forgotten the scope of Carnot’s discussions on what we now call 2LoT. A nice presentation can be found in FLoP V.1 Chp.44 : http://www.feynmanlectures.caltech.edu/I_44.html
– Inasmuch as the dQ/T integral can only measure differences in entropy between states, without agreement to a value of entropy at a specific state, the entropy would be undefined to within an additive constant. Nernst’s heat theorem (3LoT) asserts that the entropy of any substance = 0 at T = 0; but this cannot be reached in actuality. In practice, if you want to define the entropy ini thermodynamical terms, you have to have a reference state for which the value of entropy is agreed.
nealjking,
“In practice, if you want to define the entropy ini thermodynamical terms, you have to have a reference state for which the value of entropy is agreed.”
For energy quantities, such as internal energy, enthalpy, and Gibbs free energy one needs to establish a reference state by convention. But we have absolute values for entropy. And those values can be shown to converge on zero at absolute zero. The Third Law is not just a convention; it is an observable fact about how the world works.
To see this, consider the Gibbs free energy, defined by
G = H -T*S.
Now consider how G changes when we change the temperature. For an infinitesimal change, we have
dG = dH – T*dS – S*dT.
We can obtain dG from changes in equilibrium constants or electrochemical cell potential. We can obtain dH and dS from heat capacity data. dT is directly measurable. So S can be determined. (Note that the familiar formula: delta_G = delta_H – T*delta_S is for an isothermal process.)
In practice, that is not a very good way to get S, although the inverse calculation provides a valuable way to check the internal consistency of empirical data (third law calculations). S is usually determined from the Third Law, with the help of statistical mechanics. At very low T, heat capacities of solids obey simple laws. For example, for an isotropic non-conducting, non-metallic solid, the heat capacity at low T obeys
Cp = B*T^3
where B is a constant that is a property of the specific substance (derived from the Debye model and extensively verified). If you get the substance cold enough that heat capacity measurements show that this law is obeyed, then you can determine the value of B and extrapolate your results to absolute zero.
For small gas molecules, entropy can be calculated from spectroscopic data by using results from statistical mechanics.
So if you look at a table of thermochemical data, you will see columns headed delta_H_f and delta_G_f with values of zero for elements (essentially, the arbitrary additive constant). But the entropy column is just headed S, with non-zero values for the elements. Those are absolute values, with no undetermined additive constant.
Mike M:
Yes, I already mentioned the 3LoT.
But one doesn’t bother to take a run at approaching the T=0 results for every experiment: It’s not practical to design experiments that way, particularly if you don’t happen to addressing a low-T issue. For example, if one is studying phenomena at 5000 Kelvin, it makes no sense to do the dQ/T integral from a state starting near 0 so that you can do a low-T procedure to approach T=0. It is literally the case that you wouldn’t want the low-T equipment in the same room with the high-T equipment.
Instead, you would use the 3LoT to find the entropy increment from a projected value of 0 at a low-T state, to a state that is a practical starting point for the bulk of your measurements.
nealjking,
You are correct that there are many cases where delta_S is fully adequate and there is no need to complicate things by bothering with absolute entropy. I was only pointing out that absolute entropies can be obtained and there are times when they are needed. And in chemical thermodynamics there are available extensive tables of measurement derived absolute entropies.
Neal and DeWitt: I don’t know if you noticed the reference about the interaction between atom beams and lasers in DeWitt’s comment dated May 31, 2016 at 1:54 pm. A non-paywalled version of the article is available at:
Deflection of an atomic beam by a laser wave: transition between diffractive and diffusive regimes.
Click to access jphysB-v17-p4623-1984.pdf
From this paper, I learned that the technical name for Neal’s experiment is an “optical Stern-Gerlach effect”. This effect has been observed – with a beam of He atoms, not ions.
http://journals.aps.org/prl/abstract/10.1103/PhysRevLett.68.1996
http://www.atomoptics.ru/Publications/Papers/Papers/1992/SternGerlach(PRL).pdf (non-paywalled version)
They used a standing laser wave to split the beam into two or three parts, depending on where the beam path passed through the standing wave.
One complication I missed is that the laser stimulates emission from the excited atoms (as well as excites them). So the phenomena is more complex that I imagined.
Frank:
It is not clear to me why they are calling this an “optical Stern-Gerlach” effect. Other papers I’ve looked at use the term to indicate a difference in displacement due to optical polarization. Maybe they mean that a path displacement is effected by some kind of interaction with light.
In any case, the displacement is a lot cleaner with a coherent source of photons, since the displacements will be more quantized. In the case of the IR thermal photons, there should be displaced smears; and the chances of a double-deflection are far smaller.
Neal: The non-paywalled link above didn’t copy and paste correctly. The correct link ends with … Gerlach(PRL).pdf
Click to access SternGerlach(PRL).pdf
This paper shows that equally opposing radiative fluxes (or photons) do not cancel because a beam of atoms passing through such as flux separates into three streams when that radiation can be absorbed by the atoms. This proves that one-way flux doesn’t exist.
We imagined doing a “classical” experiment that used the momentum from absorption of photon to prove that radiation is coming from two directions. In an ideally controlled experiment, we would observe: a) some atoms being deflected to the right when the radiation comes from the left, b) some atoms being deflected to the left when the radiation comes from the right, and deflection in both directions when the equal amounts of radiation arrive from both directions.
Above I asked you want would happen if the radiation were coherent. Would the probabilities of being deflected add, or would the amplitudes produce interference. The experiment in this paper is analyzed from the purely non-classical QM perspective with atoms interacting with a radiation field (not individual photons). The coherent radiation produces standing waves in this field and a gradient in the field on a scale wider than the width of the beam of atoms. If this experiment were analyzed in terms the amplitude for an atom interacting with a photon, I would understand it much better, but then we might need to add these amplitudes.
In any case, Wikipedia description of the Stern-Gerlach experiment provides some parallels (which I capitalized) which might justify calling the experiment I found the optical Stern-Gerlach effect.
“The Stern–Gerlach experiment showed that the spatial orientation of ANGULAR MOMENTUM IS QUANTIZED. It demonstrated that atomic-scale systems have intrinsically quantum properties, and that measurement in quantum mechanics affects the system being measured. In the original experiment, silver ATOMS were sent through a NON-UNIFORM MAGNETIC FIELD which deflected them before they struck a detector screen. Other kinds of particles can be used. If the particles have a magnetic moment related to their spin angular momentum, the magnetic field gradient deflects them from a straight path. The screen reveals discrete points of accumulation rather than a continuous distribution, owing to the quantum nature of spin. Historically, this experiment was decisive in convincing physicists of the reality of angular momentum quantization in all atomic-scale systems.”
I tried to modify the text from Wikipedia for the optical Stern-Gerlach experiment. I may not have translated the concepts correctly.
The OPTICAL Stern–Gerlach experiment showed that the ELECTROMAGNETIC FIELD is quantized (IN THE FORM OF PHOTONS). It demonstrated that atomic-scale systems have intrinsically quantum properties, and that measurement in quantum mechanics affects the system being measured. In the original experiment, HELIUM atoms were sent through a non-uniform ELECTROMAGNETIC field, which deflected them before they struck a detector screen. If the particles have ELECTRIC DIPOLE MOMENT, the ELECTROMAGNETIC field gradient deflects them from a straight path. The screen reveals discrete points of accumulation rather than a continuous distribution, owing to the quantum nature of LIGHT.
I currently don’t understand why a magnetic field GRADIENT and an electromagnetic field GRADIENT is specified for both experiment. If I were smarter, I would understand Wikipedia’s explanation for why a magnetic field gradient is needed and then maybe I could translate that rational into the reason why an electromagnetic field gradient is needed.
Life would be much simpler if we could observe an atom beam passing between opposing non-coherent beams of light.
The original Stern-Gerlach experiment needs a B-field gradient to give the magnetic dipole something to push against. If you imagine the magnetic dipole as a “N” pole and a “S” pole separated by a short distance, the pull on the two poles cancel unless the B-field is stronger at one than the other.
Likewise, the electric dipole won’t do much without a difference in the intensity of the E-field at the “+” and “-” poles.
You could arrange for the beams from the left and right to be mutually non-coherent: Use two lasers. But you need to have them produce the same frequencies at the same time.
Also: the same intensities.
You can see why it’s much easier to run the experiment with just one laser.
Neal wrote: “Likewise, the electric dipole won’t do much without a difference in the intensity of the E-field at the “+” and “-” poles.”
So electric fields deflect a particle with a charge, but an electric field gradient is required to deflect a particle with a dipole. The authors of this paper used coherent radiation to create a standing wave whose gradient varied with location. So non-coherent radiation – which is absorbed by atoms and molecules – must have an electric field gradient(s) even though that field isn’t localized in space. Which goes back to the theory that radiation consists of oscillating, self-propagating electric and magnetic fields – with the oscillations producing the gradients (at random).
Wave/particle duality implies that we can explain these phenomena using fields or particles, but particles/photons seem much easier to understand to me – assuming that one handles the amplitudes correctly. Which is why I would prefer to use a non-coherent light source. In that case, when the light comes from both sides, we expect a simple superimposition of the results from light from the left and light from the right. It won’t matter is the intensity from each side is exactly the same.
Coherent light from both directions is needed to produce an effect similar to the Stern-Gerlach effect, which relied on a magnetic field gradient in space to separate particles with a magnetic dipole. That magnetic gradient didn’t come from radiation. I wasn’t envisioning am electric field gradient in the experiment you proposed. So, the “optical Stern-Gerlach” effect was not an obvious name for the experiment we originally discussed.
Do you understand why they chose to use triplet He atoms for their target.
Frank,
The answer is in the paper. It has to do with the transition being exited by the laser frequency. Look in the second column of page 1997 about half way down. Helium only has two electrons, so it’s capable of having a triplet state when excited.
DeWitt wrote: “The answer is in the paper. It has to do with the transition being exited by the laser frequency. Look in the second column of page 1997 about half way down. Helium only has two electrons, so it’s capable of having a triplet state when excited.”
If I can translated the symbols correctly, an electron is promoted from the 2S to a 2P orbital while the remaining electron presumably is in a 1S orbital making both triplet states of He. Getting He atoms into the correct ground state appears to represent a lot of unnecessary experimental work. Why couldn’t they have used ground-state He, or He ions? The momentum delivered by the 1.083 um photons they used is about 1/5 or less than the shorter wavelength absorptions (violet or ultraviolet) available in these species. Part of the problem may be that they need an electric field gradient from a standing wave that is spread out over a region wider than the width of their atom beam. So 1 um radiation might have been the optimum choice given their ability to focus an atom beam with a tight spread of velocity.
Figure 1 also looks a little strange to me with all of the triplet He being deflected up and down and all of the singlet He passing through unchanged. In an NMR experiment (something I supposedly understand), the only eigenstates have the magnetic dipole of a nucleus aligned with or against a magnetic field. Herein, the electric dipole of the entering triplet He is aligned with or against the electric field and therefore deflected by the field differently. This is a very different picture from the “classical” idea that SOME He atoms are deflected in one direction or another when they absorb a photon.
Above we discussed the difference between non-coherent and coherent radiation coming from both directions from the “classical” perspective. I’m not sure we got the correct answer.
Frank,
Looking at the energy level diagram, it looks like the 1.083μm transition is the most convenient of the allowed transitions from the triplet state of helium. You’d need a UV laser, 338.9nm for the other allowed transition in the diagram.
Mike wrote: “Based on my reading of Chapter 17, it seems that Denker’s understanding of thermodynamics leaves much to be desired. In the heating of a potato in a microwave, the energy transfer is in the form of work, not heat.”
From my less-experienced point of view, deciding whether radiation (or electricity) is transferring heat or work leaves a lot to be desired. For the moment, I’m going to ask myself by what mechanism produced the excited states emitting photons.
There is certainly heating involved, as the dissipation of the EM energy produces entropy. The difference between microwave heating and solar heating (for instance) is that the EM is coherent; this would also be true of a laser used for heating.
Frank,
You wrote: “From my less-experienced point of view, deciding whether radiation (or electricity) is transferring heat or work leaves a lot to be desired.”
I must emphasize that what is being transferred is energy. Whether the energy is transferred as heat or work is needed to find changes in entropy. Energy transfer without a change in entropy is work; energy transfer with a change in entropy is heat. Whether the transfer is as heat or work can be different at each end of the process.
Consider the transfer of a quantity of energy from A to B. The amount of energy added to B must be the same as that removed from A. But the amount removed as work (or heat) from A need not be the same as the amount delivered as work (or heat) to B. The only constraints are that total energy be conserved and that the total entropy not decrease.
You wrote: “For the moment, I’m going to ask myself by what mechanism produced the excited states emitting photons.”
I think that is correct for the change in the thing emitting the radiation. But you must also consider what happens in the thing receiving the radiation. If the energy is thermalized (potato baking in a microwave) the energy is received as heat. If the energy is not thermalized (increasing the population of electrons in the conduction band) it is received as work.
Mike: Thanks for the reply. Things often seem clearer when you comment.
You finished by reminding me of the importance of what happens to the energy that is absorbed. If the receiving object is in LTE, the energy is thermalized. If not, then possibly it can produce work and thereby escape the limitations of the 2LoT. Which brings us back to my original speculation about what might allow us to make use of (do work with) the energy in DLR. The absorber can’t be in LTE.
Is a conventional solar panel (or photosynthetic apparatus in plants) in LTE? Presumably no. These devices work precisely because they harvest the energy of the excited state (which quickly becomes an electron-hole pair) before it is thermalized. Unfortunately, any material which will produce an electron-hole pair after absorbing a DLR photon also produce electron-hole pairs from thermal collisions and therefore not be a semi-conductor.
I would just say: That is why glass is so good an idea.
If you have a square meter hole in the wall you will have 400 to 500 W passing in and out, and every temperature difference between inside and outside would give 5W/K heat transfer.
Put a glass/window in and it will have the mean temperature between outdoor and inside. The radiation loss to the outdoor would be only half.
Glass is mostly a blackbody for infrared, else it would not work.
Besides the effect on radiation it has an effect on ventilation.
It’s not entirely fair to compare glass and no glass because no glass would not limit convective energy transfer. But yes, glass is better than, say a polyethyene film or a clear rock salt window. Even better is glass with a low thermal IR emissivity coating on the outside, what’s called low-e glass. It keeps you warmer in the winter and cooler in the summer.
Neal, DeWitt and anyone following our discussion of experimental proof of the two-way flux of radiation might be interested in the following very short video featuring Serge Haroche, who received the Nobel Prize in 2012 for probing the quantum behavior of light using a beam of atoms. He, of course, isn’t wasting his time on the possibility of one-way flux. However, he used a beam of atoms to probe aspects of the quantum behavior of light – in his case decoherence. As he says, normally we use light to see matter, but we can use atoms to “see” light (individual photons) and probe its fundamental behavior.
https://www.scientificamerican.com/video/how-physicists-trapped-photons-in-a-box1/