In an earlier post – Why Global Mean Surface Temperature Should be Relegated, Or Mostly Ignored – I commented:
There’s a huge amount of attention paid to the air temperature 6ft off the ground all around the continents of the world. And there’s an army of bloggers busy re-analyzing the data.
It seems like one big accident of history. We had them, so we used them, then analyzed them, homogenized them, area-weighted them, re-analyzed them, wrote papers about them and in so doing gave them much more significance than they deserve. Consequently, many people are legitimately confused about whether the earth is warming up.
Then we looked at some of the problems of measuring the surface temperature of the earth via the temperature of a light ephemeral substance approximately 6ft off the ground.
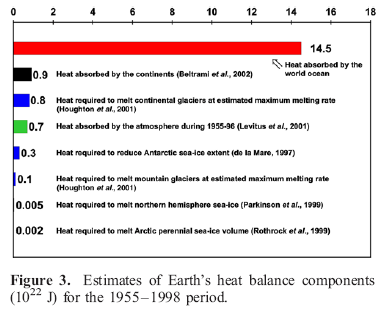
In Warming of the World Ocean 1955-2003, Levitus (2005) shows an interesting comparison of estimates of absorbed heat over almost half a century:
Once you find out that the oceans have around 1000x the heat capacity of the atmosphere, the above chart won’t be surprising.
For those who haven’t considered this relative difference in heat capacity before:
- if the oceans cooled down by a tiny 0.1°, transferring their heat to the atmosphere, the atmosphere would heat up by 100°C (it wouldn’t happen like this but it gives an idea of the relative energy in both)
- if the atmosphere transferred so much heat to the oceans that the air temperature went from an average of 15°C to a freezing -15°C, the oceans would heat up by a tiny, almost unnoticeable 0.03°C
So if we want to understand the energy in the climate system, if we want to understand whether the earth is warming up, we need to measure the energy in the oceans.
An Accident of History
Measuring the temperature of the earth’s surface by measuring the highly mobile atmosphere 6ft off the ground is a problem. By contrast, measuring ocean heat is simple..
Except we didn’t start until much later. Sea surface temperatures date back to the 19th century, but that doesn’t tell us much. We want to know the temperature down into the deep all around the world.
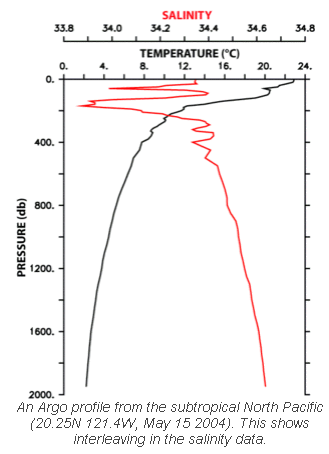
Here is a typical sample. Unlike the atmosphere, the oceans are more “stratified” – see Why Global Mean Surface Temperature Should be Relegated, Or Mostly Ignored for more on the basic physics of why the ocean is warmer at the surface. However, the oceans have complex global currents so we need to take a lot of measurements.
Measurements of the temperature down into the ocean depths didn’t really start until the 1940s and progressed very slowly since then. Levitus says:
Most of the data from the deep ocean are from research expeditions. The amount of data at intermediate and deep depths decreases as we go back further in time.
Fast forward to 2000 and the Argo project began to be deployed. By early 2010, over 3300 sensors have been moved into place around the world’s oceans. The Argo sensors drop to 2km in depth every 10 days and automatically measure temperature and salinity from the surface to this 2km depth:
Why salinity? Salinity is the other major factor apart from temperature which affects ocean density and therefore controls the ocean currents. See Predictability? With a Pinch of Salt please.. for more..
As we go back from 2010 there is progressively less data available. Even during the last 10 years measurement issues have created waves. But more on that later..
The Leviathan
It’s often best to step back a little to understand a subject better.
In 2000, Science published the paper Warming of the World Ocean by Sydney Levitus and a few co-workers. The paper has a thorough analysis of the previous 50 years of ocean history.
Now and again the large number of joules (unit of energy) are turned into a comparison W/m2 absorbed for the time period in question. 1W/m2 for a year (averaged over the entire surface of the earth) translates into 1.6×1022J.
But it’s better to get used to the idea that change in energy in the oceans is usually expressed as 1022J.
The graphs above show a lot of variability between oceans but still they all demonstrate the similar warming pattern.
Here is the data shown (from left to right) as the energy change in the deeper 3000m, 800m and 300m.
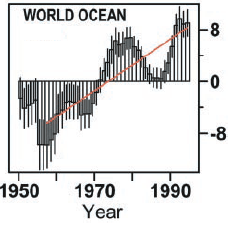
We are used to seeing temperature graphs, even sea surface temperature graphs that go up and down from year to year. Of course we want to understand exactly why, for example see Is climate more than weather? Is weather just noise? It’s easy to think of reasons why that might happen, even in a warming world (or a cooling world) – with one of the main reasons being that heat has moved around in the oceans.
For example, due to ocean currents colder water has been brought to the surface. The measured sea surface temperature would be significantly lower but the total heat hasn’t necessarily changed – because we are only measuring the temperature at one vertical location (the top).
So we wouldn’t expect to see a big yearly decline in total energy.. not if the planet was “warming up”.
So this is quite surprising! See the change downward in the 1980’s:
What caused this drop?
Here’s a another fascinating look into the depths that we don’t usually get to see:
Here we see changes in the deeper North Atlantic in two comparison periods about 15 years apart. (As a minor note the reason for the comparisons of averaged 5-year periods is the sparsity of data below the surface of the oceans).
See how the 1990 period has cooled from 15 years earlier.
Levitus, Antonov and Boyer updated their paper in 2005 (reference below).
They comment:
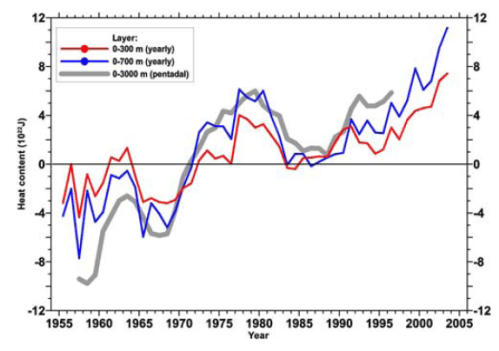
Here we present new yearly estimates for the 1955– 2003 period for the upper 300 m and 700 m layers and pentadal (5-year) estimates for the 1955–1959 through 1994–1998 period for the upper 3000 m of the world ocean.
The heat content estimates we present are based on an additional 1.7 million temperature profiles that have become available as part of the World Ocean Database 2001.
Also, we have processed approximately 310,000 additional temperature profiles since the release of WOD01 and include these in our analyses.
(My emphasis added). Think re-doing GISS and CRU is challenging? And for those who like to know where the data lives, check out the World Ocean Database and World Ocean Atlas Series
Here’s a handy comparison of the changing heat when we look at progressively deeper sections of the ocean with the more up-to-date data.
The actual numbers (change in energy) from 1955-1998 were calculated to be:
- 0-300m: 7×1022J
- 0-700m: 11×1022J
- 0-3000m: 15×1022J
- 1000-3000m: 1.3×1022J
So the oceans below 1000m only accounted for 9% of the change. This gives an idea of the relative importance of measuring the temperatures as we go deeper.
In their 2005 paper they comment on the question of the early 80’s cooling:
One dominant feature .. is the large decrease in ocean heat content beginning around 1980. The 0–700 m layer exhibits a decrease of approximately 6 x 1022 J between 1980 and 1983. This corresponds to a cooling rate of 1.2 Wm2 (per unit area of Earth’s total surface).
Most of this decrease occurs in the Pacific Ocean.. Most of the net decrease occurred at 5°S, 20°N, and 40°N. Gregory et al. [2004] have cast doubt on the reality of this decrease but we disagree. Inspection of pentadal data distributions at 400 m depth (not shown here) indicates excellent data coverage for these two pentads.
And they also comment:
However, the large decrease in ocean heat content starting around 1980 suggests that internal variability of the Earth system significantly affects Earth’s heat balance on decadal time-scales.
So far so interesting, but as the article is already long enough we will come back to the subject in a later post with the follow up:
How Big Should Error Bars be and the Sad Case of the Expendable Bathythermographs.
And for one reader, in anticipation:
Update – follow up post – The Real Measure of Global Warming – Part Two – How Big Should Error Bars be, and the Sad Case of the Expendable Bathythermographs
References
Warming of the World Ocean, Levitus et al, Science (2000)
Warming of the World Ocean 1955-2003, Levitus et al, GRL (2005)






[…] Update – check out The Real Measure of Global Warming […]
Could perhaps a greater than usual daytime cloud coverage over much of the oceans caused the decrease in total ocean heat content in the early 80s?
As you pointed out, the oceans – in general – are thermally stable. That is cold on the bottom, warm on the top, so not much mixing.
To some extent it doesn’t matter in human terrestrial terms how much heat there is in the ocean. The depths could be much colder or hotter and it wouldn’t affect us much.
What affects us is the surface temperature and surface evaporation. Things that affect that are net solar flux, wind patterns, and wave structure, plus a small component of tidal motion that creates some mixing in shallow water zones. There are also non-tidal currents that tend to meander and bring warmer and cooler water to regions, changing the regional scale climate.
So on any reasonable time scale we are most interested in the top 10 metres of the sea surface. I’m not presently up to speed on rates of energy transfer between sea layers, but I expect that you start getting into high decades, century and millenium scales for any significant mixing.
Jerry:
I am afraid that I disagree. In particular with the “so not much mixing”. If by stable you refer to the tendency of the oceans to move towards an equilibrium condition, all I can say is that the form of the equilibrium condition is the result of mixing.
The thermal properties, in particular the diffusion coefficient of still water and still sand (non-mixing water and land) are actually quite similar but the bulk diffusivity of the ocean is about three orders of magnitude greater than that of still water, and it is so because of mixing.
Mixing very much makes the oceans go round, and is key to understanding of the bulk properties.
Alex
Jerry,
My take is that oceans cannot be much colder than they are due to basics like geo-heating, and the freezing point of water.
An interesting question for me is this:
Where does the heat go?
I see El Nino’s as huge events that move heat out of the oceans and into the atmosphere. La Nina’s seem to be recharging events- putting heat back in the oceans.
But the thermal mass of the oceans seems to be, along with the ocean capacity for CO2 and other ghg’s, what really controls the climate.
Both appear to be huge beyond (nearly) comprehension.
Like Scientific American on a good day.
Pertinent, substantial stuff and no hawafeena.
I learn a lot here- thanks!
scienceofdoom,
Your comment showing the differences in heat capacity of air vs. water (atmospheric ocean vs. water ocean) is striking.
Thank you very much for putting this together.
Dave McK makes a strong compliment that I would second.
He also makes a sad indictment of what has happened to far too much of our media.
I was wondering when somebody would get round to talking about the elephant in the room, now that we’ve nearly caught that pesky mouse. I look forward to you further revelations.
P.S.
A bathythermograph is much simpler than its name might lead one to suspect! Good to know these things too.
Cheers
What % of ohc is from direct radiation from the sun?
Steve Koch:
That seems like a difficult question to answer. Ultimately all of the heat comes from the sun. Then the oceans warm the atmosphere in some places. The atmosphere warms the oceans in some places.
And the water has been receiving heat from the sun and exchanging heat with the atmosphere for the entire history of the planet.
Is this what you meant, or did you have something else in mind?
No, I mean what % of ohc is from sun rays hitting the water directly and heating it (as opposed to heating from infrared energy, for example).
Steve Koch
At any one time there is a mix of solar energy and downward longwave energy incident on the ocean’s surface.
On average (globally annually) about 170W/m^2 of solar radiation is absorbed by the earth’s surface. And about 300W/m^2 of downwards longwave radiation. (Some of this is from the atmosphere absorbing shortwave radiation, heating up and radiating out longwave, the rest is the “greenhouse” effect).
Of this, on average around 390W/m^2 is radiated upwards, and the balance is moved by latent heat (water vapor evaporation) and sensible heat (conduction).
But OHC is the integration of these effects over all time. So when we are talking about OHC this is not an easy question to answer.
Perhaps you were really asking a slightly different question..
The ability of the oceans to absorb solar radiation energy is far greater than that of land surfaces, correct? In terms of heating water beneath the surface of the ocean, solar radiation is more efficient than long wave radiation, correct? Long wave radiation heats the very top of the ocean, the part of the ocean that reradiates that energy back into the atmosphere as long waves.
It sounds like, in terms of heating the ocean, long wave radiation is a net energy loss for ohc and that the source of the energy that can actually be efficiently retained in the ocean is the solar radiation.
What I’m trying to get at is the relative importance of solar radiation vs long wave radiation for changing the ohc. Is there anywhere on the web that describes the detailed thermodynamics of ocean heat?
heats water more So if the absorption factor for the earth is 170W/m^2, the absorption for the ocean is > than 170 and the absorption for the land is lower than 170, right?
If the focus is on how ohc changes, that is the change in ohc with respect to time, then we can look at a very small time interval rather than all time.
Steve Koch:
Interesting question(s).
1. How deep does each wavelength (or shortwave vs longwave) penetrate the ocean surface?
2. If there is a difference what impact does that have on ocean heat retained?
Once the energy has been turned into heat of course it doesn’t make any difference where it came from.
But if took two different ocean surfaces and one was heated in the top 1m at 500W/m^2 and the other was heated in the top 10m at 500W/m^2 would it have any difference to ultimate heat retained?
Note: I’m not saying those 2 numbers reflect the difference between SW and LW, but thinking about the consequences if that was the case.
The top 10m are well mixed due to wind and wave action.
I have 2 graphs in front of me for shortwave absorption in the ocean under 2 situations – and they are quite different as one – the Baltic – had lots of sediments. I don’t have one for longwave absorption by depth but I’m sure I can find one (eventually).
Then the final question you ask is a little mixed up.
“..heats water more. So if the absorption factor for the earth is 170W/m^2, the absorption for the ocean is > than 170 and the absorption for the land is lower than 170, right?”
Take a look at Radiative Forcing, Thermal Lag and Equilibrium Temperatures for a bit more background.
In brief, if a body absorbs 170W/m^2 it absorbs 170W/m^2. The specific heat capacity of the body affects how fast it heats up, not its equilibrium temperature or whether it can actually absorb this energy.
And a body at a given temperature radiates an amount according to the Stefan-Boltzmann law (proportional to absolute temp ^4) – regardless of its specific heat capacity.
(Note for the purists – if systems aren’t in equilibrium then specific heat capacity will affect how the system operates and moves energy around)
Did I understand and rephrase the first questions correctly?
scienceofdoom:
“I don’t have one for longwave absorption by depth but I’m sure I can find one (eventually).”
I think this may help:
http://disc.sci.gsfc.nasa.gov/oceans/additional/science-focus/modis/MODIS_and_AIRS_SST_comp.shtml
IR (longwave) is very much a skin effect (millimetric to micrometric in scale).
Alex
Thanks
My point is that given that solar radiation penetrates much more deeply than long wave radiation, doesn’t it heat the ocean much more efficiently than the same amount of long wave energy? The reason is that the long wave radiation stays at the surface of the ocean where it can easily radiate out to space.
Doesn’t most long wave radiation end up radiating out to space much faster than solar radiation? Doesn’t the concept of a kind of half life apply to any long wave energy because as it keeps get reradiated after heating CO2 (for example), some of that energy goes out to space at each reradiation.
So the time that a joule of energy stays on earth makes a huge difference. If that joule gets stored in the ocean, it might stay in ohc for a century.
If the joule gets converted to long wave energy, it is unlikely to get stored in the ohc for a long period and more likely to radiate out to space sooner rather than later.
So some joules are more important than other joules.
The last sentence in my previous message was actually a typo.
Degrees can’t be converted to watts. That’s all there is to that…lol.
So I’m animating Sweden’s data that I got from http://data.smhi.se/met/climate/time_series/day/temperature/
ScienceOfDoom- send me an email if you want to know how it’s going.
Steve Koch:
Ok so I’m pretty sure I understand the question.
First, what makes you think that shortwave does penetrate much more deeply into the oceans? What do you think the relative depths are for shortwave and longwave?
Second, even if it does, the first thing that would happen is that this deeper section of water would warm up and it would expand, and then rise to the surface because it was less dense. That’s why the surface of the ocean is the warmest.
The top 10m of ocean are also well-mixed by wind and waves so any heat in this top layer gets moved around very effectively.
Once the surface of the ocean has heated up it is now heat. This heated surface will radiate longwave energy regardless of what the original source was.
Perhaps this is the source of some confusion? Take a look at CO2- An Insignificant Trace Gas? for explanation about shortwave and longwave.
But an ocean surface at 15’C will radiate energy at a total of 390W/m^2 across a spectrum of wavelengths with the peak wavelength around 10um. We call this (conventionally) “longwave”. This is true no matter how it was heated up. The only factor that affects the amount radiated out is the surface temperature and the only factor that affects the peak wavelength is the surface temperature (but it doesn’t change much from 0’C to 30’C).
[…] post picks up from The Real Measure of Global Warming which in turn followed Why Global Mean Surface Temperature Should be Relegated, Or Mostly […]
Long wave radiation penetrates water to about .1 meter.
Solar radiation can penetrate water up to 100 meters.
The ratio is about 1000 to 1.
Steve Koch:
I believe you are right. S/w absorption varies a lot depending on the ocean quality, but apparently longwave absorption is very shallow. The graphs I have for shortwave absorption with depth also show a strong wavelength dependence which is interesting. For example, in subtropical waters:
400nm – 90% absorbed in about 40m
500nm – 90% absorbed in 75m
600nm – 90% absorbed in about 8m
700nm – hard to tell but much less than 8m (maybe 3m)
500nm penetrates furthest and the longer wavelengths are absorbed much more quickly (ie in the upper levels of the water).
A similar graph for the Baltic, with lots of sediments shows:
400nm – 90% absorbed in about 3m
500nm – 90% absorbed in 10m
600nm – 90% absorbed in about 7m
700nm – 90% absorbed in about 3m
What effect this has in terms of heat retained vs radiated requires some thought and research.
Look forward to a future post on this subject. And thanks for the question.
trivia:
sea water shows a significant transparency at 420nm.
That’s the familiar blue of the deep sea critters’ lignts, approximately.
You can get LED diodes that emit at 420nm.
With these you can perform high speed optical communication to @ 100 meters, depending a lot on opacity from particulate matter.
more trivia:
the safety glasses for a CO2 laser are ordinary clear perspex.
Most things are fairly opaque to IR except for cubic crystals which one uses for the lens on CO2 lasers.
That means that everything absorbs and reradiates the IR, even though CO2 has the spectral match for a particular wavelength of it.
That means that EvErYThInG is either a greenhouse gas, a greenhouse liquid or a greenhouse solid.
The only thing is, the earth isn’t enclosed in glass, so there is no greenhouse.
I had some spare time.
Sweden temperature data 2000-2008, UTC06, UTC12, UTC18, overlaid.
Daily observations for all reporting stations converted to color and plotted precisely on coordinates of each station on satellite map.
Each daily plot, then becomes one frame of a yearly animation.
Yearly animations combine to decadal, etc.
The bottleneck is drive speed and drivespace.
Unfortunately youtube degrades it a lot.
[…] Some interesting remarks on ocean temps and heat capacity: The Real Measure of Global Warming […]
[…] climate change is warming the oceans. The vast majority of absorbed heat in the past 150 years has gone to the oceans. Given their enormous volume, they’ve only warmed a little bit compared to their long-term […]
A recent update on this subject appeared on Nature and was the subject of a RealClimate post.
I wanted to see the source for Bigg 2003, but this was not spelled out in your source cites at the end. (maybe you’ve cited this fully elsewhere- I’m new here.) I presume this textbook is the Bigg 2003 you’re referring to?
http://catdir.loc.gov/catdir/description/cam032/2003043956.html
fortunately our physics dept. library has a copy, so some day i hope to find time to have a look.
Very informative thread uncluding the comments, thanks!
Sorry typo, i meant INcluding (no intent to diss the thoughtful and relevant questions and repkies from commenters!)
Bigg and co authors of this textbook also published a substantial (33 pg) review article on the same topic that year:
Click to access The%20role%20of%20the%20oceans%20in%20climate.pdf
I’m using that as a starting point until I get time to check out the full textbook.
Jim Prall:
You were correct originally, the Bigg reference is “Ocean and Climate”, Grant Bigg, Cambridge University Press, 2003 2nd edition.
I think it is a good book. See also Find Stuff Out and Book Reviews for other books recommeded.
scienceofdoom,
Is rise in sea level a good indicator of ocean heat and therefore also global temp.? I think sea level rise since the last Ice age is due to thermal expansion of seawater. There was a fast rise in sea level between 15,000 to 8,000 yrs ago averaging 142 cm per century. I suppose that was due to melting of land ice.
In the last 7,000 yrs, sea level rise averaged 4 cm per century. That could be due to thermal expansion of seawater. It requires 140 times more heat for water to expand by 1 liter than for an equivalent 1 kg of ice to melt. So the same amount of heat would result to lower sea level rise due to thermal expansion vs. due to melting of land ice.
But in the last century sea level rose by 20 cm, much faster than the ave. 4 cm per century. Is that indicative of unprecedented global warming? Is sea level a better proxy for past global temp. than tree rings and ice cores that measures air temp.?
The paper below suggests that the heat needed to cause the global warming associated with the recent El Nino came from ocean heat content in the Western Pacific Warm Pool (WPWP) and the waters just to the north (Kwajalein). To satiate my innate skepticism, I tried to do some calculations with there ocean heat content data = which I don’t understand. Globally, ocean heat content is usually reported in 10^22 J. The paper shows maps of with changes of tens of 10^16 J. The units must be J/area, not J, but what area. Wikipedia says J/m2. It also discussing integrating temperature from one depth to another, but doesn’t specify degC or degK or anomaly. Can anyone help?
Figure 2 shows the key info. I don’t have much faith in OHC prior to Argo, especially in a highly variable regions like the WPWP, but there appears to be a very dramatic release of heat from a fairly small area (down to 700 m) around Kwajalein, and I was wondering just how much global warming that could produce.
They also show a 20 cm drop in sea level when the heat was lost. Since I believe water runs downhill, I’d like to attribute this to a change in wind, but their are formulas for converting ocean heat content to steric rise.
https://agupubs.onlinelibrary.wiley.com/doi/pdf/10.1002/2017GL076500
A 0.24°C jump of record warm global mean surface temperature (GMST) over the past three consecutive record-breaking years (2014–2016) was highly unusual and largely a consequence of an El Niño that released unusually large amounts of ocean heat from the subsurface layer of the northwestern tropical Pacific. This heat had built up since the 1990s mainly due to greenhouse-gas (GHG) forcing and possible remote oceanic effects. Model simulations and projections suggest that the fundamental cause, and robust predictor of large record-breaking events of GMST in the 21st century, is GHG forcing rather than internal climate variability alone. Such events will increase in frequency, magnitude, and duration, as well as impact, in the future unless GHG forcing is reduced.
Frank wrote: “Globally, ocean heat content is usually reported in 10^22 J”.
That is an anomaly relative to some baseline year. Sometime in the 80’s, I think.
Frank wrote: “The paper shows maps of with changes of tens of 10^16 J. The units must be J/area, not J, but what area. Wikipedia says J/m2.”
My guess is that it would be J in that grid cell. It is ridiculous that they do not specify what it is. You might be able to find that in the files here: https://www.nodc.noaa.gov/OC5/3M_HEAT_CONTENT/
Frank wrote: “It also discussing integrating temperature from one depth to another, but doesn’t specify degC or degK or anomaly.”
Since they are looking at change in heat content, all that matters is change in temperature.
Frank wrote: “Figure 2 shows the key info. … a very dramatic release of heat from a fairly small area (down to 700 m) around Kwajalein, and I was wondering just how much global warming that could produce.”
I consider it bad science to call that a “release of heat”, at least not without careful justification (the fault lies with the authors, not Frank). It is probably mostly just warm water moving around. Note that figure 2 shows some areas warming while others cool.
It looks like there was some reduction in global ocean heat content during the El Nino: https://en.wikipedia.org/wiki/File:Ocean_Heat_Content_(2012).png
The 3 month average looks like it has a seasonal component. The one year average has a dip of about 2e22 J. Over one year averaged over the globe, that would be 1.3 W/m^2. With an TCR of 1.3 C, that would be about 0.5 C. But, warming basically means SST warming. The heat capacity of the mixed layer of the ocean is something like 10-15 W yr /K /m^2. So the heat released would only warm the surface ocean by about 0.1 C. There is an obvious ambiguity here that I have not tried to resolve.
Frank wrote: “They also show a 20 cm drop in sea level when the heat was lost. Since I believe water runs downhill, I’d like to attribute this to a change in wind, but their are formulas for converting ocean heat content to steric rise.”
I am pretty sure that drop in the western tropical Pacific is due to the reversal of wind direction during an El Nino.
At least some main stream climate scientists attribute El Nino warming to a redistribution of clouds caused by the redistribution of sea surface temperature. I think that much more likely than direct warming due to heat being released.