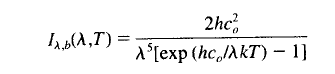In Radiation Basics and the Imaginary Second Law of Thermodynamics I covered a fair amount of ground because it started in answer to another question/point from a commenter.
We all agree that the net effect of radiation between hot and colder bodies is that heat flows from the hotter to the colder body, but many people have become convinced that this means radiation from a colder body has no effect on a hotter body.
After explaining a few basics about emission and absorption, I concluded:
Therefore, there is no room in this theory for the crazy idea that colder bodies have no effect on hotter bodies. To demonstrate the opposite, the interested student would have to find a flaw in one of the two basic elements of thermodynamics described above.
And just a note, there’s no point reciting a mantra (e.g., “The second law says this doesn’t happen”) upon reading this.
Instead, be constructive. Explain what happens to the emitting body and the absorbing body with reference to these elementary thermodynamics theories.
One of our regular commenters has finally explained what happens.
This was a great day of joy because in three other articles on this blog (The Imaginary Second Law of Thermodynamics, How Much Work Can One Molecule Do? and On the Miseducation of the Uninformed by Gerlich and Tscheuschner (2009)) and one on another blog the subject has been much discussed.
I have asked many many times:
What happens to radiation from the colder body when it “reaches” the hotter body?
But I had never been given an answer – until now. (Note: the general consensus from the imaginary second law advocates – as much as I can determine – is that the colder body does emit radiation so at least there is agreement on the first step).
Finally, the answer is revealed:
Back to thermodynamics and electromagnetic radiation.Scienceofdoom and others think that the hot surface has no option but to absorb a photon from the cold surface.I think a lot of this radiation is in fact scattered from the hot surface and is not absorbed.
“A Lot” is Not Absorbed?
Before we dive into the fascinating topic of absorptivity and absorption, I hope people don’t think I am being pedantic for drawing attention to the fact that one of our most prominent advocates of the theory (the Imaginary Second Law) has actually failed to support it.
Science is about detail.
If no radiation from the colder body is absorbed by the hotter body then the imaginary second law stands. That is, if any radiation emitted by a colder body “reaches” the hotter body and is absorbed by the hotter body then the colder body has transferred energy to the hotter body.
The colder body has had “an effect”.
It’s hard to be certain about the imaginary second law of thermodynamics because I can’t find it in a text book. In fact, on another note, it has been a day of double joy, because another imaginary second law advocate stepped up to the plate when presented with this from a thermodynamics textbook:
And said:
It is absolutely in error.
Which was wonderful to hear because up until now everyone else had simply ignored the question as to whether Incropera and DeWitt didn’t understand the basics of radiation (for reasons that are all too easy to imagine).
But I digress.
I can’t be 100% certain what the imaginary second law of thermodynamics teaches but it has appeared up until now – from the comments by many advocates – that colder bodies have no effect on hotter bodies.
How can it be that the first time someone explains what happens to the incident radiation they agree, at least in principle, with the rest of the world? We (the rest of the world) all think that cold bodies have an effect on hotter bodies.
Perhaps more advocates can comment and vote on this idea..
[Stop press – after writing this a newer entrant to the field has promoted a new idea but this one can wait until another day]
Absorptivity and Absorption
There are a few basic concepts in traditional thermodynamics that are worth explaining, even though they might appear a little tedious.
I was already thinking about writing an article on this after I had earlier explained:
According to Kirchoff’s law, emissivity = absorptivity for a given substance at the same wavelength (and for some surfaces direction needs to be defined also).
And the same commenter responded:
You have a particularly naive view of heat transfer.
When a green plant absorbs some em radiation to make starch does it simultaneously emit the same radiation?
You have not grasped the fact that if the absorption of 4um radiation was exactly balanced by the emission of 4um radiation the net effect would be zero.
Which would shoot a massive hole in the case for AGW.
I prefer to think of my understanding of physics as traditional rather than naive. So, a few basics need explaining so that readers can judge for themselves.
Just a comment, as well, for new people looking into this subject. The basics often seem a little dull, but hopefully I can persuade a few to dig deep and work hard to get the very basics clear. I can promise unrelenting joy down the track as you realize that you understand more than most other people who had jumped ahead and are now writing confused comments.. (assuming this will give you unrelenting joy).
Let’s look at snow in sunlight and use it to “shed light” on a dull subject.
First of all, there are two important factors involved in the absorption of radiation:
- the radiation incident on the snow (i.e., the radiation value as a function of wavelength)
- the absorptivity – the property of the snow (as a function of wavelength) which determines how much radiation is absorbed and how much is reflected
We will focus on the snow being heated by sunlight and ignore terrestrial radiation – even though the material will also be heated by terrestrial radiation. This is because we aren’t trying to work out a complete energy balance for this particular material, just illustrating the important points.
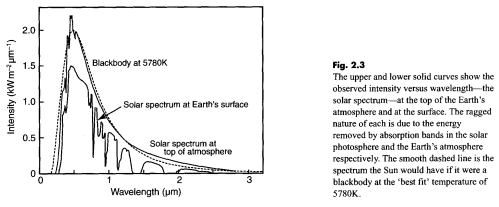
Solar radiation has a spectrum which looks something like this:
This shows how the radiation incident on the snow varies with wavelength. The actual amplitude is dependent on the time of day, the latitude, the amount of clouds, and so on.
The important point is that radiation from the sun peaks at a wavelength of around 0.5μm and above 2μm is much reduced (91% of solar radiation is below 2μm). Therefore, to find out how much radiation the material actually absorbs we need to know the absorptivity at these wavelengths.
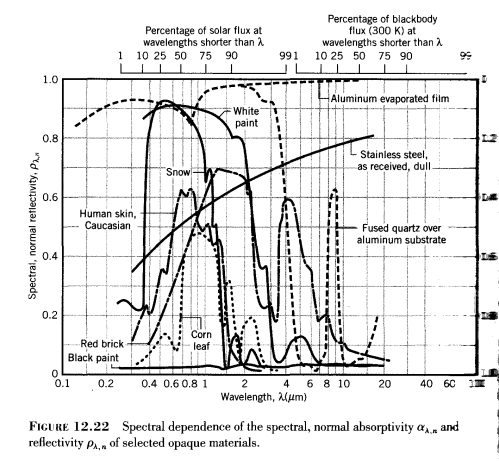
Here are some examples of reflectivity and absorptivity of various materials across quite a wide spectral range:
The scale on the left is reflectivity and on the right (harder to see) is absorptivity from 1.0 at the bottom up to 0.0 at the top. Absorptivity = 1-reflectivity.
Absorptivity is a function of wavelength and is the proportion of incident radiation at that wavelength which is absorbed.
Absorptivity is an inherent property of that material
Let’s take snow as an example. Sunlight on snow will be mostly reflected and not absorbed. That’s because the incident sunlight is mostly between 0.2μm to 2μm – and if you check the reflectivity/absorptivity graph above you will see that the absorptivity is quite low (and reflectivity quite high).
Snow has a high albedo for sunlight – around 60% – 80% is reflected, meaning only 20%-40% is absorbed.
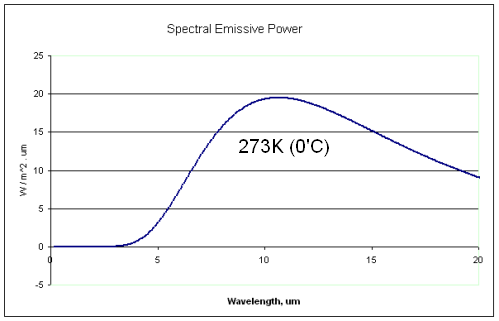
Now let’s consider the snow at a temperature of 0°C (273K). How much thermal radiation does it emit?
If it was a blackbody, it would emit radiation as the Planck function at 273K:
The first thing you notice is that the snow is radiating at completely different wavelengths to the solar radiation. The solar radiation is mostly between 0.2μm to 2.0μm, while the snow is radiating between 5μm and 50μm.
So we need to know the emissivity between these wavelengths to work out the actual emission of radiation from the snow. Emissivity is a value between 0 and 1 which says how close to a blackbody the material is at that wavelength. Emissivity is equal to absorptivity – see the next section – so we can just look up the absorptivity instead.
In the graph from Incropera the absorptivity of snow at higher wavelengths is not shown. But it’s clear that it has changed a lot (and in fact absorptivity is very high – and reflectivity very low – at these higher wavelengths, which climate scientists call “longwave”).
Kirchhoff’s Law
Kirchoff’s law says that emissivity = absorptivity as a function of wavelength – and sometimes direction. That is, these two intrinsic properties of any material have the same value at any wavelength. (The derivation of this formula isn’t something that will be discussed here).
Kirchoff didn’t say that emission = absorption
That’s because emissivity is not the same as emission. And absorptivity is not the same as absorption.
Of course, if a body is only gaining and losing energy by radiation (i.e., no conduction or convection), and the body is not heating up or cooling down then absorption will equal emission. This is due to the first law of thermodynamics or conservation of energy.
But if absorption increases, the body will heat up until the new value of emission balances the increase in absorption. However, the absorption might be in one wavelength range and the emission in a totally different one.
It’s not so difficult to understand, but it does require that you grasp hold of the basics.
So (digressing) back to our commenter, it’s clear that his “reasons” for ditching Kirchhoff’s law weren’t because Kirchhoff was wrong..
And Kirchhoff’s law is very strong. It would need a monumental effort to overturn this part of thermodynamics basics. Reasonable people might expect that if over-turning Kirchhoff’s law is necessary to support the imaginary second law of thermodynamics, then this might imply that the imaginary law is, well, imaginary..
However, even stranger concepts are necessary to support the imaginary second law.
Would Sir like to Absorb this Radiation? No? Very Good Sir, I’ll take it Back.
Now that we have covered a few basics, perhaps some points might start to make sense.
And perhaps this comment might seem a little flawed:
Back to thermodynamics and electromagnetic radiation.Scienceofdoom and others think that the hot surface has no option but to absorb a photon from the cold surface.I think a lot of this radiation is in fact scattered from the hot surface and is not absorbed.
Let’s examine the idea that radiation from the cold surface is “not absorbed”, and see it in its comedic glory.
Consider a surface of 0°C (273K).
And now consider one body at 10°C radiating towards this 0°C surface. According to the imaginary second law advocates the radiation from the 10°C body is accepted.
Now consider a similar scenario but the 10°C body has been replaced with a
-10°C body. According to the imaginary second law advocates the radiation from the colder -10°C body is not accepted. And according to its strongest advocate, “it is scattered and not absorbed“.
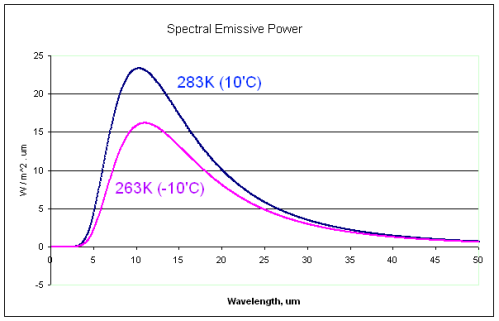
Here is the comparison spectrum for the two radiating bodies:
Notice the very similar radiation curves.
And remember that absorptivity is simply a function of the material receiving the radiation.
And ask yourself, how can the 0°C surface reflect the 10μm radiation from the colder -10°C body, and yet absorb 10μm radiation from the hotter +10°C body?
If the absorptivity at 10μm is 0.9 then it will accept 90% of the radiation at this wavelength from a +10°C body and 90% of the radiation at this wavelength from a -10°C body. It can’t check the menu and “send it back”.
Likewise for each wavelength in question.
[By the way, the fact that the body at -10°C emits radiation that is absorbed by the 0°C surface doesn’t mean that the real second law of thermodynamics is violated. Simple, the 0°C surface is also radiating, and at a higher intensity than the -10°C surface. The net is from the hotter to the colder.]
Conclusion
It took many many requests to finally hear the explanation as to why radiation from a colder body has no effect on a hotter body.
It’s not an explanation that will stand the test of time – except for the wrong reasons. It requires the advocate to believe amazing things about materials. Perhaps that’s why it took so long to get the answer.
Yet more ridiculous ideas have recently been proposed. All in the cause of supporting the imaginary second law of thermodynamics. (These need considering in another post).
Just a digression on the perpetual motion machine (because I don’t want to write a whole post on it). For some reason, perhaps the Gerlich and Tscheuschner miseducation, many confused people think that the absorption and re-emission of longwave radiation by the atmosphere constitutes a perpetual motion machine – and therefore this proves the inappropriately-named “greenhouse” effect can’t exist! Well, we all agree that there is no perpetual motion machine.
But why would the atmosphere radiating towards the earth constitute “perpetual motion”?
Think for a minute before answering, if you claim this.
Right now the earth is around the same temperature it was 100 years ago and also 1000 years ago. Is that a perpetual motion machine – a machine that can’t exist? No. The sun warms the earth. And the sun is powered by internal reactions.
Ok. So if the sun turns off what happens? The earth cools down.
For people who think that the earth’s surface is radiating towards the colder atmosphere, and the colder atmosphere is radiating less energy back towards the earth, and the earth is absorbing this radiation.. we expect the same thing to happen when the sun turns off. The earth will cool down. Just a little slower.
No perpetual motion machine.
Well, the second law of thermodynamics is quite a basic one but misunderstood by many who think they are supporting it.
For newcomers to this debate the approach I have taken is to take a specific example and ask the advocates of their theory how specific well-understood physical properties can possibly support their argument. Mostly I get no response.
I have finally had two answers. One is considered here and it’s hard to understand how anyone can believe it.
See the followup article – The First Law of Thermodynamics Meets the Imaginary Second Law
And the later article – The Real Second Law of Thermodynamics