This post will suffer from the unfortunate effect of too much maths – something I try to avoid in most posts and certainly did in The Imaginary Second Law of Thermodynamics. It’s especially unfortunate as the blog has recent new found interest thanks to the very kind and unexpected words of Steve McIntyre of Climate Audit.
However, a little maths seems essential. Why?
Some of the questions and triumphant points some commentators have made can only be properly answered by a real example, with real equations.
It’s something I commented on in American Thinker Smoking Gun – Gary Thompson’s comments examined, where I explained that a particular theory is not usually a generalized statement about effects but rather a theory is usually a set of mathematical equations to be applied under certain well-defined circumstances.
In The Imaginary Second Law of Thermodynamics the example was of a sun radiating into the nothingness of space, when a new star was brought into the picture. And the new star was hotter. So the question was, would the radiation from the colder star actually have an effect on the hotter star.
Some Gerlich and Tscheuschner apostles thoughtfully spent some time trying to enforce some discipline about terminology of heat vs energy and whether radiation was a vector – but forgot to answer the actual question.
Recently a commentator suggested that the real answer lay in considering two stars of equal temperature which were brought into proximity.
As for the sun and the other star, only the sun at T10,000 will go up in T if you insert another T11,000 star, never mind how hard one thinks about it. Maybe this becomes obvious once you make the sun and the new star equally hot at T10,000 . They don’t become both hotter, which is what is predicted in the thought experiment. If the sun heats up the newly inserted star it should not matter really if that new star is at T11,000 or T10,000 does it?
And I said:
Unless all of the radiation is reflected it will increase the surface temperature. It might be 0.1K, 1K, 0.0001K – it all depends on the W/m^2 at that point – and the absorptivity at the wavelengths of the incident radiation.
If that isn’t the case, then you have a situation where incident radiation is absorbed but has zero effect.
And our commentator responded:
Congratulations on sticking with it! I think you just discovered the endless source of energy we are all so desperately looking for. When you expect two equally hot bodies to keep heating each other, where is the limit? and could we not syphon off some off that excess heat.
Which bring us to here. Many people gets confused around these basic points, which is why we need a post with some maths. The maths can prove the point, unlike “talk”.
Conceptual understanding is what everyone seeks. I hope that this article brings some conceptual understanding even though it has a core maths section.
Some Unfortunate but Necessary Maths
Let’s first of all consider one “star” out in the nothingness of space.
The star has a radius of 1000m (1km) and a temperature of 1000k (727°C). This temperature is identical all over the surface and is powered from internal stellar processes. This internal heat generation is constant and not dependent on any changes in surface temperature. We will also assume – only necessary for the second part of the experiment – that the thermal conductivity of this star is extremely high. This means that any radiation absorbed on one part of the surface will conduct rapidly around the surface of the star – to avoid any localized heating.
We also assume that its emissivity is 1 – it is a blackbody across all wavelengths.
A few derived facts about this star, which we will give, in true mathematical style, the exotic name of “1”.
Surface area:
A1 = 4πr2 = 1.256×107m2
Flux from the surface of star 1, from the Stefan-Boltzmann equation:
F1 = εσT4 = 1 x 5.67×10-8 x 10004 = 56,700 W/m2
How the radiation emission varies with wavelength:
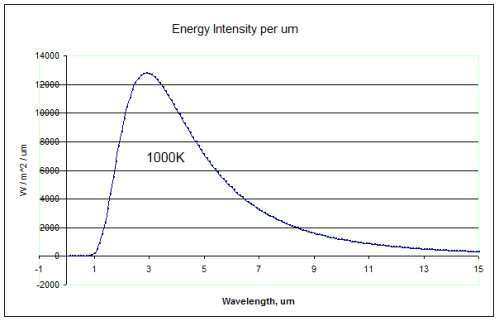
Total thermal energy radiated:
E1 = A1. F1 = 7.12 x 1011W
If this is the thermal energy radiated, and star 1 is at equilibrium, then the heat generated within the star must also be this value. After all, if the heat generated was higher then the star’s surface temperature would keep increasing until steady state was reached.
For example, if the internal energy source increased its output (for some reason) to 8 x 1011W then the output of the star would eventually reach this value. So F1(new) = 8 x 1011/A1 = 6.37 x 104 W/m2
And from the Stefan-Boltzmann equation, T = 1,030K. Just an example, for illustration.
And now, two stars brought into some proximity
So what happens when two identical stars are brought into some proximity? According to our commentator, nothing happens. After all, if “something” happens, it can only be thermal runaway.
The only way we can find out is to use the maths of basic thermodynamics. For people who go into “fight or flight” response when presented with some maths, the conclusion – to relieve your stress – is that the system doesn’t go into thermal runaway, but both stars end up at a slightly higher temperature. Deep breaths. See a later section for “conceptual” understanding.
We define E1 = the energy from star 1 before star 2 (an identical star) appears on the scene.
And E1‘ = the energy from star 1 after star 2 appears on the scene.
The distance between the two stars = d
The radius of each star (the same) = r = 1000m
Consider star 2, radiating thermal energy. Some proportion of star 2’s thermal radiation is incident on star 1, which has an absorptivity (= emissivity) of 1.
To calculate how much of star 2’s thermal radiation is incident on star 2, we use the very simple but accurate idea of a large sphere at radius d from star 2. This large sphere has a surface area of 4πd2.
On this large sphere we have a small 2-d disk of area πr2, which is the area projected by the other star on this very large sphere. And so the proportion of radiation from star 2 which is incident on star 1:
b = πr2 / 4πd2 = r2 / 4d2 [equation 1]
This value, b, will be a constant for given values of r and d.
So, our big question, when star 2 and star 1 are “wheeled in” closer to each other, at a distance d from each other – what happens?
Well, some of star 2’s radiation is incident on star 1. And some of star 1’s radiation is incident on star 2.
Will this – according to the crazy theories I have been promoting – lead to thermal runaway? Star 1 heats up star 2, which heats up star 1, which heats up star 2.. thermal runaway! The end of all things?
Thermal Runaway? Or a Slight Temperature Increase of Both Stars?
To work out the answer, it’s all about the maths. Not that the subject can’t be understood conceptually. It can be. But for those who are convinced this is wrong, “conceptual” just leads to “talk”. Whereas maths has to be disputed by specifics.
When our two stars were an infinite distance from each other in the vastness of space, E1 = E2 – with the values calculated above.
Now that the two stars are only a distance, d, from each other, a new source of thermal energy is added.
Consider star 1. If this star absorbs thermal radiation from elsewhere, it must emit more radiation or its temperature will rise. If its temperature rises then it will emit more radiation. (See note 1 at end).
So:
E1‘ = E1 + E2‘ b [equation 2]
E2‘ = E2 + E1‘ b [equation 3]
This is simply showing mathematically what I have already expressed in words.
And because the stars are identical:
E1 = E2 [equation 4], and
E1‘ = E2‘ [equation 5]
So, from [2] and [4], E1‘ = E1 + E1‘ b, or (rearranging):
E1 = E1‘(1-b), so E1‘ = E1 / (1-b) [equation 6]
So once new equilibrium is reached, we can calculate the new radiation value, and from the Stefen-Boltzmann equation, we can calculate the new temperature.
These equations don’t tell us how long it takes to reach equilibrium, as we don’t know the heat capacity of the stars.
Let’s put some numbers in and see what the results are:
Let d=1000km = 1,000,000m or 1×106m
Therefore, from [1]:
b =10002/4x(1×106)2
b = 2.5×10-7
And, from [6]:
E1‘ = E1/0.99999975 = 1.00000025 E1
Do we even want to work out the change in temperature required to increase the radiation from the star by this tiny amount? Just for interest, the new surface temperature = 1000.00006 K
But this is the new equilibrium for both stars.
Note that there is no thermal runaway.
The approach can now be subject to criticism. (So far no one has checked my maths, so it’s quite likely to have a mistake which changes the numerical result). I can’t see how there can be a mistake which would change the main result that no thermal runaway occurs. Or that would change the result so that no change in temperature occurs.
For more interest, suppose the stars were only 10km or 10,000m apart. Strictly speaking, while the distance between the stars is “much greater” than the radius of the stars we can use my equations above. The mathematical expression for this “much greater” is, d>>r. Once the stars are close enough together the maths gets super-complicated. This is because the distance from one point on one star to one point on another star is no longer “d”. For example, as a minimum it will be d-2r (the two closest points)
No one wants to see this kind of “integral” (as the required maths is called). Least of all, me, I might add.
Well, we’ll ignore the complexities and how it might change the result, just to get a sense of roughly what the values are.
If d=10,000, b=0.0025 and so E1‘ = E1 / 0.9975 = 1.0025 E1
Consequently the change in surface temperature to increase the temperature by this amount, T=1000.6K
Not very exciting, and still no thermal runaway.
Conceptual Understanding and Some Radiation Theory
Understanding this conceptually for most people won’t be too difficult. If you add energy to a body it will warm up. And it will emit more radiation. There will be a new equilibrium.
Two bodies doing this to each other will also just reach a new equilibrium – they can’t go into thermal runaway. Of course, no one believes that thermal runaway will result, least of all the person who made the original comment – that was their whole point. They just didn’t realize that a new equilibrium could exist. The only way I can prove it is mathematically.
Conceptual thinking is very valuable. Maths is very tedious. But because Gerlich and Tscheuschner have made such a huge contribution to the misunderstanding of basic thermodynamics it needs some extended explanation, including some maths.
Many people have got confused about the subject because
Heat flows from the hotter body to the colder body
We all agree.
Many people have taken the statement about heat flow and imagined that thermal radiation from a colder body cannot have any effect on a hotter body. This is where they go wrong.
A body with a temperature above absolute zero will radiate according to its emissivity (and according to the 4th power of temperature). This property is dependent on wavelength and sometimes on direction. The emissivity of a body is also equal to the absorptivity at these same wavelengths and directions.
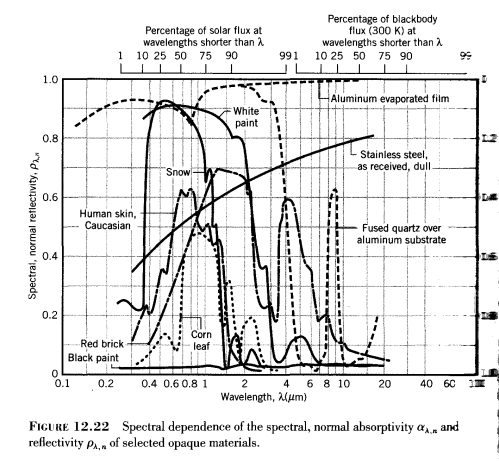
The wavelength dependence of emissivity and absorptivity is very striking:
Absorptivity is the scale on the right from 1 at the bottom to zero at the top and is 1-reflectivity. (See note 2).
Here you can see that snow is highly reflective at solar wavelengths (shortwave) and absorbs little radiation, whereas it has a high absorptivity at longer wavelengths (and therefore does not reflect much longwave radiation).
The same goes for white paint. It reflects sunlight but absorbs terrestrial radiation.
The equation for how much radiation is emitted by a body – εσT4 – does not include any terms for where the radiation might end up. So whether this radiation will be incident on a colder or hotter body, it has no effect on the radiation from the source. (See note 3).
Similarly, when radiation is incident on a body the only factor which affects how much radiation is absorbed and how much radiation is reflected is the absorptivity of the body at that direction and wavelength. The body cannot put out traffic cones because the incident radiation has been emitted by a colder body.
This is elementary thermodynamics. Emissivity and temperature determine the radiation from a body. Absorptivity determines how much incident radiation is absorbed.
Therefore, elementary thermodynamics shows that a cold body can radiate onto the surface of a hotter body. And the hotter body will absorb the radiation – assuming it has absorptivity at that wavelength and direction.
And once thermal radiation is absorbed it must heat the body, or slow down a loss of heat which is taking place. It cannot have no effect. This would be contrary to the first law of thermodynamics.
Two bodies at different temperatures in proximity both radiate towards each other. Heat flow is determined by the net effect. As standard textbooks indicate:
Why the Original Misconception?
I think that the original comment about two bodies with the same temperature being unable to heat each other is an easy misconception for two reasons:
First, the most likely mental image immediately conjured up is of two pots of water at say 50ºC. When these two pots of water are mixed together the temperature is obviously still at 50ºC.
Second, the two stars are probably pictured as already in equilibrium at the original temperature. Well, if that’s the case then nothing will change. The change only occurs when they are brought closer together and so the mutual radiation from each has a slight increase on the temperature of the other.
It’s just my guess. But what actually happens in the thought experiment probably isn’t intuitively obvious.
Conclusion
When two bodies have an energy source which has created a constant surface temperature and they are subsequently brought into proximity with each other, there will be an increase in each other’s temperature. But no thermal runaway takes place, they just reach a new equilibrium.
Basic thermodynamics explains that bodies emit thermal radiation according to temperature (to the fourth power) and according to emissivity. Not according to the temperature of a different body that might happen to absorb this radiation.
And basic thermodynamics also explains that bodies absorb thermal radiation according to their absorptivity at the wavelengths (and directions) of the incident radiation. Not according to the temperature (or any other properties) of the originating body.
Therefore, there is no room in this theory for the crazy idea that colder bodies have no effect on hotter bodies. To demonstrate the opposite, the interested student would have to find a flaw in one of the two basic elements of thermodynamics described above. And just a note, there’s no point reciting a mantra (e.g., “The second law says this doesn’t happen”) upon reading this. Instead, be constructive. Explain what happens to the emitting body and the absorbing body with reference to these elementary thermodynamics theories.
Update – now that one advocate has given some explanation, a new article: Intelligent Materials and the Imaginary Second Law of Thermodynamics
Notes
1. I said earlier: “If this star absorbs thermal radiation from elsewhere, it must emit more radiation or its temperature will rise. If its temperature rises then it will radiate more energy.” Strictly speaking when radiation is absorbed it might go into other forms of energy. For example, if ice receives incident radiation it may melt, and all of the heat is absorbed into changing the state of the ice to water, not to increasing the temperature.
2. Incident radiation can also be transmitted, e.g. through a thin layer of glass, or through a given concentration of CO2, but this won’t be the case with radiation into a body like a star. The total of reflected energy plus absorbed energy plus transmitted energy has to equal the value of the incident radiation.
3. The Stefan-Boltzmann equation is the integral of the Planck function across all wavelengths and directions:
Where h, c0 and k are constants, T is temperature and λ is the wavelength.
The Planck function describes how spectral intensity changes with wavelength (or frequency) for a blackbody. If the emissivity as a function of wavelength is known it can be used in conjunction with the Planck function to determine the actual flux.
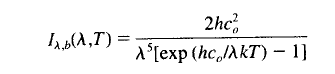


What happens when you go to the extreme case where the two stars are coincident in space – by some magical means.
[…] – a worked example with the maths, Radiation Basics and the Imaginary Second Law of Thermodynamics Possibly related posts: (automatically generated)CO2 – An Insignificant Trace Gas? Part OneThe […]
The joint temperature rise is correct qualitatively. Two points.
1. You don’t earn a free energy lunch of two hotter stars. It will take work to move the stars closer together through the radiation field. In a sense you are pushing against a radiation pressure. So the total work required to move the stars closer together should equal the increase in energy due to the higher temperature.
2. This analysis completely ignores gravitational field effects That’s OK since it’s not the point here. Let’s say stars have zero mass, and all temperature changes will be basically instantaneous.
The run away conclusion-confusion has a Zeno’s pardoxian feel to it. Thanks for the rust removing math. Been almost 30 years
You are getting quite close to repeating Pictets Experiment.
www2.ups.edu/faculty/jcevans/Pictet’s%20experiment.pdf
Considering this problem in the realm of ‘Sources and Sinks’:
Sources: the stars (high degree K numbers and source of initial flux)
Sinks: space (deep space: low deg K numbers and eventual ultimate destination of all flux appearing locally)
In betwixt, exists an energy flux ultimately determining the temperature of each body, including the ‘bodies’ of each stars …
I would introduce the concept of S-parameters (Scattering Parameters AKA Scattering Matrix Parameters) we RF/microwave engineers use to characterize uWave components and the direction and relative intensity of energy flux densities in/thru components irrespective of physical quantities (like voltage and current) for that concept would seem to be ideal to characterize the action of “greenhouse gases” (incl CO2 and H2O) and their affect on solar and earth-sourced energy (heat) flux –
– but no time to delve deeper, for a friend is going to gift me a 1/2 tank of filtered WVO (waste veggie oil) for the old diesel Mercedes 300TD
.
.
For some reason this link did not work try again
www2.ups.edu/faculty/jcevans/Pictet’s%20experiment.pdf
3rd time lucky
Click to access Pictet%27s%20experiment.pdf
I think this is Bryan’s link
The third comment you quoted was interesting. He is confusing power with energy (you are encouraging this a bit by using E for power, and describing power as “energy”, but giving it in correct units of Watts). Minor point.
The “total energy radiated” is given of course by the integral of the power over time. For a hypothetical source of constant radiated power, the integral over all times of this is infinite. That is obviouslyunphysical, but it is what mathematical assumptions of “constant” give you if you don’t include any more physics, like E=m c^2 which is the real “speed limit” on how much energy can be emitted by an isolated star.
“Consequently the change in surface temperature to increase the temperature by this amount, T=1000.6K
Not very exciting, and still no thermal runaway.”
– – – – – – – – – –
Have you taken into account time? That temperature is the result after a nanosecond (or whatever span of time) after the experiment started. After the second nanosecond the temperature increase is bigger and will continue increasing as time keeps rolling…
A problem with getting in love with one’s theory is that too much maths is getting too close to the tree. The forest dissapears.
By this theory, the famous IPCC oven with perfectly reflecting inner walls will roast a chicken starting with its own ambient temperature. Nonsene.
Edward
The math was being done from the perspective that equilibrium had been reached; whether that happens in one or many nanoseconds. Generally speaking you would expect the approach to equilibrium to be asymptotic, unless you start adding temperature sensitivity to things that have been assumed constant in the above equations. In which case the approach to equilibrium can be differently interesting
As to the forest and the trees – it was just looking at the forest that led people to make fantastic thermodynamic claims; necessitating looking at the “trees”.
The math, tedious as it might be, clarifies since the assumptions are stated. One can go back and loosen the assumptions, if necessary.
DEEBEE –
I missed your answer before I added mine. Looks like mine wasn’t necessary.
SoD , sylas or anyone.
Could you point me to a source of a diagram in reasonable resolution showing the full spectrum of downwelling backradiaton taken a few hours after sunset.
Edward:
This is the equilibrium response.
As the article says:
So we don’t know whether it’s a nanosecond or a million years. That isn’t important. We know that this is the new temperature at which equilibrium finally occurs.
Eppur si muove!
– – – – – – – – – – – – – –
“So once new equilibrium is reached, we can calculate the new radiation value, and from the Stefen-Boltzmann equation, we can calculate the new temperature.
These equations don’t tell us how long it takes to reach equilibrium, as we don’t know the heat capacity of the stars.”
“So we don’t know whether it’s a nanosecond or a million years. That isn’t important. We know that this is the new temperature at which equilibrium finally occurs.”
– – – – – – – – – – – – – –
The equilibrium is a function of time! The equilibrium is reached after some measurable time has elapsed after the last temperature increase; that is, when there is no further increase in temperature. I guess you can put that in mathematical terms. Can you?
When would there be an equilibrium reached? When both the energy emitted and absorbed are the same. No rocket science here, simple high school physics.
After the minimum unit of time needed for the temperature increase (and forget about million years, please), energy is still flowing, so, according to your postulate, both stars will increase their heat. Then, instead of emitting K=1000 Star A will irradiate 1000.6K to star B. At the same instant star B is irradiating in a new level of energy (1000.6K) because it was heated by star A in 0.6K. As this process is simultaneous, both stars will increase its absorption and irradiation in an exponential way. Which is nonsense.
This comment illustrates the problem with people who try to do physics using rhetorical argument.
I would suggest that either Edward put his argument in terms of equations that have well defined meaning, so they can be rigorously tested. Or if he is not able to do so, he should just say so and move on.
There is a baseline level of training to do science properly. I know some people take offense to that, but it is a fact.
Carrick,
you’re wrong. You are assuming I don’t understand radiation and the rest. of the field. You would be surprised.
On the contrary, the burden of the proof is resting on YOUR shoulders, those who claim that can explain anything through maths. It is you who are among those pushing the postulate who must provide the proof.
And rethorics, or logics, is the base of mathematics. I don’t know if you knew that. It was extensively used by Archimedes, Pythagoras, Ptolomeus and the rest of the gang.
Edward:
Of course we’ve advanced more than a little bit since then in how we practice science. That’s why philosophy isn’t considered part of the physical sciences.
Since you bring up the ancients, Plato’s Republic for comments on rhetoric (note spelling), and then research what happened to Socrates and why. The ancients were as well as the moderns of the limitations of rhetoric, and the need for geometrical proof to replace it. They didn’t have modern analytic based proofs or they certainly would have used that too. Apologies for this being so OT.
As to the responsibility being on my shoulders… um.. no. You are making claims, it’s your responsibility to substantiate them, not mine.
Bryan:
Exactly how long after sunset? Why not daytime?
scienceofdoom
Exactly how long after sunset? Why not daytime?
Say after three hours or so.
Reason being daytime Downwelling also contains solar input as well as “back” radiation.
I am only interested in the truly “back”component of radiation for the moment.
Bryan:
We can differentiate solar radiation from terrestrial radiation. 99% of solar radiation is less than 4um.
99.9% of terrestrial radiation is greater than 4um.
That’s because solar radiation from a body at 5780K has a maximum at 0.5um and terrestrial radiation from a body around 288K has a maximum at 10um.
Here’s the solar spectrum:
So downward longwave radiation at the earth’s surface that is greater than 4um is from the atmosphere.
Would the figure/’curve’ describing upwelling surface LWIR be a static or a dynamic figure: Ans: Dynamic; as the upwelling ‘flux’ (EM enrgy) would diminish as the temperature of the surface falls (after sunset) according to the 4th power (of temperature in the Planck’s law and S-B equation). Note also the peak of the ‘spectral curve’ is changing to longer wavelengths as cooling takes place.
There is an observable example that may help some understand the issue conceptually.
Some deep space missions for which solar panels were not practical, used Pu 238 pellets as an energy source. Those Pu 238 pellets were sort of hot when laying out on a table. However, when they were placed in an insulated box, they became red hot. Sorry, I don’t know the specific change in temperatures, but no need to know that to understand that the temperature of the pellet increased when placed in the insulated box. So the pellet temperature was increased because heat generated by the pellet was being re-absorbed. Some may say its because the heat cannot be transferred to the surrounding environment as efficiently when inside the box, but what is actually happening is that the box is returning much of the heat to the surface of the pellet, even though its cooler than the pellet. So in that sense, its similar to the star thought experiment.
The PU 238 pellets irradiate energy (or heat) to the surrounding medium (air or water, as nuclear spent fuel does in nuke stations repositories) therefore they are cooling. In an enclosed container ambient temperature raises until it equals the heat released by pellets. As pellets are no longer cooling as before to a cooler medium, their surface reflects its real temperature and acquire their red hot color. You can see that on a kitchen stove and a steel pan over the fire: with water the color is normally light because water is cooling the pan. In turn, water is cooled by evaporation.
When the water evaporates completely the steel becomes red hot because air does not cool the pan as effectively as water (different density and mass).
I forgot to point out that the pan is heated by an energy source below it, ie.: the stove flame. The pellet’s surface are heated by an internal source of heat, PU-238 decay.
Hmm, so the main reason the pellet gets hotter is because the delta T between the pellet and its immediate environment gets smaller and therefore the rate of transfer of heat to the environment decreases until it gets hot enough for equilibrium (transfer rate = generation rate) to be reached again. I can buy that. Thanks. Guess its not that similar to the star though experiment.
Not really; there is release of energy at IR wavelengths as the Pu238 decays. Because no energy can escape from the box, the temp rises. If the Pu pellets were unconstrained, as with stars’ energy sources, different scenario. Mind you, the irradiation of each star’s mass, if the photons were absorbed, would increase the temp thereof, thus increasing the amount radiated from each, eventually reaching some equilibrium point. One could split hairs and argue that SOME losses thru the box will eventually result in an equilibrium being reached (max temp).
Good point. My example wasn’t a good analogy. See my response to Edward above.
scienceofdoom
“So downward longwave radiation at the earth’s surface that is greater than 4um is from the atmosphere.”
I think that this is to ignore the effects that radiation solar radiation uv,visible secondary effects from upper atmosphere ionisation,scattering and so on will produce.
I know that nighttime measurements are taken but I cannot find a decent reasonable resolution graph, hence the question.
I take it you find yourself in the same position!
scienceofdoom
Its OK Ive found what I’m looking for
Click to access TownEtAl_2005.pdf
An alternative way of looking at it is to say that the same power is being generated but must radiate to infinity from a smaller surface area. Two spheres widely separated have total area A1+A2, but as they come together, the view each has of outer space is partially blocked by the other. If you bring them into contact, the areas facing one another disappear entirely.
To play devil’s advocate for a moment, suppose instead that you left the stars where they were, but connected then physically with a thermally conducting wire – and let’s pretend it can conduct enough heat to make a difference. Now again the net heat flow is from the hotter star to the cooler one, but heat diffusion is only the net result of a bidirectional random walk in which energy flows both ways, so just as in the radiative case, we can say that both stars are receiving a heat input that they were not previously.
They are also each outputting more energy, because of the introduction of the wire, with the effects offsetting against one another. The net result of course is that the cooler star will warm, and the warmer star will cool.
[However, interestingly, the net result (assuming perfect conductivity) won’t be half way between the two temperatures. Instead, it will be the “L4 norm”: the fourth root of the mean of the fourth powers of the temperatures. So it’s still not like mixing warm pots of liquid at different temperatures.]
This example shows that the effect being discussed is a feature of the special geometry of radiating objects close to one another, not a general result of thermodynamics.
When other non-radiative heat transfer mechanisms can make a contribution, entirely different behaviour may result.
Edward:
As the article states:
It is very common to find the equilibrium solutions to this kind of problem without finding out the changing values on the way to equilibrium as a function of time. These time based calculations are usually much more challenging.
See for a simple and different example – Radiative Forcing, Thermal Lag and Equilibrium Temperatures
In the maths in the article there is no “t” so nothing is a function of time. (The values of E as Carrick pointed out are in units of watts, or J/s. But these values are not a function of t).
I think that your conceptual thinking is leading you astray.
The reasons – conceptually – that the stars change from their original equilibrium is that both have a new source of energy when they are moved into positions of proximity to each other.
But as I explained in the article, the reason for the maths, is to prove something when conceptual thinking is indicating something opposite.
So to demonstrate that the values E1′ and E2′ are functions of time and will continue to increase you need to show some flaw in the above maths – as this is the mathematically proven equilibrium solution for this new scenario.
******************
Quote: “In the maths in the article there is no “t” so nothing is a function of time. (The values of E as Carrick pointed out are in units of watts, or J/s. But these values are not a function of t).”
****************
That’s why the equation and the postulate are invalid because they are incomplete. You are making a sloppy abstraction with selected factors and leaving out vital ones: time and absorbance index for both stars. Without time and absorbance in the equation you’ll never know when a state of equilibrium is reached. Your postulate is an elegant exercise of twisting and spinning the science, dodging some basic 2nd Law strict requirements for energy radiation and transmission. We find that in the present AGW way of doing “their” science.
BTW: The intensity of radiation is given by I = I0 x exp(-A) + B [1 – exp(-A)]
I0 is the incident intensity, B is the Planck term and applies only to the particular frequency being considered. The absorption and emission are both determined by the value of A, the absorbance. For a system in equilibrium with no resultant absorption or emission, I = I0 and therefore I0 = B.
This is really an alternative statement of the Kirchhoff radiation law. Any argument going against this goes against the 2n Law of Thermodynamics. Are you brave enough to contradict the 2nd Law? If you can demonstrate the 2nd Law is wrong and that you are right you should apply for a Nobel in Physics. If you can’t, then follow the advice given to me and just move on.
The second law, is that entropy increases, chaos increases, energy disperses. This doesn’t contradict that. It is a result of the increasing entropy of two systems interacting.
I think conceptually this can be a confusing subject(it is something i believe i “get ” however.
But a simple every day example that most of us experience on a yearly basis may help….
Ok, so you have a house and a fire, conduction and subsequent convection from the materials wont change. You have the fire going for twenty four hours, the outside temp hasnt changed(as happens here with southerly windstorms, and id imagine is the same in the northern hemisphere from the north)… but in the day time sun streams in through the windows. Will the house get warmer in the day than during the night? I think we all know the answer. And the reason why, the shortwave radiation from the sun is absorbed by the interior of the house, and the subsequent long wave radiated through the house… even though the “warmth” of the winter sun is less than the house warmth, it will increase the equilibrium temperature of the house.
This maybe a bit o a pointless comment, but i think its easier to “get” stuff when it can be compared with everyday experiences… even if other factors can complicate things as far as quantifying everything exactly in this example.
If we combine the two suns, we double the total power and the volume, but we don’t double the surface area. The surface area increases by a factor of ~1.587 or the square of the cube root of 2. If I did my sums right, the surface temperature will be 1122.5 K
DeWitt Payne said
I guess this is a cooler temperature than that generated by the limiting value of simply bringing the two stars closer together. For one thing the geometry calculations certainly don’t assume that the stars physical size changes once they start overlapping.
The simple geometry model says the resultant star would be the same diameter as the original star, but with twice the power output. By calculation 1189 K
More complex is the transition between first touching and finally merging. I’d probably have to get my third year calculus books out to figure that one out.
I didn’t ask my original question as a flight of fancy, but to get some handle on the limiting value of temperature. we have two possible values based on the energy output – DeWitt’s and my naive version. Plotting the temperature using SoD’s formulae would – we hope – produce a temperature that was limited at one of those values – I suspect my naive version.
If the temperature from the SoD formulae limit at some other value then we would have an interesting situation.
Congratulations for the great blog.
I’m am engineer and I worked for a while with physiology modelling, with mathematical techniques not much different from the ones used in climate science.
Although I think glboal warming is probably happening, the real questions non-scientists should be asking is “how much we trust those results? well enough to spend trillions of dollars on policies based on it? shouldn’t we wait until it becomes a little more clear?”
I think global warming is an issue serious enough for governments to create focused an well funded research programmes, but nothing as certain enough for being the main driver of world politics or economics.
Sorry for any grammar mistakes. English isn’t my first language.
The science of Doom,
The following is an attempt to make more simple in words what you have shown in maths:
The two stars have internal sources of energy. The surface temperature results from the generated power being radiated from the area of the surface. The proximity of two stars will result in some radiation that will be absorbed at each star from the other star, just as a planet absorbs energy from a star to heat up. The amount absorbed would also be like that absorbed by a planet of the same angular size and distance (assuming same absorption coef.), and for reasonable separation distances, would be small. This will still result in a slightly higher temperature for both stars independent of which is hotter or colder, as both are receiving slightly more energy on their surface than just that internally generated. In the case of a planet, it is heated only by the star (ignoring internal energy from its formation or from radioactive decay). Obviously bringing a planet close to a star heats it up. However, even the relatively cold planet surface will heat the star up also, although it would be a very small amount. This would be due to reflected star radiation, or long wave planetary radiation at the lower planet temperature. It is still energy and is absorbed by the star.
Two facing walls in a room, that are at the same temperature (and not being heated on their back side) would not heat each other up, as heat emission and absorption would balance (and all surfaced above absolute zero always emit). If one were hotter initially, the walls would tend toward equal levels over time. No perpetual motion here or runaway. The difference between the stars and walls is that one is a continual generator of energy, and the other is not.
Seems like a good explanation.
The reason for using an internal source of energy for toy models is that the atmosphere is relatively transparent for incoming short wavelength solar radiation but much less transparent for outgoing long wavelength IR radiation. So if one just wants to look at outgoing long wavelength IR, one constructs a model with an internal heat source to represent the energy from the absorption of incoming short wavelength solar radiation.
Jerry and DeWitt Payne:
The limiting value of the temperature increase when both stars are touching can probably determined without performing the double integral. “View factors” have been calculated for many shapes and are listed in text books. I should have the opportunity to look it up in a few days.
I think a lot of people are mislead by the following (incorrect) chain of reasoning:
(1) If the two stars will increase each other’s temperature, this constitutes a system with positive feedback;
(2) If the system has positive feedback, the stars’ temperatures will increase without bound; and
(3) Since this kind of runaway (unbounded) increase in T is obviously unphysical, statement (1) must be incorrect.
I think this is the same mistaken assumption that Edward is making in this thread, and the same one that’s implicit in his “IPCC oven” joke.
In actuality, a positive feedback doesn’t imply runaway. If the magnitude of the feedback factor is between 0 and 1, the system will simply increase or decrease asymptotically towards some new limit.
Actually, the oven joke is even more confused. It’s hard to believe that Edward was citing that seriously as an analogy for the topic at hand.
Good points. I would add one other misconception:
4) Since the two stars increase each other’s temperature, that this means they are emitting a larger net amount of energy into space.
In fact, the ‘system’ of stars emits the same amount of energy as the two stars when they are far apart. The extra radiation absorbed at star1 (from star2) is the exact same amount of energy that star2 absorbs from star1’s emission, and so this additional bit of radiation from star1 (due to its increased temperature) is not available for absorption outside the ‘system’ of stars.
The funny thing is that “Edward” went boasting to a private climate sceptic email list about how he had taught you all a lesson. He appears to have been rapidly brought round by some of the less insane people there who explained patiently and politely how he was talking nonsense. Not that he actually shows any signs of understanding, but he blindly trusts a few of the others.
I’m sure there is a lesson there somewhere.. [moderators note – I appreciate where you are coming from with this comment, but we try and keep away from “stuff people have done” on this blog and stay with the science, that way all are welcome even those who are “wrong”]
Science of Doom,
I am puzzled by your comment “the blog has recent new found interest thanks to the very kind and unexpected words of Steve McIntyre of Climate Audit”. Why do you consider this unexpected? Many serious scientists are skeptics because they honestly have examined the facts and conclude the science of AGW is not settled. In fact it is mainly the non-skeptics that seem to be close minded to reason (you and only a few others seem to be the exception to that closed mindedness). While there are outliers on both side of the issue that are neither scientists nor unbiased, this is true on all issues, and should not lead one to conclude that the outliers are the main people to argue against. My case is probably typical of serious scientists that are skeptics. I started out accepting as a hypothesis the claims of AGW, but after examining the literature, reduced my belief to the fact there was some GW, but the AGW claims were exaggerated and especially the CAGW claims were unsupportable by any facts. Since the entire world economy would be affected by decisions relating to results of the debate, it is not surprising that skeptics are very vocal, as they are trying to find the actual facts and lead to a valid conclusion.
Leonard Weinstein:
Because I wasn’t expecting it.
scienceofdoom:
My kudos for maintaining an informative, respectful site.
Your additive energies, [equations 2 & 3] seem to be at odds with my understanding of radiant heat exchange. From A Heat Transfer Textbook v3 by Lienhard:
Radiant heat exchange between two finite black body is given by:
Q1=A1F1(eb1-eb2) Where Q1 = net loss of energy to surface 2 (if negative, gaining energy)
A1 = area of surface 1
F1 = view factor of surface 1 to 2
eb1 = back body flux from star1
eb2 = back body flux from star2
As you can see Q1 equals zero based upon your problem criteria. Applied to equations 2&3 E1’=E2’=E1=E2…no increase in temp.
Side note:
I checked your view factor calculations via alternative equitation and arrived at the same answer. Further, for d=2r F12=0.067. Working this value through your equations achieves an equilibrium temp of 1017.5K.
“via alternative equitation”
equitation…I wasn’t riding a horse, damned auto correct.
scienceofdoom,
Steve and others have complemented Judith Curry and others when they gave reasonably balanced positions and engaged in fair conversation. I have also complemented your site, although I do not have a separate blog. It was not a surprise to me that Steve specifically mentioned your site. I was just curious why you seemed surprised. I realize you did not expect it, but the serious skeptics are very much interested in reasonable interactions and good scientific analysis, and you have a very interesting and fair blog.
This is off topic, but … I sure wish there was a more neutral term we could use in place of “skeptic”. Most scientists, regardless of their position on AGW, are or should be “skeptics” and it grates a bit to see the term used in a way that implies that the virtue of skepticism is the exclusive property of one side in this debate. The term “denialist” is worse, of course … and referring to people as “pro” or “anti-AGW” doesn’t make sense either.
It’s a real head-scratcher….
[note-sorry this got incorrectly trapped in the spam queue]
Jenn
I think “contrarian” is pretty much neutral… I’ve seen people using this around.
FWIW, I think of a skeptic as someone who doubts conclusions derived or extrapolated from facts and a –peoplewhodontagreewithusist— [moderator’s note, please check the Etiquette ] as someone who ignores or disagrees with the facts, often in the effort to come to some other conclusion.
Being skeptical is healthy; denial is…commonly a defense mechanism.
J. Lanier:
In my example also the net heat exchange is zero.
The point is that the emission of thermal radiation from star 1 is already considered in reaching the original surface temperature of 1000K.
But star 2 has not yet appeared on the scene. So the equilibrium state prior to the arrival of star 2 is 1000K – and this is WITH the emission of thermal radiation from the surface of the star 1.
Now star 2 arrives and so star 1 now has an additional source of energy.
But E1=E2 and E1’=E2′
Isn’t Q1 the same as E1b?
I didn’t have a Q1 or E1b. Your Q1 = 0, which I also agree with because E1=E2 and E1’=E2′ (but E1 is not equal to E1′). What is E1b?
Why not find the flaw in my calculations? If your equation demonstrates that my result is wrong, it’s just a matter of pointing out which step is incorrect.
It’s the spherical geometry that makes the problem difficult. Take two disks of arbitrary but equal diameter, where the diameter is much, much greater than the thickness so emission from the edge can be neglected, oriented with the surfaces parallel. Put an internal energy source in each disk such that the surface temperature is 1000K at infinite separation. Now bring them very close together. There can be no energy lost on the facing surfaces. So each disk will have to radiate twice as much energy from the surfaces that are not facing and the surface temperature of each disk will be the fourth root of two higher or 1189K. The temperature goes up, not because there is an exchange of energy between the disks or spheres or whatever, but because there is a loss of effective radiating area for the same total energy flux.
“The temperature goes up, not because there is an exchange of energy between the disks or spheres or whatever, but because there is a loss of effective radiating area for the same total energy flux.”
I believe the point of the thought experiment was that temperature would rise before contact of the radiating bodies… So if you have the disks 1mm apart, its not changing the surface area per mass, but it will change the equilibrium temperature of both bodies, because they would essentially(approximately) be absorbing the same as they are radiating. So basically it is changing the radiating area… but through energy/radiation exchange?
What im really asking, is, are you predicting that there will be no change in temperature of both disks til contact is made?
No. The temperature of each disk will go up as the spacing between the disks decreases. Once the spacing gets small enough that the radiation escaping from the gap between the disks can be neglected, there will be no further increase in temperature. At infinite separation, each side of the disk sees a solid angle of pi steradians at absolute zero. As the disks approach, each disk sees less area at absolute zero and more area at the disk temperature. But the radiant flux remains the same so the temperature goes up. It should be fairly easy to graph the temperature vs separation with planar geometry, certainly a lot easier than with spherical geometry. I’ll see if I can do it tomorrow.
To calculate the result as the stars get closer and closer, I searched for a handy “view factor” for 2-spheres and don’t have one. I have the view factor for 2 cylinders of different radii separated by a distance and a number of other geometries but not the one we require.
I’m sure that they have been worked out and are in a reference book somewhere.
Alternatively, someone who enjoys the challenge of doing the necessary double integral can look forward to the praise and adulation of all of us on completing the challenge.
This is the solution for parallel disks, not two spheres. It probably isn’t exactly correct, but it shouldn’t be too far off. What I did was calculate the fourth root of the ratio of the solid angle at infinite separation, 2*pi, to the remaining solid angle. Take two very thin parallel disks separated by a distance x, each with a radius of 1 m and an internal power source that provides sufficient energy to keep the surface at a temperature of 1000 K when exposed to space at absolute zero and infinite separation. The equation I used in Excel was 1000*power(2*pi()/(2*pi()-2*atan(1/x)),0.25). power calculates the value of the first argument raised to the power of the second argument, in this case 0.25 or the fourth root, atan is the arctangent function which returns the angle in radians of the argument and x is the separation distance in meters.
Graph.
scienceofdoom:
If there is no net accumulation then adding any portion, b, of new energy level, which is based on the accumulation of energy, would yield zero…no change in temperature. That is, neither star accumulates energy from the other at infinite distance or when d=2r…and regardless of time to equilibrium. No increase in temperature.
To better illustrate the problem with equations 2&3, try this. Star 1 is as you outlined. Star 2 is now a spherical mass 2, with a radius equal to that of star 1, a temp of 0 K and an ε of 0.
Now E1 = E1′ E2= E2’=0 yet an equal amount of energy is incident on star 1 via reflection.
Should the equation now be E1’=E1+E1b?
Dewitt Payne said
Now that is a good result! Your limit value of 1189 from geometry is the same as the physics model of doubling the power output.
I was a bit concerned about the difference in surface area of a sphere rather than a disk but I think that your assumptions of ‘sufficient power’ in the disks negates that
If we assume that the spheres cannot overlap, then the upper bound of the temperature would be the same as for two parallel disks with the same radius as the spheres at a separation of twice the radius, or 1040.7 K.
“Therefore, there is no room in this theory for the crazy idea that colder bodies have no effect on hotter bodies. To demonstrate the opposite, the interested student would have to find a flaw in one of the two basic elements of thermodynamics described above. And just a note, there’s no point reciting a mantra (e.g., “The second law says this doesn’t happen”) upon reading this. Instead, be constructive. Explain what happens to the emitting body and the absorbing body with reference to these elementary thermodynamics theories.”
Agreed.
Now, will SOMEONE please provide me with a “radiation cartoon” that shows what is happening (i.e., the radiation “scenario”) in the waters off Fiji at noon on a clear day. I would like a diagram similar to the K&T diagram, but for this actual spot on Earth. Please remember that the water is radiating according to almost a blackbody at about 30 C. How much “backradiation” is present in this situation? Why don’t I cook in the presence of all this IR?
And will someone tell me why it is not as hot in those areas with copious amounts of GHGs (Fiji) as it is in deserts where there is very little GHGs.
Hint: you can consider convection and evaporation.
Aw, nuts. I didn’t specify the time. Need diagrams for noon and midnight. It has to be very frigging hot at noon with all the radiation from the surface + solar radiation + backradiation, eh?
Silence, as usual. I know, don’t feed the trolls and talk to the insane, I guess….
To delve into my own dilemna: If I put a glass-covered greenhouse with a black floor on Fiji, I will get temperatures on that floor in excess of 160-180 F at 1:00 on a clear day. This makes perfect sense, since the solar radiation at that time of day is in the neighborhood of 900 wm-2, and the SB calculations predict such a temperature for the blackbody floor. The glass will block the IR “backradiation.”
Now, if I open the windows and allow convection, the temperature goes back to approximately ambient, which is about 33 C there. There is no “trapping” of IR in a greenhouse. Wood did the requisite experiments to prove all this way back in 1909, IIRC.
Now, if I replace the glass on the greenhouse with a material that passes IR, such as NaCl (polyethylene??), I will wager that the temperature goes no higher than in the glass greenhouse, DESPITE the fact that the total radiation seen by the floor is now solar PLUS “backradiation.”
WTF?
In your ‘polythene’ scenario there will be roughly equal IR flux in and out of the greenhouse. In that case the air inside is radiatively interacting with the air a few metres outside. What you are most likely to see is a reduction of the internal temperature.
The overall heating is of course mostly due to absorbtion of all frequencies of light by the floor and subsequent heating of the internal air by convection.
Jerry:
“What you are most likely to see is a reduction of the internal temperature.”
No, Wood’s experiments showed that there was no difference in temperature. Google Wood greenhouse effect.
But IF you can add backradiation to all other forms of radiation (solar, “surface”) the temperature should clearly be higher, no?
Of course, if that were true, the solar heating guys would have pounced on this 50 years ago, and the “enclosures” for all direct solar energy systems (like hot-water heaters) would be constructed of IR-transparent materials. But, alas, they are usually simple glass!
There is simply something wrong with the common concept of the “atmospheric greenhouse effect.” Period.
Actually, the Wood greenhouse experiment showed similar, not identical warming.
His experiment had many sources of error and heat loss, including air leakage from the glasshouse and conduction through the structure and transparent panes.
What I am talking about is the IR radiative difference between say 60C internal air and 20C external air which is a T^4 relationship.
The hotter internal air will be radiating appreciably more strongly than the external air – at IR frequencies. However the total insolation power of all light frequencies well exceeds the LW IR power transmission. Hence I expect only a smallish temperature drop
Jerry: we seem to be on different wavelengths here. But, first off, can you support (with science) your notion that Wood had “many sources of error and heat loss????” A reference to the literature would be great. If you don’t have that, goodbye.
Well if it helps, here is Wood’s description of the initial setup
So we have immediately, imperfect insulation – he could have used a Dewar flask for instance.
We also know from direct measurement that objects in space – e.g. the moon get to very high temperatures in direct sunlight – up to +123C and down to -233C. So ignoring radiation there is clearly heat leakage from his apparatus otherwise it would have trended to the higher temperature.
He also did not control for thermal conductivity of the transparent plates.
JAE:
Your questions aren’t ignored. But there isn’t a defined service level agreement on Science of Doom..
Previously, on Sensible Heat, Latent Heat and Radiation you asked a similar question about the difference between Atlanta and Phoenix.
Maybe I can ask you a question, which might make solving the problem easier – from both questions I sense that you don’t believe this “back radiation” exists. Is that correct?
If so, what about this radiation measured at the earth’s surface, unfortunately I don’t have one for a desert or Fiji, but hopefully Canada will do for now:
And in the article where you first posted there was a measurement of longwave radiation (greater than 4um) over a 24 hour period:
And if not, clearly there is something bothering you about the idea.. at least that’s how it seems. Rather than riddles, why not explain your theory.
Three identical metal cylinders of mass one kilogram with a little hole drilled in each to accept a thermometer.
Common apparatus in Schools and colleges.
Three identical thermometers.
The cylinders and thermometers were interchanged but no differences could be found in either group.
Two of the cylinders were placed in a large perfectly reflecting box.
After some time the two cylinders reached thermal equilibrium (indicated by each thermometer reading 25degrees C)
Outside the box the temperature was adjusted to maintain a constant 25degrees C.
The third cylinders thermometer read 25degrees C and this was now placed in the box.
The cylinders were situated at each apex of an equilateral triangle centred two cylinder diameters apart.
What changes if any would we find in the thermometer readings?
Scienceofdoom: Of course I “believe” in backradiation. All substances absorb/emit radiation in accordance with their spectral characteristics. My problem is that the backradiation seems to have little or no effect on the air temperature (Phoenix/Atlanta). This may be due to the effects of convection and evaporation. However even this does not explain why the temperature in the clear greenhouse is not higher; why is it not equivalent to the sum of the solar radiation plus the backradiation (it is obviously not)?
If I have a theory, it is tha the “greenhouse effect” is nothing more than the storage of heat by the surface and atmosphere. The backradiation is just an effect of this heat and does not “cause” any increase in temperature.
Jerry:
I don’t think it makes much sense to compare the moon with the earth, since it has no atmosphere and has a different albedo.
Any thermal conductivity differences would probably make very little difference in this case.
JAE,
In an IR-opaque greenhouse, you will get back-radiation from the glass. In an IR-transparent greenhouse, you get back-radiation from the sky, which will generally be cooler than the greenhouse glass.
A greenhouse with an IR transparent cover actually gets colder on average on a clear night than if there were no cover. This is particularly true with the tunnel type. That’s why one company makes IR absorbent polyethylene sheet. The reason is that with convection blocked, a temperature inversion always forms inside the greenhouse.
http://www.extension.org/article/18367
Nullius: The point is that the entire temp. inside either greenhouse is explainable by considering ONLY solar insolation. Where does the backradiation come in to play? Shouldn’t it add to the solar insolation wattage in the clear greenhouse to make the temperatrue even higher?
That’s only true of the maximum temperature during the day. The minimum temperature at night is entirely dependent on the brightness temperature of the sky and the optical properties of the cover. Convection and conduction are blocked by the cover so the greenhouse can and does cool mainly by radiation from the surfaces inside.
“That’s only true of the maximum temperature during the day. ”
No, it’s true whenever the Sun is shining on the greenhouse. Forget the nighttime, for the moment. Are you ignoring my question about the effects of backradiation during the DAY? Why is it not hotter than can be explained simply by the solar insolation?
JAE:
On Fiji vs a desert. Why one climate is different from another depends on lots of factors and not just the surface radiation budget.
Why do the poles radiate out more than they receive from the sun? Because energy is carried from the equator and sub-tropics to the poles by the oceans and the atmosphere (split about 50/50).
On average – globally, annually – the horizontal movement of energy balances out to zero and you get a diagram like the Trenberth and Kiehl one. But it’s not the case in any one location.
Why is Fiji cooler than the desert?
Fiji is in the middle of the ocean which has a large heat capacity and thermal conductivity, whereas sand has low values. Therefore, with an equivalent amount of heat received, the oceans don’t increase in temperature as much as deserts. The ocean takes longer to warm up and longer to cool down than the desert.
Fiji 18’S
– Hottest (max) month – Feb 31’C av max, 24’C av min
– Coldest (max) month – Jul 27’C av max, 20’C av min
not sure of the basis period this is just a rough guide from http://www.tourismfiji.com/fiji-weather.html
Desert at 18’S?
The closest I could find from remembering where deserts were was the Kalahari at 23’S in Namibia.
– Hottest (max) month – Oct 35’C av max, 18’C av min
– Coldest (max) month – Jun/Jul 25’C av max, 6’C av min
but some days max gets up to 45’C
source –
http://www.kalahari-desert.com/kalahari_desert_weather_climate.asp?varTourName=EcoAfrica
What do we find – the desert has a larger diurnal range and a larger seasonal range.
You see this with continental interiors vs maritime climates. Day/night and summer/winter variation is much higher.
Also, lots of other factors affect climate. Clouds, for example, generally have a net cooling effect – more downward longwave radiation (heating) and more reflected solar radiation (cooling). Net effect on average (global annual) is -18W/m^2.
Over deserts clouds don’t have as much cooling effect as over oceans because the albedo of the desert is similar to the albedo of the cloud, so minimal shortwave cooling but some longwave heating.
How cloudy is the Kalahari vs Fiji?
How much moisture is in the air in the Kalahari vs Fiji?
Climate is complex. Microclimate is complex. But it’s not that physics lacks mechanisms and equations, just lots of data is needed to calculate the difference between Fiji and Kalahari on any one day.
I there! I guess the news of my death was greatly exaggerated.
I have seen a lot of misunderstanding going on:
Woods experiment:
Salt is a well known hygroscopic material. It absorbs humidity from the air. Evaporation then cools the rock salt plate that becomes cooler than the transparent plate that does not absorb humidity. Was Wood not using his brain?
This leads also to the common myth that glass is opaque to IR radiation.
I somebody believes that, hurry go and tell all photographers to stop losing their time making infrared photography because IR won’t pass through their glass lenses!
All wavelengths in the spectrum pass through glass. Visible wavelengths go through glass, and radio waves too, so what’s the reason for a very narrow band in the IR region not to pass through glass?
The question is in the use of the widely used term: relatively. They say “Glass is relatively opaque to IR”, but they forget to say that all materials are “relatively” opaque to IR. The fact is all materials absorb radiation in more or less amounts and transmit heat in different amounts and speed. Glass absorbs all radiations and will let pass most of IR and visible light. If enough layers are superimposed the amount of light passing will decrease and there will be a point where all light will be absorbed and no light will pass through the thick glass block.
http://www.newton.dep.anl.gov/askasci/phy00/phy00890.htm
From a physics forum of 2005:
http://www.physicsforums.com/showthread.php?t=76246
****************************
True. CO2 or any other material does not produce heat. Only heat from the sun (dismissing internal heat from Earth’s core), and transferred by different mechanisms, heat the Earth and everything in it.
Edward writes:
You’re confusing different regions of the EM spectrum. “Infrared” photography employs near-infrared wavelengths (0.7 to ~1 micron wavelength). This is far shorter than the longwave thermal infrared emitted by the earth’s surface and atmosphere (on the order of ~10 microns).
One could probably dig up an absorptance spectrum for glass in the thermal infrared. I remember seeing one for Corning glass — transmittance was near 0 in the 8-10 micron range and I think it averaged about 50% in the entire 8-14 micron window. In other words, Corning glass is much more opaque to outgoing longwave thermal infrared than it is to incident visible/near-IR solar irradiance. Glass used for making windows or greenhouses is probably a bit different.
Note, IR photographers are most likely using near-IR around 1-2um, where glass is indeed transparent.
can you please explain your statement in light of the following spectra
http://www.korth.de/transmiss/sio2.htm
and for fused sio2
thanks.
JAE
It will be difficult to prove/disprove your theory by trying to work out the temperature changes in one location when you don’t have all the data for that location.
Let me ask this question –
If the “backradiation” from the atmosphere doesn’t cause any increase in heat at the surface, then how is it that the annual global average upward longwave radiation from the surface is 396W/m^2?
Where does it come from? The solar insolation averaged globally and annually is only 240W/m^2.
How is the extra heat transported to the surface and what is the source?
science of:
Well, first, I take note that you are still avoiding my question about the greenhouses. As is everyone else. As has been the case for about two years.
Relative to your last comment, the big problem is that you and most of the rest of the blogosphere are blinded by/hung up in a stupid AVERAGE radiation diagram, which does not apply at any real spot on Earth. You, especially, are smart enough to know that the relationship between temperature and radiation varies by the FOURTH [–moderator’s note: swearing is not allowed on this blog–] POWER. Therefore, “average radiation diagrams” mean absolutely NOTHING! Even I am that smart.
There is an enormous surplus of heat absorbed inthe tropics–WAY MORE than 396 Wm-2, and it is distributed over the planet by various well-known mechanisms (as you know very well). There is enough heat in the water in Fiji to keep the air at the ambient temperature. No reason to invoke some mysterious greenhouse effect. Simple radiation from a blackbody.
But for now, please answer my question about backradiation in a greenhouse.
[note: retrieved from the spam queue]
Scienceof: I marvel that a person that can produce all these brilliant posts would ask me about the AVERAGE 396 wm-2. LOL You know very well that this average radiation value has ABSOLUTELY no meaning for any point on Earth at any time, ever. It is a purely misleading abstract piece of [–moderator’s note – please read the Etiquette]. There is plenty of heat received in the tropics to make up for some supposed deficit based on this flawed nonsense. Give me a break, please.
As it is relevant to this topic, I’d like to remind ScienceOfDoom about the promise on another topic to discuss night-time cooling by radiation from the earth and the subsequent development of inversions.
The fact you can get ice forming in open bowls of water exposed to the night sky in the Sahara – at the same time as the air above is significantly warmer – is a pretty good indication that there are more radiative processes than just long wave IR interacting with the CO2 and H2O in the atmosphere.
Jerry:
Sorry, thought I had covered it in Sensible Heat, Latent Heat and Radiation but maybe it needs more..
However, as a quick explanation, your description of the Sahara inversion sounds like it will be very well explained by radiative processes. The ground is a more effective radiator than the atmosphere as it has an emissivity close to 1 for longwave. Therefore radiation from the ground is higher than radiation from the atmosphere and there is no solar radiation to keep the ground heated.
Does that explain it?
The key element is that the air immediately above the ground is not cooling much at all, while the ground is getting cool very quickly.
To me that means that the ground is radiating at frequencies that are not absorbed/re-emitted by the air. And especially, these frequencies are radiating straight out to space without being intercepted by any pesky CO2 or H2O molecules.
If the air simply emitted less than the ground then – I expect – it would absorb less as well so there would be negligible effect on ground radiation.
Relative emissivity may be relevant, but frequency distribution seem to me to be much more relevant
Jerry, I will try and look at this in more detail at some stage. Blog popularity is making it harder..
JAE
Well, clearly you are upset about this. Perhaps you should take it up with all of those people.
You first appeared on this blog on April 16th 2010 on Sensible Heat, Latent Heat and Radiation and asked a question not about greenhouses but why the temperature in Atlanta was different from the temperature in Phoenix.
I attempted to answer this question but pointed out there wasn’t enough data to be sure of answering it.
Then you came onto this post 2 days ago and asked why deserts were hotter than Fiji.
Which I attempted to answer as you see above. Again we are probably lacking enough information at this stage.
Perhaps you need to go and antagonize the people who haven’t answered you for 2 years instead of making angry comments here.
I will attempt to answer reasonable questions – as you can see from the many hundreds of replies I have made on this blog – but I also expect some courtesy in response.
There are plenty of blogs to go and be angry on.
Scienceof:
Sorry about the anger. I do appreciate your comments. I just get frustrated because it often seems that folks are responding to questions I did not ask and ignoring those I do ask. Forget it.
New here, but if I may…
Using math as the language of the argument is great, but if you want to bring the point of no-runaway, increased-temperature home to a layman, no math required, if might be better to use an example with human bodies instead of stellar ones.
If there is another body next to yours in bed, is it easier to stay warm than when you are by yourself?
Do you both burst into flames if you stay there too long?
The answers are obviously ‘yes’ and ‘no’, respectively.
Why?
It’ll be apparent that some energy is exchanged between the two bodies as opposed to being simply lost to the surroundings. However, there is loss to the surroundings and the amount of that loss increases with temperature; kind of why it is so hard to feel warm when you have a fever.
True, it’s lousy with issues of whether convection or radiation play a bigger role, but it still works for bodies A and B, of approximately equal temperature, being warmer in proximity than not, and with no runaway heating.
Is this site for real?
1)- the 2nd Law of Thermodynamics IS A LAW OF SCIENCE.
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON.
—-
2)- ALL Radiation (including Infrared Heat radiation) is accomplished by Propagating Electromagnetic Fields which CARRY Photon energy from one place to another.
Photon
“In physics, a photon is an elementary particle, the quantum of the electromagnetic field and the basic “unit” of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force. The effects of this force are easily observable at both the microscopic and macroscopic level, because the photon has no rest mass; this allows for interactions at long distances”
http://en.wikipedia.org/wiki/Photon
Properties of electromagnetic waves
“An electromagnetic wave, although it CARRIES no mass, does CARRY energy.”
“A more common way to handle the energy is to look at how much energy is CARRIED by the wave from one place to another.”
http://physics.bu.edu/~duffy/PY106/EMWaves.html
—-
3)- Electromagnetic Fields (including Infrared Heat radiation) are VECTOR QUANTITIES that have a Magnitude and a Direction.
Heat flux
“Heat flux or thermal flux, sometimes also referred to as heat flux density or heat flow rate intensity is a flow of energy per unit of area per unit of time. In SI units, it is
measured in [W·m-2]. It has both a direction and a magnitude so it is a vectorial quantity.”
http://en.wikipedia.org/wiki/Heat_flux
—-
4)- Scalar “accounting math” does NOT APPLY. One needs to use VECTOR MATHEMATICS when summing VECTOR FIELDS.
Vector addition of fields
http://hyperphysics.phy-astr.gsu.edu/hbase/electric/mulpoi.html#c3
This link shows how resultant field vectors are calculated.
Using superposition, many, many sources can analysed at any point in space to produce a SINGLE RESULTANT VECTOR.
—-
5)- Computing many VECTOR points around radiating sources will give a RADIATION PATTERN.
This link shows Sound Wave radiation patterns produced by multiple sources.
Radiation from a dipole sources and multiple sources showing cancellation of sound..and an animation
http://www.kettering.edu/~drussell/Demos/rad2/mdq.html
This example shows radiation patterns produced by sound wave sources.
The same patern types are produced for Antennas using the Poynting Vectors of Electromagnetic Fields.
Notice the NULLS and PEAKS calculated by vector mathematics.
NOTE: The dipole radation pattern is the same radiation pattern that the TWO IDENTICAL STARS example will produce.
There will be ZERO heat radiation transfered between the Two Stars because the EM Fields will cancell.
—–
6)- Light Interference (including ALL EM fields) is a very common phenomena that demonstrates Vector addition of EM fields.
Here is an example of complete light cancellation in a soap bubble.
The Physics is explained as wave propagation in the time domain and Vector analysis (no time variable) explains the same Physics.
Cancellation of Light
http://www.exploratorium.edu/ronh/bubbles/bubble_colors.html
Notice the light can be completely cancelled in the Soap Bubble.
There is also a good explaination of Constructive and Destructive Interference.
—-
7)- Radiative Heat Transfer equations also show this:
Heat Radiation
Radiation is heat transfer by the emission of electromagnetic waves which CARRY energy away from the emitting object. For ordinary temperatures (less than red hot”), the radiation is in the infrared region of the electromagnetic spectrum. The relationship governing radiation from hot objects is called the Stefan-Boltzmann law:
Heat Transfer by Radiation using the Stefan-Boltzmann Law
P = e*BC*A(T^4 – Tc^4)
Where P = net radiated power (Watts), e = emissivity, BC = Stefan’s constant, A = area, T = temperature of radiator and Tc = temperature of the surroundings or another body.
..when rearranged gives
P/A = e*BC*T^4 – e*BC*Tc^4 (Watts/m^2)
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html#c2
This is an obvious subtraction of two Electromagnetic Fields
It also complies with the Vector subtraction of Electromagnetic Fields which are Vectors.
The resultant Electromagnetic Field will have a magnitude of P/A and have a direction of propagation in the direction of the larger field produced by the hotter body.
There is absolutely no energy flow from cold to hot, complying with the 2nd Law of Thermodynamics.
——
8)- If ANY heat could flow from Cold to Hot it would violate the Law of Conservation of Energy and create a “perpetual motion machine”.
Example:
– A hot tungsten filament of a light bulb radiates light to a reflective wall in the room.
– If ANY of the reflected light were absorbed by the hotter filament, it would increase in energy, temperature and produce more light.
– The increase in light would then be reflected back to the hotter filament further increasing it’s energy, temperature and produce even more light.
– The cycle would continue until the filament’s energy and temperature reached infinity!
Perpetual motion
“The term perpetual motion, taken literally, refers to movement that goes on forever. However, the term more generally refers to any closed system that produces more energy than it consumes. Such a device or system would be in violation of the law of conservation of energy, which states that energy can never be created or destroyed.”
“Perpetual motion violates either the first law of thermodynamics, the second law of thermodynamics, or both”
“A perpetual motion machine of the first kind produces energy from nothing, giving the user unlimited ‘free’ energy. It thus violates the law of conservation of energy.”
http://en.wikipedia.org/wiki/Perpetual_motion
In fact, all that is required is that the filament be above absolute zero and the “heat flowing from cold to hot” will produce an infinite filament temperature.
This is exactly the same fictional process that the “Greenhouse Effect” claims.
——-
Like I said in (1)- the 2nd Law of Thermodynamics IS A LAW OF SCIENCE.
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON!!!
I’d like to thank Gord for this highly informative comment,
If it wasn’t provided I would not have really understood the ‘why’ of the original post by Science Of Doom.
Now I see the two arguments I am satisfied that the different points of view have been expressed clearly and concisely and I can now make an informed decision on their merits.
Gord,
I don’t know if it will help, but I believe you have confused the net effect of a large number of exchanges with behavior at the individual quantum level. When an individual energy quanta encounters a body that could absorb it, it doesn’t care if the mean temperature of the body that it came from was higher or lower than the body it is encountering. It will get absorbed or not according to whatever happens between it and the body. However, warmer bodies emit more quanta per surface area than cooler bodies; so, when you consider the net effect of an exchange between to bodies, yes, the flow of energy is toward the cooler body.
Also, heat and energy don’t always mean the same thing, and you appear to using them as though they were.
Gord,
Also, it might help to remember that a body radiates energy in all directions, but the energy emitted by a neighboring star or off of a wall only comes from some limited subset of all directions.
Gord, there’s no problem with the 2nd law. There IS a problem with people (like yourself) misapplying it. All of your points here have been dealt with clearly in the post and the comments.
“The cycle would continue until the filament’s energy and temperature reached infinity!”
Er, no. The filament loses energy through radiation. It gains energy through absorption. In your imaginary example it absorbs exactly the same quantity of energy that it radiates, keeping its temperature constant.
“This is exactly the same fictional process that the “Greenhouse Effect” claims.”
With all due respect, you’re confused about this. Reread the original posting and the comments in this thread. The GHE is not at all in contradiction to the 2nd law.
No, Gord is not confused. The filament has a constant power supplied to it, PLUS, the erroneously assumed ABSORBED back radiation. If the filament has the initial power PLUS the back radiation, it has a higher temperature and emits more radiation, if it emits more radiation, then the back radiation increases, so on and so forth. I think the major paradox in back radiation absorption hypothesis is that there would never be a point of equilibrium.
I mean, this is SO easy to demonstrate experimentally by taking two very hot objects and moving them closer to each other, if this blog is correct then the two objects should heat up. No assumptions are needed, just a simple experiment with a very sensitive thermal camera. Anyone know of an actual experiment demonstrating this?
Where are you all wrong? With the assumption that the back radiation is simply absorbed by the molecules in the hotter object. Thermal radiation can be absorbed by a molecule ONLY when said absorption results in the molecule vibrating to a higher quantized state, otherwise the molecule is transparent to that radiation. Therefore, your error in the math above is the assumption that the two stars are absorbing all of the incident radiation from each other. Admittedly, QM is difficult to understand, so if there is something missing then please enlighten.
RWturner wrote: “No, Gord is not confused. The filament has a constant power supplied to it, PLUS, the erroneously assumed ABSORBED back radiation. If the filament has the initial power PLUS the back radiation, it has a higher temperature and emits more radiation, if it emits more radiation, then the back radiation increases, so on and so forth. I think the major paradox in back radiation absorption hypothesis is that there would never be a point of equilibrium.”
If you surround a filament with a perfect insulator and you provide a constant input of work (electricity) then the temperature will increase without limit (actually until something melts, vaporizes, or otherwise fails). There will never be a point of equilibrium. There is nothing paradoxical in that. There is no violation of the Second Law. The nature of the insulator does not matter. There is, of course, no such thing as a perfect insulator (or a perfect reflector) but that is immaterial to the hypothetical argument.
Gord seems to claim that the runaway will occur even without an input of energy. That is wrong, as pointed out by Jenn.
RWturner wrote: “I mean, this is SO easy to demonstrate experimentally by taking two very hot objects and moving them closer to each other, if this blog is correct then the two objects should heat up. No assumptions are needed, just a simple experiment with a very sensitive thermal camera. Anyone know of an actual experiment demonstrating this?”
Is there a constant input of energy to the hot objects, with a matching constant loss? In that case they will heat up. Nothing strange there. Pack a whole bunch of people into a poorly ventilated room. It will get quite warm. I have participated in the experiment. 🙂
RWturner wrote: “Where are you all wrong?”
We aren’t. You are.
Cancellation is associated with the WAVE nature of photons, and is spatially dependent upon the energy / wavelength of the photons and their locations relative to their sources. See Phase Cancellation.
The location and energy of a photon are statistical probabilities, are they not? Quantum mechanics? How about the probability distribution of energy states of an array of molecules – all the same? A vanishingly small number at a much higher state than the “average”, but still non-zero?
Doesn’t it boil down to the probability that a low temperature body will emit a photon of sufficient energy to raise the energy level of a molecule in another body, being smaller than the reverse?
I know I’m missing a lot (and a lot of years since school), but isn’t the “cancellation” thing above off base?
Regarding,
“Doesn’t it boil down to the probability that a low temperature body will emit a photon of sufficient energy to raise the energy level of a molecule in another body,…”
Well, if I remember correctly, black bodies (most solids and liquids) absorb energy at all wavelengths. That is, the energy level of the photon does not matter as far as whether it gets absorbed or not. If energy gets absorbed by a body, the energy level of that body is increased.
Of course. I’m talking about the (minute) probability of a cool body emitting a photon of energy sufficient to be absorbed by the warmer one. Lots of lower energy ones, just very few high enough to matter in the low-high direction.
Chris G
New here, but if I may…
New here too, but reading your comment (and not being a physicist, hence no maths), I thought about putting two heat lamps next to each other. I think that is more radiative than convective, but there is still no thermal runaway. They reach an equilibrium and everyone is happy.
This matter of two lamps, stars, blackbodies, plates, etc, being closer together and radiating energy/heat or whatever, and absorbing or not, or increasing their temperature or not, or running amok or reaching an equilibrium, is an extremely boring byzantine discussion about how many angels can dance on the top of a pin. A discussion where everyone wants to show how much he knows about physics and radiation and how ignorant are the others.
Everybody has forgotten that all their warm bodies are getting their energy from an inner source, fusion, electricity, etc; almost no one has taken into account the absorbance of those bodies and time variables, (no time variable in the formula, no equilibrium will ever be known), also the fact that a weaker photon going towards a warmer source is overwhelmed and neutralized by the stronger photons coming out from the hotter body preventing the weak one from reaching the stronger source, and many more factors.
The formula being used makes no use of those factors and variables so it is useless. Now start all over again and see what happens with two equaled heated bodies of the same size and shape and see if there is a math equation that can explain this and solve the problem once and for all.
Bye, and have a nice discussion.
Sorry for commenting so late, I just found this blog.
Edward said:
a weaker photon going towards a warmer source is overwhelmed and neutralized by the stronger photons coming out from the hotter body preventing the weak one from reaching the stronger source.
Please, PLEASE go take a beginners optics course. This is NOT how photons work.
Chris G
I don’t understand your use of “….it doesn’t care if the mean temperature of the body that it came from was higher or lower than the body it is encountering.”
Energy “doesn’t care” ever, it simply follows rules that we humans have observed, measured and have called Laws of Science.
Heat energy flow is always from Hot to Cold objects unless work is done to reverse the flow (ex. a Refigerator) and there has NEVER been ANY measurement done to dispute this.
If you can’t understand the vector nature of EM fields, I’m sure you are fimiliar with the fact that ALL FORCES are vector quantities as well.
If you have a block of wood with two unequal and opposing forces applied to it, I’m sure you know that the block of wood will move in the direction of the stronger force.
It is NOT POSSIBLE for the block of wood to move in the direction of the weaker force.
The block of wood “doesn’t care” either, it is simply the case that all measurements, ever done, shows that the the block of wood will ALWAYS move in the direction of the stronger force.
—
Did you know that Electromagnetic Fields are FORCE FIELDS?
In fact, the Electromagnetic Force is one of the four fundamental forces.
Electromagnetic force
“The electromagnetic force is one of the four fundamental forces. The other fundamental forces are: the strong nuclear force (which holds quarks together, along with its residual strong force effect that holds atomic nuclei together to form the nucleus), the weak nuclear force (which causes certain forms of radioactive decay), and the gravitational force. All other forces are ultimately derived from these fundamental forces.”
“In physics, the electromagnetic force is the force that the electromagnetic field exerts on electrically charged particles. It is the electromagnetic force that holds electrons and protons together in atoms, and which hold atoms together to make molecules. The electromagnetic force operates via the exchange of messenger particles called photons and virtual photons.”
“The electromagnetic force is the one responsible for practically all the phenomena one encounters in daily life, with the exception of gravity.”
http://en.wikipedia.org/wiki/Electromagnetic_force
The Electromagnetic Field is a FORCE that moves zero mass photons from one place to another.
Hot objects produce a stronger Electromagnetic Field and a stronger FORCE than Cold objects.
When you have opposing Electromagnetic FORCES, one from a hot object (the larger force) and one from a cold object (the weaker force), the zero mass photons will ALWAYS move in the direction of the larger force.
It is NOT POSSIBLE for the zero mass photons to move in the direction of the weaker force.
That’s why the 2nd Law of Thermodynamics (a LAW OF SCIENCE) states:
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON!!!
——————
Heat is Energy in transit.
Heat
“Heat may be defined as energy in transit from a high temperature object to a lower temperature object. An object does not possess “heat”; the appropriate term for the microscopic energy in an object is internal energy. The internal energy may be increased by transferring energy to the object from a higher temperature (hotter) object – this is properly called heating.”
http://hyperphysics.phy-astr.gsu.edu/Hbase/thermo/heat.html
Heat energy is the same as ALL Electromagnetic Fields that move photon energy from one place to another.
——————-
All bodies that absorb ANY, I repeat ANY, heat energy will increase in temperature and radiate the same amount of energy.
This is called the Stefan-Boltzmann Law (another LAW OF SCIENCE).
P/A = BC*T^4 (Watts/m^2)
Where P = net radiated power (Watts), BC = Stefan’s constant (5.67 X 10^-8), A = area, T = temperature of radiator
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html#c2
Let’s assume the original radiating body has surface area of 1 m^2 and a temperature of 100 deg C or 373 K
From the equation above it will radiate 1097.59 watts/m^2 in ALL directions.
If there was a mirror with an area of 1 m^2 on a wall 5 meters away.
The watts/m^2 at the mirror would be 1097.59/(4*pi*5^2) = 1097.59/314.16 = 3.49 watts/m^2
Reflecting back to the original radiating body, the watts/m^2 would be 3.49/314.16 = 0.0111 watts/m^2
(The reduction of the watts/m^2 both ways is 0.0111/1097.59 = 0.000010113.)
The 0.0111 watts/m^2 would be absorbed by the original radiating body for a total of 0.0111 + 1097.59 = 1097.6011 watts/m^2
The temperature of the original radiating body will now rise to very slightly and will now radiate 1097.6011 watts/m^2
—
Next cycle:
After being reflected back the original radiating body will receive an additional 1097.6011 X 0.000010113 = 0.0111 watts/m^2
The 0.0111 watts/m^2 would again be absorbed by the original radiating body for a total of 0.0111 + 1097.6011 = 1097.6122 watts/m^2
The temperature of the original radiating body will now rise to very slightly and will now radiate 1097.6122 watts/m^2
—
Next cycle:
After being reflected back the original radiating body will receive an additional 1097.6122 X 0.000010113 = 0.0111 watts/m^2
The 0.0111 watts/m^2 would again be absorbed by the original radiating body for a total of 0.0111 + 1097.6122 = 1097.6233 watts/m^2
The temperature of the original radiating body will now rise to very slightly and will now radiate 1097.6233 watts/m^2
Etc, Etc, Etc.
As you can see the original radiating body is radiating more and more Energy with each cycle.
The eventual outcome is an infinite increase in temperature and energy!
—————————
That’s why the 2nd Law of Thermodynamics (a LAW OF SCIENCE) states:
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON!!!
I ran the numbers and found that the above math resulted in the creation of 151,992,878 W after the stars became closer. Though, this could have simply been rounding errors, using scientific figures would have resulted in 0.00 W increase of EM 0.00 K increase in temperature.
RWturner,
You seem to have a problem differentiating between power and energy. Power is Watts, or joules/second. Energy is joules. Energy is conserved, not power.
If an object is radiating a constant 1000W/m², then something must be supplying that power or it will cool because it’s losing energy. If you put that object along with its power supply into a perfectly reflecting cavity, the temperature will indeed increase. Note ‘with its power supply’. It’s the power supply that is causing the temperature to increase, not just reflection and absorption.
If you put an object at a specific temperature into a perfectly reflecting cavity, the temperature won’t go up or down. The energy radiated from the object will be exactly matched by the energy reflected from the walls and absorbed again.
Science of Doom…
I case you have not realized it, your example of the two identical stars has CREATED energy.
E1 = E2 that you started with became 1.00000025 E1 = 1.00000025 E2
Where did the extra energy come from???
If you repeat your analysis again using 1.00000025 E1 = 1.00000025 E2 the energy will increase AGAIN.
Repeat the cycle a multitude of times and eventually each star will have infinite energy.
By any definition, that is called THERMAL RUNAWAY!
Gord
No energy was created. There is just less energy availble to the rest of the universe (less “lost” radiation).
No extra energy is created, even though boths stars are emitting slightly more radiation (ie. are slightly higher temperature).
ie. The same amount of extra energy emitted from star1 (as it reaches equilibrium at a higher temperature) is being absorbed from star2. And that extra energy emitted from star1 is absorbed by star2 (and so is not being emitted from the star ‘system’). The total energy emitted from the two stars to space is exactly the same even though they are higher temperature.
Mike Blackadder
You said…
“No extra energy is created, even though boths stars are emitting slightly more radiation (ie. are slightly higher temperature).
ie. The same amount of extra energy emitted from star1 (as it reaches equilibrium at a higher temperature) is being absorbed from star2. And that extra energy emitted from star1 is absorbed by star2 (and so is not being emitted from the star ‘system’). The total energy emitted from the two stars to space is exactly the same even though they are higher temperature.”
Gee, how did BOTH of the two stars manage to both increase in temperature?
WHERE DID THE EXTRA ENERGY COME FROM?
Place ANY two objects of the same temperature near each other.
Do they increase in temperature?
NO, THEY DO NOT!
Further, Scienceofdoom has both stars starting with equal energy E1 = E2 and finishing with a higher but equal energy (1.00000025 E1 = 1.00000025 E2).
What prevents the same process repeating???
If you repeat the same analysis again using 1.00000025 E1 = 1.00000025 E2 the energy will increase AGAIN.
Repeat the cycle a multitude of times and eventually each star will have infinite energy.
By any definition, that is called THERMAL RUNAWAY!”
Gord Said
Gord, consider yourself and a friend in the midst of the arctic wastes.
Sitting well apart you freeze on all sides. Getting closer together you are exposed to the slight warmth from the other person. As you get closer you get more and more warmth on that side. Now not touching but getting very chummy, assume half of your body is almost in contact with the other person.
Now your metabolic system doesn’t need to worry about heat loss on all sides. Instead of freezing all over, the bit facing your chum actually does warm up because you aren’t radiating all your heat to the coldness.
So yes, two bodies at the same temperature – in the middle of a cold climate – do warm up when they get closer.
Jenn…
Please show me where I have misapplied the 2nd Law of Thermodynamics.
1)- the 2nd Law of Thermodynamics IS A LAW OF SCIENCE.
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON.
——
I have provided the Physics links that show that:
2)- ALL Radiation (including Infrared Heat radiation) is accomplished by Propagating Electromagnetic Fields which CARRY Photon energy from one place to another.
3)- Electromagnetic Fields (including Infrared Heat radiation) are VECTOR QUANTITIES that have a Magnitude and a Direction.
4)- Scalar “accounting math” does NOT APPLY. One needs to use VECTOR MATHEMATICS when summing VECTOR FIELDS.
5)- Computing many VECTOR points around radiating sources will give a RADIATION PATTERN.
6)- Light Interference (including ALL EM fields) is a very common phenomena that demonstrates Vector addition of EM fields.
7)- Radiative Heat Transfer equations show there is absolutely no energy flow from cold to hot, complying with the 2nd Law of Thermodynamics.
8)- If ANY heat could flow from Cold to Hot it would violate the Law of Conservation of Energy and create a “perpetual motion machine”.
I have read the posts on this thread and I didn’t see any that was backed by any Physics to support their “opinions”.
That includes your Post.
The facts are:
– The Physics links I have posted are fundamental Physics used by Electrical Engineers and Physicists for over a hundred years.
– The fantasy “Greenhouse Effect” is not supported by EVEN ONE Law of Science and VIOLATES many, many established Laws and Principles of Science.
– The fantasy “Greenhouse Effect” is not supported by EVEN ONE measurement, EVER, that shows that a Colder Atmosphere can HEAT UP a Warmer Earth.
– Every measurement, EVER DONE, confirms that a Colder Atmosphere CANNOT HEAT UP a Warmer Earth.
So, if you disagree with the above facts:
– Please post ANY Law of Science that supports the fantasy “Greenhouse Effect”.
– Please post ANY measurement, EVER, that shows that a Colder Atmosphere can HEAT UP a Warmer Earth.
There has been Billions of dollars spent on AGW/Greenhouse Effect “research” and thousands of “scientific papers” written on it so you should have no problem posting these….RIGHT??
I look forward to your next post.
Mark F…
All light and all EM fields are subject constructive and destructive interference.
This includes Diffraction fringes as well.
Here are some more links:
Interference (wave propagation)
Theory
“The principle of superposition of waves states that the resultant displacement at a point is equal to the vector sum of the displacements of different waves at that point.
If a crest of a wave meets a crest of another wave at the same point then the crests interfere constructively and the resultant wave amplitude is increased. If a crest of a wave meets a trough of another wave then they interfere destructively, and the overall amplitude is decreased.
This form of interference can occur whenever a wave can propagate from a source to a destination by two or more paths of different length. Two or more sources can only be used to produce interference when there is a fixed phase relation between them, but in this case the interference generated is the same as with a single source; see Huygens’ principle.”
Constructive and destructive interference
“Consider two waves that are in phase, with the same amplitudes A1 and A2. Their troughs and peaks line up and the resultant wave will have amplitude A = A1 + A2. This is known as constructive interference.
If the two waves are π radians, or 180°, out of phase, then one wave’s crests will coincide with another wave’s troughs and so will tend to cancel out. The resultant amplitude is A = |A1 − A2|. If A1 = A2, the resultant amplitude will be zero. This is known as destructive interference.”
http://en.wikipedia.org/wiki/Interference_(wave_propagation)
———-
SINGLE SLIT DIFFRACTION PATTERN OF LIGHT
http://www.math.ubc.ca/~cass/courses/m309-03a/m309-projects/krzak/index.html
Physical Optics – Interference and Diffraction Patterns
http://dev.physicslab.org/Document.aspx?doctype=3&filename=PhysicalOptics_InterferenceDiffraction.xml
Gord:
Why not take it a step at a time, so I can understand your point of view better.
Do you think that the diagram (just above the subtitle “Why the Original Misconception?”) from Incropera and DeWitt is in error?
I think that the main problem is that of applying concepts of equilibrium thermodynamics to a system not at equilibrium, but in a steady state. Leonard Weinstein touched on that point a bit, and I will try to reframe the problem.
In the above example, consider the star as a gas which has energy coming in from nuclear fusion and energy going out as radiation. When the rates of both are equal, the system is in a steady state at a given temperature. Note that the gas is just an itermediary, converting the energy of fusion into radiation. Ultimately, the radiation is just energy coming from the fusion.
Now bring in a colder star. It is emitting radiation, part of which is absorbed by the hot one. The hot one has now two sources of energy, its own fusion plus the radiation of the other star or, in other words, its own fusion plus the fusion of the other star. It is nothing but logical the, since more energy comes in, the temperature of the hot star will go up. This in turns increases the output of radiation, and a new, hotter steady state will be reached when output balances input.
Notice that there is no heat flowing from cold to hot. You are only taking some energy from the fusion in the cold star, which was being wasted anyway, and giving it to the hot star. In more technical terms, the transfer from the cold star to the hot one is not due to the temperature gradient between the two.
The argument reminds me of the creationists’ argument that the origin of life and its evolution into more and more complex organisms violate the 2nd law. This is is not true, as you have to take into account the entropy generated by the Sun, as the Earth is not an isolated system. Overall, the total entropy of the universe is increased by the Sun+Earth system, even though entropy is reduced locally on Earth. Even if there was a transfer of heat from cold to hot (which I just argued there isn’t), you would have to take into account the total entropy created by the fusion before considering any violation of the 2nd law.
scienceofdoom
You said…
“Why not take it a step at a time, so I can understand your point of view better.
Do you think that the diagram (just above the subtitle “Why the Original Misconception?”) from Incropera and DeWitt is in error?”
——–
It is absolutely in error.
The 2nd Law is A LAW OF SCIENCE!
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON.
Treat the EM fields as the Vector Fields that they ABSOLUTELY ARE and you will ALWAYS get the correct answer….something that Incropera and DeWitt apparently don’t understand!
If the Solid surface has a temperature “Ts” that is greater greater than the Surrounding surface temp “Tsur”
By Stefan-Boltzman Law Ts will produce more w/m^2 “Ts w/m^2” than than the colder “Tsur w/m^2”.
Summing the two EM field vectors will produce a Resultant EM field vector with a Magnitude of [Ts w/m^2 – Tsur w/m^2] and have a Direction of propagation Towards the Surrounding surface.
There will be ZERO EM field propagation towards the the warmer Solid Surface, totally complying with the 2nd Law of Thermodynamics.
“It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow.”
This is fully explained in the Heat Transfer Link I posted earlier.
—–
7)- Radiative Heat Transfer equations also show this:
Heat Radiation
Radiation is heat transfer by the emission of electromagnetic waves which CARRY energy away from the emitting object. For ordinary temperatures (less than red hot”), the radiation is in the infrared region of the electromagnetic spectrum. The relationship governing radiation from hot objects is called the Stefan-Boltzmann law:
Heat Transfer by Radiation using the Stefan-Boltzmann Law
P = e*BC*A(T^4 – Tc^4)
Where P = net radiated power (Watts), e = emissivity, BC = Stefan’s constant, A = area, T = temperature of radiator and Tc =
temperature of the surroundings or another body.
..when rearranged gives
P/A = e*BC*T^4 – e*BC*Tc^4 (Watts/m^2)
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html#c2
This is an obvious subtraction of two Electromagnetic Fields
It also complies with the Vector subtraction of Electromagnetic Fields which are Vectors.
The resultant Electromagnetic Field will have a magnitude of P/A and have a direction of propagation in the direction of the larger field produced by the hotter body.
There is absolutely no energy flow from cold to hot, complying with the 2nd Law of Thermodynamics.
————-
If ANY heat energy could flow from Cold to Hot, it will Violate The Law of Conservation Energy and CREATE energy.
If ANY heat energy could flow between objects that have the same temperature, it will also Violate The Law of Conservation Energy and CREATE energy.
Just like your Two Identical Stars calculation did.
Gord:
Nice to see a thermodynamics textbook in error. Six editions and no one has picked it up.
So the next step if we can.
Using the Incropera and DeWitt extract: will the colder surrounding emit thermal radiation?
Claude
Your denial of established Laws of Science like the 2nd Law of Thermodynamics and the obvious connection to The Law of Conservation of Energy is astounding.
In case you have not heard, these Laws of Science are called LAWS OF SCIENCE for a reason….THEY HAVE NEVER BEEN SHOWN TO BE WRONG!
Engineers and Physicists have accepted LAWS OF SCIENCE as VALID first principles in ALL their designs and research for over a HUNDRED YEARS.
If you, for some odd reason, dispute these PROVEN LAWS OF SCIENCE then POST the Phantom Laws of Science that has REPLACED them!
Why not Post some PHYSICS that support your “fantastic opinions”???
Scienceofdoom
All objects that have a temperature will emit thermal radation.
Now answer these questions for me.
Do you accept that EM fields are Vector quantities and must use Vector Mathematics when summing EM fields?
Do you accept that the 2nd Law of Thermodynamics is a Valid Law of Science and that there has NEVER been ANY measurement done, EVER, that shows that heat can flow from Cold to Hot objects unless work is done done to accomplish this flow?
Gord
Nearly done.
So in the diagram by Incropera and DeWitt the colder surrounding emits thermal radiation. Seems like everyone agrees with this, which is good.
Does this radiation from the colder surroundings “reach” the solid body in the middle of the diagram?
Scienceofdoom
I have already answered two questions for you.
I’m still waiting for your answers to my questions:
Do you accept that EM fields are Vector quantities and must use Vector Mathematics when summing EM fields?
Do you accept that the 2nd Law of Thermodynamics is a Valid Law of Science and that there has NEVER been ANY measurement done, EVER, that shows that heat can flow from Cold to Hot objects unless work is done done to accomplish this flow?
—–
I will answer your question “Does this radiation from the colder surroundings “reach” the solid body in the middle of the diagram?” right after.
Come on, don’t be shy.
You know more about radiation than I. Im no scientist, my interest/understanding of thermodynamics is purely from a mechanical engineering perspective.
And i think this is where the confusion is arising, thermodynamics at its inception was focused on the science of “machines”. And the second law does refute the possibility of perpetual motion, basically stating that energy conversion to work will never be at 100%, there will always be loses. (and there is no such thing as free energy)
For an example, take a compressor expander, compress air to 10bar, raises T to around 900C, injected water will expand around 1800 times, so inject water, and we theoretically get back exactly the same amount o energy that was put into compression, so we have perpetual motion… if we could convert heat to work at 100%.. but this is what the second law contradicts, we will have loses, through noise, vibration/friction, radiation etc. This is perpetual motion, changing the equilibrium state is not perpetual motion.
An engine works by being out of equilibrium with its surroundings, and converts the flow of energy/heat moving towards equilibrium into kinetic energy. An engine will run more efficiently the greater the difference between its energy state and its surroundings. So if you increase the energy state of its surroundings, it will decrease the differential. And decrease the energy the engine is able to produce/ loose. But if you are applying the same amount o work, it will increase the engines state proportionally… so it increases its T, but it doesn’t increase the differential, its not free energy….. It is not a contradiction to the second law. But this is what a few people seem to be implying.
Gord:
EM fields are vector quantities – yes, because they have a direction and a magnitude.
I suspect your idea of “summing” EM fields is going to be the key factor in your next step..
2nd law of thermodynamics is a valid law of science – yes. You can read that in the article.
So back to the question that helps everyone see how it is that people who all believe in the 2nd law of thermodynamics really differ:
And just a reminder, this is the question I asked in the original article:
Gord:
If you go back to my original post, I mention frequency, which is the inverse of wavelength, and it shouldn’t take too much thought to appreciate that fringes, aka zones of cancellation, are wavelength (energy level) dependent, and thus have a spatial parameter. You may have a null at a specific infrared frequency (wavelength, energy level) at one location, but not at all simultaneously. Unless I’m missing something. That’s why, I think, refractive and diffractive fringes have visible color bands. No?
I agree overall with Gord here, but I can sense that other readers are interested in what happens to the radiation from the cold surface when it reaches the hot surface.
This topical analogy might give some physical structure or picture to think about.
Someone with a vivid imagination suggests that the BP oil leak “hot surface flux” can be mitigated by a water jet ” cold surface flux” in the other direction.
What size of “flux” would be required? .
Back to thermodynamics and electromagnetic radiation.
Scienceofdoom and others think that the hot surface has no option but to absorb a photon from the cold surface.
I think a lot of this radiation is in fact scattered from the hot surface and is not absorbed.
Far fetched analogies apart there is very little evidence to support the idea that the colder atmosphere in any way heats the warmer Earth surface.
Scienceofdoom
I see that while you agree that EM fields are vector quantities, you have avoided “..must use Vector Mathematics when summing EM fields”.
Also, while you agree that 2nd law of thermodynamics is a valid law of science, you have also avoided “…there has NEVER been ANY measurement done, EVER, that shows that heat can flow from Cold to Hot objects unless work is done done to accomplish this flow?”
However, I will continue on and answer your question. “Does this radiation from the colder surroundings “reach” the solid body in the middle of the diagram?”
Answer: No, the colder body radiation cannot reach and be absorbed by the warmer solid body causing the warmer solid body to heat-up.
This is verified by the 2nd Law, The Law of Conservation of Energy (as demonstrated by your own Two Identical Stars calculation), Electomagnetic Vector properties and Vector Mathematics.
——————————–
Now I will offer proof in the context of AGW literature, the fantasy Greenhouse Effect and actual measurements.
With reference to Kiehl and Trenberth’s Earth Energy Balance Diagram
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/Earthebal.html#c1
This Diagram is widely used by AGW’ers including the IPCC.
First notice that the ONLY real energy source is the Sun.
The diagram shows that the in-coming Solar Energy at the top of the atmosphere is 342 w/m^2.
The reflected Solar radiation is 107 w/m^2. This leaves 235 w/m^2 to heat the Earth and atmosphere.
235 w/m^2 is ALL THE ENERGY AVAILABLE !!! and only 168 w/m^2 is AVAILABLE TO HEAT THE EARTH’S SURFACE !!
The out-going Longwave Radiation at the top of the atmosphere is also 235 w/m^2 is also 235 w/m^2, so there is no violation of the Law of Conservation of Energy at the top of the atmosphere.
However, this is mis-leading because below the top of the atmosphere there are numerous violations of the Law of Conservation of Energy.
Example:
– The Earth’s Surface Radiation (390 w/m^2) exceeds 235 w/m^2 and 168 w/m^2.
– The Back Radiation from the atmosphere (324 w/m^2) exceeds 235 w/m^2 and 168 w/m^2.
The diagram shows the total Solar Energy absorbed by the Earth’s surface as 168 w/m^2.
Trenberth clearly shows the colder Atmosphere Back Radiation of 324 w/m^2 being ABSORBED by the warmer Earth’s surface.
Anytime a body absorbes heat energy it’s temperature has to increase, the warmer Earth’s surface was warmed by the colder atmosphere.
A CLEAR Violation of the 2nd Law.
Remember, The Sun (THE ONLY ENERGY SOURCE) only provides 168 w/m^2 of energy that is absorbed by the Earth’s surface.
Is the Earth an Energy source?…NO IT IS NOT!
Is the Atmosphere an Energy source?….NO IT IS NOT!
The 168 w/m^2 FROM THE SUN IS ALL THE ENERGY THAT IS AVAILABLE!
Trenberth shows the Atmosphere Back Radiation absorbed by the Earth’s surface is 324 w/m^2.
Is 324 greater than 168?
ENERGY WAS CREATED.
————————————–
Now on to some actual Measurements.
————————————–
The Kiehl and Trenberth’s Earth’s Energy Budget Diagram shows:
– The Solar Energy absorbed by the Earth’s surface is 168 w/m^2 + reflected by the Earth’s surface 30 w/m^2 = 198 w/m^2
– The Back Radiation from the colder atmosphere that is absorbed by the warmer Earth’s surface is 324 w/m^2
The Back Radiation exceeds Solar Energy and BACK RADIATION IS AVAILABLE DAY AND NIGHT.
—
NOTE: Solar Ovens are Parabolic Mirrors that can concentrate Solar Energy and IR Back Radiation at a focal point.
If Back Radiation actually reached and heated the Earth as Trenberth shows, then Solar Ovens would produce heating at Day and Night!
Here is an experiment done by the Physics Dept. Brigham Young Unversity that PROVES that Back-Radiation CANNOT heat the Earth.
—
Solar Cookers and Other Cooking Alternatives
“The second area of solar cookers I looked at was their potential use for cooling. I tested to see how effective they are at cooling both at night and during the day. During both times, the solar cooker needs to be aimed away from buildings, and trees.
These objects have thermal radiation and will reduce the cooling effects. At night the solar cooker needs to also be aimed straight up towards the cold sky. During the day the solar cooker needs to be turned so that it does not face the Sun and also points towards the sky.
For both time periods cooling should be possible because all bodies emit thermal radiation by virtue of their temperature. So the heat should be radiated outward.
Cooling should occur because of the second law of thermodynamics which states that heat will flow naturally from a hot object to a cold object.
The sky and upper atmosphere will be at a lower temperature then the cooking vessel. The average high-atmosphere temperature is approximately -20 °C.
So the heat should be radiated from the cooking vessel to the atmosphere.”
http://solarcooking.org/research/McGuire-Jones.mht
—-
This link shows that heating of the Earth’s surface cannot occur from the colder atmosphere.
In fact, the article shows how to COOL items placed in the Solar Oven at NIGHT AND DAY!
All you have to do is point the Oven away from the Sun during the Day and the Oven will transfer heat from the WARM object in the Oven to the COOLER atmosphere!
It can even be used to produce ICE when the ambient air temp is +6 deg C!
“If at night the temperature was within 6 °C or 10°F of freezing, nighttime cooling could be used to create ice. Previous tests at BYU (in the autumn and with less water) achieved
ice formation by 8 a.m. when the minimum ambient night-time temperature was about 48 °F.”
And, this also confirms the validity of 2nd Law of Thermodynamics….heat energy CANNOT flow from Cold to Warm objects.
——————
Summary:
——————
AGW theory and the Greenhouse Effect has been proven to violate the 2nd Law of Thermodynamics and the Law of Conservation of Energy.
Actual measurements confirm this.
If Back Radiation actually reached and heated the Earth as Trenberth shows, then Parabolic Mirror Solar Ovens would produce heating Day and Night!
After all, they claim this Back Radiation is responsible for heating the entire Earth’s surface from -18 deg C to +15 deg C!!
Why do the AGW so called “scientists” not promote the use of Back-Radiation as a clean energy source available DAY and NIGHT and solve all our energy problems?
BECAUSE IT IS “NOT POSSIBLE” FOR BACK RADIATION FROM A COLDER ATMOSPHERE TO HEAT UP A WARMER EARTH…THAT’S WHY!
AGW/Greenhouse Effect has been presented to the public as some sort of ‘scientific fact’ when it is actually a FRAUD.
Mark F
Perhaps you did not see the link I provided earlier about complete light cancellation in a soap bubble.
Cancellation of Light
“Given a certain thickness of the bubble wall, a certain wavelength will be cancelled and its complementary color will be seen. Long wavelengths (red) need a thicker bubble wall to get out of step than short wavelengths (violet). When red is cancelled, it leaves a blue-green reflection. As the bubble thins, yellow is cancelled out, leaving blue; then green is cancelled, leaving magenta; and finally blue is cancelled, leaving yellow. Eventually the bubble becomes so thin that cancellation occurs for all wavelengths and the bubble appears black against a black background.”
http://www.exploratorium.edu/ronh/bubbles/bubble_colors.html
The magnitude of reflected EM radiation varies with the thickness of the bubble wall and the wavelength of the EM radiation.
The light “color” or wavelength leaving the bubble is determined by the Vector addition of the incident wave magnitude less the magnitude of the reflected wave.
As the bubble thickness reduces more and more light “colors” are first reduced and then completely cancelled due to reflection.
Finally, the entire spectrum of light is cancelled with no light leaving the bubble and it appears black.
I hope this answers your questions.
Gord:
Interestingly, one of our other “imaginary” second law advocates, Bryan, believes that the colder radiation “reaches” the warmer body.
I’m just noting that for interested observers in the whole debate. No reason for us to expect a monolithic voting block..
So, rather than a 20 week course in EM fields, leaving many readers behind with vector calculus, we can – as with so many discussions – actually cut to the chase.
In the context of the earth’s surface and atmosphere, which is our main objective here, perhaps you can explain what this is – downward longwave radiation at the earth’s surface:
If the fields cancel out we shouldn’t measure any downward longwave radiation at the earth’s surface. By the way, you can see more about the measurement in CO2 – An Insignificant Trace Gas? Part Six – Visualization.
scienceofdoom
Interesting that you totally ignored the the Parabolic Mirror Solar Oven measurements which prove that it is NOT POSSIBLE for the Back-Radiation to reach and heat-up a warmer Earth.
You also avoided this”…there has NEVER been ANY measurement done, EVER, that shows that heat can flow from Cold to Hot objects unless work is done done to accomplish this flow?”
Did you really think I have not looked at the AGW’ers “measurements” of Back-Radiation?
I might as well Expose this whole [moderator’s note – please check the Etiquette ] in one post.
———-
Here is a link to NASA’s website:
Climate change: How do we know?
Certain facts about Earths climate are not in dispute:
-The heat-trapping nature of carbon dioxide and other gases was demonstrated in the mid-19th century. Their ability to affect the transfer of infrared energy through the atmosphere is the scientific basis of many JPL-designed instruments, such as AIRS. Increased levels of greenhouse gases must cause the Earth to warm in response.
http://climate.nasa.gov/evidence/
TWO fabricated outright [moderator’s note – please check the Etiquette ].
——
#1
“Increased levels of greenhouse gases must cause the Earth to warm in response.”
The AIRS instrument (used to measure Back Radiation from a -20 deg C atmosphere) uses IR detectors that have been CRYOGENICALLY COOLED far below the -20 deg C atmosphere temperature to make the direct measurement POSSIBLE!
Just like the 2nd Law clearly states!!
———-
Example: The AIRS instrument NASA talks about above.
The AIRS Instrument:
Notice the amount of “Cooling” modules?
They are used to cool the IR detectors in the Focal Plane Assembly.
Cryogenic Cooling Systems
-Dewar Assembly
-Cryocooler Assembly
-Radiators and Earth Shield Assembly
http://airs.jpl.nasa.gov/technology/instrument/
—
Dewar Assembly
“The focal plane assembly operates at 58 K for high sensitivity and is packaged in a permanent vacuum dewar which mates directly to the 155 K grating spectrometer.”
58K =-215 deg C !!
http://airs.jpl.nasa.gov/technology/instrument/dewar/
—
Cryocooler Assembly
“Low vibration, long life focal plane operation near 58 K is critical to the success of AIRS..”
http://airs.jpl.nasa.gov/technology/instrument/cryocooler/
—-
Radiators and Earth Shield Assembly
“It extends around the spectrometer and operates in the 171-190 K temperature range. The second stage radiator provides extended cooling to 145-160K.”
“The AIRS Earth Shield Assembly provides shielding of the cold radiator surfaces to earth radiation.”
145K =-128 deg C, 160K =-113 deg C !!!
http://airs.jpl.nasa.gov/technology/instrument/radiators/
—–
Focal Plane Assembly
“The PV modules consist of 1, 2 or 4 bi-linear arrays of back-side-illuminated HgCdTe detectors,…”
“The AIRS FPA is unique in its hybrid PV/PC approach and required special care in the routing, shielding and grounding of very low noise (nV) PC signals in the presence of high level (V) PV signals. A total of 526 leads interconnect to the motherboard assembly using a series of 10 high-density, thin-film flex cables specifically designed for cryogenic operation.”
http://airs.jpl.nasa.gov/technology/instrument/focal_plane/
—————
Here is another instrument that NASA uses to measure Back-Radiation.
TES Instrument Specifications
“Individual detector array spectral coverage (cm-1) 1A (1900-3050), 1B (820-1150), 2A (1100-1950), 2B (650-900) All mercury cadmium telluride photo voltaic at 65 K ”
65K =-208 deg C!
http://tes.jpl.nasa.gov/instrument/instrumentspecs/
——
#2
NASA’s statement that “The heat-trapping nature of carbon dioxide and other gases was demonstrated in the mid-19th century. Their ability to affect the transfer of infrared energy through the atmosphere is the scientific basis of many JPL-designed instruments, such as AIRS.” is an outright [moderator’s note – please check the Etiquette ].
The mid-19th century “Science” they are refering to is the obsolete Science produced by Fourier 1824, Tyndall 1861 and Arrhenius 1896 who believed that heat could flow from cold to hot objects and certainly had ZERO influence on the “scientific basis of many JPL-designed instruments, such as AIRS.
Maxwell discovered EM fields in 1864 and has formed the basis of modern Heat Radiation Physics ever since.
—————
In the end, all your “Greenhouse Effect” measurements are [moderator’s note – please check the Etiquette ]!
ALL Back Radiation direct measurement instruments use COOLED IR detectors because:
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not
flow spontaneously from a low temperature object to a higher temperature object.”
All the measurements, including NASA’s, PROVE that IT IS NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow.
IT IS NOT POSSIBLE for Back Radiation from a COLDER ATMOSPHERE to reach a WARMER EARTH let alone HEAT IT UP by 33 deg C !!!!
The TRUTH is contained in the 2nd Law of Thermodynamics
Gord:
Perhaps you and Bryan can chat about this for a while among yourselves.
Bryan says the radiation from the atmosphere “reaches” the surface. And thinks that Gerlich and Tscheuschner agree with him.
You think the radiation can’t reach the surface and the experiments that demonstrate this are a con.
Fascinating.
Well, first I will finish the post devoted to some recent comments from Bryan. Watch out for it.
Then we can have a look at some experimental work that you question.
It’s a new and quite amazing world for me.
scienceofdoom
-It’s funny that your TWO IDENTICAL STARS calculation wound up CREATING ENERGY….just like Trenberth’s Earth Energy Budget Diagram does.
-Interesting that you totally ignored the the Parabolic Mirror Solar Oven measurements which prove that it is NOT POSSIBLE for the Back-Radiation to reach and heat-up a warmer Earth.
-You also avoided this”…there has NEVER been ANY measurement done, EVER, that shows that heat can flow from Cold to Hot objects unless work is done done to accomplish this flow?”
-Then “no comment” about NASA’s claim:
“The heat-trapping nature of carbon dioxide and other gases was demonstrated in the mid-19th century. Their ability to affect the transfer of infrared energy through the atmosphere is the scientific basis of many JPL-designed instruments, such as AIRS. Increased levels of greenhouse gases must cause the Earth to warm in response.”
….when they CLEARLY use CRYOGENICALLY COOLED far below the -20 deg C atmosphere temperature to make the direct measurements of Back-Radiation POSSIBLE!
Sounds like you have a LOT OF EXPLAINING to do.
FACINATING.
And, I’m sure It’s a new and quite amazing world for you.
Gord:
When the thickness of the bubble wall is one half the wavelength of a given color, the wave will be cancelled and REFLECTED, not transmitted. Other wavelengths, however, will be transmitted – passed through. There is some finite chance that some will be absorbed, but if you can’t see blue, it isn’t being reflected. As the wall thickness decreases to less than the half-wavelength of violet, the shortest visible one, no more visible light will be reflected. Not only will a black object be visible as black, but a rainbow-colored object will appear to be rainbow-colored. All visible light is being transmitted. BUT UV will be reflected at shorter wavelengths, just as a really thick bubble would block radio waves. My point being that as the bubble becomes thinner, it reflects ONLY one wavelength, not all. It passes (transmits) the rest. Multi-coated optical lenses have multiple coatings, each of several thicknesses, to deal with reflections. But there are other phenomena at work there.
Somebody better call the Royal Swedish Academy of Sciences and tell them that Penzias and Wilson must have faked their experiments and should have to return their Nobel Price. After all, they measured the 3K background radiation without cooling their antenna below that temperature.
Radiometers are not cooled to be able to receive EM waves, they are cooled to reduce noise and give more accurate readings.
The only thing the solar oven ‘proves’ is that there is no _net_ exchange of energy. As long as the temperature of the object in the cooler is below atmospheric temperatures, it simply radiates more energy away than it receives from the atmosphere.
I can only repeat the original poster’s question: if the energy of the cooler object that is radiated towards the warmer object does not reach it, where does it go to? It can’t just vanish or the principle of energy conservation would be violated.
Destructive interference can not be the answer, since it is very unlikely that the two radiation sources have the required distance and their light is out of phase exactly by 180d at all times for all wavelengths. And even then you would only not have waves in the space _between_ the two radiating bodies. At the endpoints the waves must exist again (or it would violate energy conservation) and thus the objects will absorb each others energy.
Another point people seem to overlook when they claim that the two-star or tungsten lamp example demonstrates runaway heating is that those objects are connected to an energy source. Turn the power off and you will see that the tungsten lamp cools down rapidly, even if it absorbs it’s own (reflected) energy. The only thing that happens is that it cools down slower.
Mark F
“Gord:
When the thickness of the bubble wall is one half the wavelength of a given color, the wave will be cancelled and REFLECTED, not transmitted. Other wavelengths, however, will be transmitted – passed through. There is some finite chance that some will be absorbed, but if you can’t see blue, it isn’t being reflected. As the wall thickness decreases to less than the half-wavelength of violet, the shortest visible one, no more visible light will be reflected. Not only will a black object be visible as black, but a rainbow-colored object will appear to be rainbow-colored. All visible light is being transmitted. BUT UV will be reflected at shorter wavelengths, just as a really thick bubble would block radio waves. My point being that as the bubble becomes thinner, it reflects ONLY one wavelength, not all. It passes (transmits) the rest. Multi-coated optical lenses have multiple coatings, each of several thicknesses, to deal with reflections. But there are other phenomena at work there.”
—
I don’t know where you are getting your Physics from.
Do you just make it up on the spot?
Reflection
“Reflection is the degree to which infrared energy reflects off a material. Polished metals such as aluminum, gold and nickel are very good reflectors.
Conservation of energy implies that the amount of incident energy is equal to the sum of the absorbed, reflected, and transmitted energy.
Incident Energy = Absorbed Energy + Transmitted Energy + Reflected Energy
http://www.optotherm.com/emiss-physics.htm
In the case of the soap bubble, the absorbtion will be very small simplifying the equation to.
Incident Energy = Transmitted Energy + Reflected Energy
The incident light wave inside the bubble travels to the bubble wall where a portion of the wave will be reflected back and the rest will be transmitted through the bubble wall.
As the bubble wall thins all the incident wave will be reflected and there will be ZERO transmitted through the bubble wall.
Just like I said..
“As the bubble thickness reduces more and more light “colors” are first reduced and then completely cancelled due to reflection.
Finally, the entire spectrum of light is cancelled with no light leaving the bubble and it appears black.”
———-
Your description above conflicts with the link:
Cancellation of Light
“Given a certain thickness of the bubble wall, a certain wavelength will be cancelled and its complementary color will be seen. Long wavelengths (red) need a thicker bubble wall to get out of step than short wavelengths (violet). When red is cancelled, it leaves a blue-green reflection. As the bubble thins, yellow is cancelled out, leaving blue; then green is cancelled, leaving magenta; and finally blue is cancelled, leaving yellow. Eventually the bubble becomes so thin that cancellation occurs for all wavelengths and the bubble appears black against a black background.”
http://www.exploratorium.edu/ronh/bubbles/bubble_colors.html
Here is another link that talks about soap bubbles, that says the same as the above link.
Interference
“If the crests of one wave coincide with the troughs of the other wave, the resultant amplitude is decreased or may even be completely canceled, as illustrated in Figure 3. This is called destructive interference. The result is a drop in intensity, or in the case of total cancellation, blackness.”
http://www.olympusmicro.com/primer/lightandcolor/interference.html
The links are correct, your description is wrong.
Why don’t you find some Physics support for your “opinions” before posting?
diessoli
Why don’t you actually use the internet to find out the Physics of Antenna Temperature before posting your “opinions”???
Antenna Temperature
“Antenna Temperature (Ta) is a parameter that describes how much noise an antenna produces in a given environment. This temperature is not the physical temperature of the antenna.
Moreover, an antenna does not have an intrinsic “antenna temperature” associated with it; rather the temperature depends on its gain pattern and the thermal environment that it is placed in.
To define the environment, we’ll introduce a temperature distribution – this is the temperature in every direction away from the antenna in spherical coordinates. For instance, the
night sky is roughly 4 Kelvin; the value of the temperature pattern in the direction of the Earth’s ground is the physical temperature of the Earth’s ground. This temperature
distribution will be written as T(theta,phi). Hence, an antenna’s temperature will vary depending on whether it is directional and pointed into space or staring into the sun.”
http://www.antenna-theory.com/basics/temperature.php
Radio Astronomy: Lecture #5
“Ta = Sc2/2kn2 4/p (D2n2/c2) = 4SD2/2pk = 4(10-26)(102)/2p(1.38 x 10-23) = 0.04 K!”
http://web.njit.edu/~gary/728/Lecture5.html
————-
You said….
“I can only repeat the original poster’s question: if the energy of the cooler object that is radiated towards the warmer object does not reach it, where does it go to? It can’t just vanish or the principle of energy conservation would be violated.”
Does a block of wood, with two opposing forces, move in the direction of the stronger force?
Did the weaker force just vanish and violate the principle of energy conservation???
It’s the same with Electromagnetic Forces, you know, one of the four FUNDAMENTAL FORCES.
The only difference is that instead of the two opposing forces acting on a block of wood, it acts on ZERO MASS PHOTONS.
Which way do you think the photons will move?
I think a 10 year old could answer that question.
A 10 year old can also tell you which way Heat flows when an Ice Cube is placed in their hand.
—————
You said…
“The only thing the solar oven ‘proves’ is that there is no _net_ exchange of energy. As long as the temperature of the object in the cooler is below atmospheric temperatures, it simply radiates more energy away than it receives from the atmosphere.”
Oh, there absolutely IS an exchange of energy…it’s from the warmer water at the Solar Oven’s focal point to the COLD ATMOSPHERE.
The water receives ZERO energy from the COLD atmosphere even though all that Back-Radiation (324 w/m^2) should be CONCENTRATED at the focal point of the Solar Oven!!!
Meanwhile, the water will BOIL when the Solar Oven receives a paltry 168 w/m^2 from the SUN!!!
Gee, I wonder why that is???
Could it be that the 2nd Law of Thermodynamics, you know, that pesky LAW OF SCIENCE is correct???
I just laugh whenever I read the AGW’er “scientific papers” when they say that this fantasy “Greenhouse Effect” has warmed the Earth from -18 deg C (the heating from the wimpy Sun) and then proclaim that the Back-Radiation from the COLD atmosphere (that is so much more powerfull than the Sun) has caused the entire Earth to increase in temperature by a whopping 33 deg C, all the way up to a balmy +15 deg C.
The REALITY is that even when this Back-Radiation is CONCENTRATED onto a tiny bit of water, the water will FREEZE!
I laugh harder when I see NASA measuring this Back-Radiation using instruments that have been CRYOGENICALLY COOLED far below the -20 deg C atmosphere temperature to make the direct
measurement POSSIBLE, and then say that this Back-Radation MUST cause the Earth to warm in response.
That’s some “Greenhouse Effect” !!
Gord,
You can buy quite cheap room temperature thermal sensors that measure radiation from the sky (and ground). They are used in an innovative application for attitude control in model aircraft using a 4 quadrant differential measurement system
The intriguing part about the system is it is pretty immune to the sun being in the sky. The sun is a relatively small player in total energy as seen by pure thermal sensors.
Check out http://store.diydrones.com/product_p/se-0002-01.htm if you want to buy one.
The point about this technology is that it is not purely theoretical, and for only $99 you too can measure sky temperature.
In fact Gord,
Here’s a way for you to do a practical experiment with your own hands to show what SoD means – no theory required and only $20 to learn the lesson of a lifetime.
Buy one of http://store.diydrones.com/product_p/mlx90247esf-dsa.htm, get a battery resistors etc and a multimeter and hook-up as required.
Then point the unit at various surfaces – say a block of ice, a wooden board, and a fire.
Note the voltage out for the different surfaces, especially note you get a non-zero reading for the ice block. Then get some dry ice and point at that. Note you get a lower reading again.
Q.E.D.
Gord,
In case the above points are a bit difficult, I alos have another application that uses room temperature thermopiles to measure lower temperatures.
The unit I append the link to is an ear thermometer. It measures ear temperatures typically 36-37C.
The operating temperature range – what air temperature can be is 10C to 40C.
That is the manufacturer says the instrument can be at 40C yet it can measure an ear temperature of 36C – i.e. colder.
This is not theory. This is something you can buy at the pharmacy. And according to what you have posted it can’t work!
oops – link
http://www.microlife.com/healthguide/fever/faq/general/
So how did P&W manage to measure 4K background radition when their receiver was much warmer than that? According to your understanding of physics the radiation from the receiver should “push” the radiation from the cold sky outwards. And how does the 4K microwave radiation actually reach the receiver on the ground of the earth with the warmer atmosphere between them?
D.
– Interferometers cool their detectors to about 77 deg K for direct measurements.
– Infrared thermometers rely on thermistors, thermocouples, cold junction semi-conductors etc… that transfer heat TO THE ATMOSPHERE for it’s measurements.
The thermistor or other sensor is biased with a current at a reference temperature and it’s resistance changes with temperature.
The drop or rise of the voltage corresponds with temperature rise or fall.
The basic equation used to describe the output of a radiation thermometer is:
V (T) = e K TN
Where:
e = emittivity
V(T) = thermometer output with temperature
K = constant
T = object temperature
N = N factor ( = 14388/(lT))
l = equivalent wavelength
http://www.omega.com/literature/transactions/volume1/thermometers1.html
———————–
The pros and cons of
RTDs and thermocouples
“Temperature is the most-measured process variable in industrial automation. The two prime sensors used to measure it are resistance temperature detectors (RTDs) and thermocouples (T/Cs). For relatively narrow temperature ranges, a variety of other temperature sensors can be employed. Examples include: diode or transistor junctions, which have a relatively narrow range of measurement and are commonly used to measure the temperature of electronic equipment; thermistors, positive or negative temperature coefficient resistors, which offer a maximum range of measurement up to about 200-300”
http://www.jimpinto.com/writings/tempsensors.html
Implementing Cold-Junction Compensation in Thermocouple Applications
“In the early days of thermocouples, the ice-bath reference served as the standard in thermocouple applications. Implementing an ice bath today is impractical in most situations. Therefore, when the cold junction is not at 0°C, the temperature of this junction must be known in order to determine the actual hot-junction temperature. The output voltage of the thermocouple must also be compensated to account for the voltage created by the nonzero cold-junction temperature. This process is known as cold-junction compensation.”
http://www.maxim-ic.com/app-notes/index.mvp/id/4026
diessoli
I have already posted links to ANTENNA TEMPERATURE for you.
READ THEM.
If you don’t understand them TAKE SOME ENGINEERING COURSES.
I’m not about to spend a lot of time trying to teach you since can’t even seem to understand the 2nd Law of Themodynamics or that a Parabola has a focal point.
You’re cute Gord. Did you go to the WUWT school of discussion? Antenna temperature has nothing to do with the question I asked. And what about the atmosphere that sits between the receiver and the microwave radiation?
Actually, never mind.
diessoli
Thank you. I’m sure you are cute too.
Ever have a discussion with a cult member?
Gee, don’t you remember this question too?
“So how did P&W manage to measure 4K background radition when their receiver was much warmer than that?”
This question is DIRECTLY related to this question that you asked:
“And how does the 4K microwave radiation actually reach the receiver on the ground of the earth with the warmer atmosphere between them?”
The answer in simplistic terms is summarized in a single word…”DIRECTIVITY”.
I have already posted links to ANTENNA TEMPERATURE for you.
READ THEM….you might even see the word “DIRECTIVITY”.
If you don’t understand them TAKE SOME ENGINEERING COURSES.
—-
PS: Notice that the Parabolic Solar Ovens, that have a focal point (that Parbolic Dish Antennas also have) managed to send the Heat energy from the water to the colder atmosphere and FREEZE even when the ambient air temperature was +6 deg C?
Hint: “DIRECTIVITY”
——-
Actually, never mind.
[…] the answer is revealed: Back to thermodynamics and electromagnetic radiation. Scienceofdoom and others think that the hot […]
Gord, Oh, Gord:
The (something less than college-level) references you cite also include the term “wavelength”, and relate this to bubble wall thickness. Each color has a different wavelength, and each will be affected by a DIFFERENT bubble wall thickness. You will find this in you references. You will also find the words “no reflection” .. when the bubble wall gets thin enough – the context is “visible” reflections – all light passes through. No interference. My point remains that a single film of constant thickness can interfere with only one wavelength of visible liight. The references refer to cancellation of REFLECTED light, and in fact, correctly describe, in lay terms, what happens AT A PARTICULAR WAVELENGTH (color). Nowhere do they mention simultaneous cancellation of ALL wavelengths, but I can see how one might become confused. As the bubble wall becomes thinner, the wavelength of light affected becomes shorter, and a different SINGLE color’s reflection is cancelled – the fact that not all of longer wavelengths are simultaneously cancelled is not clarified. I prefer textbooks to microscope brochures, just to clarify that point.
Gord:
As an aside, it’s interesting how often people who lecture other people on physics don’t understand the meaning of the word “net”. These people are often the same ones who capitalize adjectives, go figure.
The correct description is there is no net flow of heat from a cold object to a warm one. If you put a cold object by a warm object, it affects the warm object too.
Example: sleep on your bed with a blanket, and without one. With the blanket on you’ll be warmer. Simple thought experiment, obvious answer.
Now here’s the kicker:
If there is absolutely no flow of heat from the colder to the warmer object, how does the colder object communicate to the warmer object that it is there?
This has caught my attention and I do work in the field professionally but don’t have all the answers and will not be able to respond to all comments. However,
here is one way to look at it:
Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface. It does, however, reduce the rate radiative cooling of the surface. The combined incoming and outgoing IR radiation is called net IR radiation and helps maintain the conservation of energy at the surface after being excessively heated by net solar radiation, at least at mid and low latitudes over the course of a year. The rest of the energy balance for those locations is achieved by atmospheric and oceanic transport to higher latitudes where this is a overall radiative loss of heat from the surface and atmosphere to space.
If one does not understanding that the earth’s surface air temperature is warmer because the atmosphere contains water vapor for example, they need to poke around a little more on this site (or visit Venus, or Mars.) Atmospheric science is very advanced and this has long been addressed but not necessarily from the angles being discussed here. The concept of additive radiation vectors can be confusing from first principles but sure does seem to work that way.
A cheaper experiment than buying anything, but maybe equally revealing, is to go out on a clear calm, preferably rather dry (low relative humidity), night and stand near a building but with clear view of the overhead sky. Then look straight up at the sky (tilt your head all the way back) and note the temperature sensation your face as compared to looking at the side of the building and noting the air temperature near your face is the same in both cases. Then repeat on a cloudy night. Yes, the clouds will still be colder than the air in contact with your face (in all but more unusual cases) but it will feel warmer relative to the wall than before because your skin is radiatively cooling less than on the clear night. The calm part of this is very important, and best clouds would be mid-level overcast (couple 1000 ft above the ground)
As widely stated and accounted for in GHW, water vapor, CO2, and several other gases all behave as do the clouds at night, just a lot less in magnitude and more difficult to sense by this method, but varying with concentration.
As for instrumentally detecting sky IR radiation, it can be done, and is routinely, at ambient temperatures by balancing the cooling rate of the detector with ambient temperature, and of course, conserving energy. The commercially available instrument is called a pyrgeometer.
BTW, the Trenberth diagram does not create energy, add it up, the balance at the surface is 0, the balance for the atmosphere is 0, the balance at the top of the atmosphere is 0 for no overall change in energy or temperature. However, the exact value of each vector there (global annual average) is still being determined and is likely varying ever so slightly because of some current changes being made to the system and there may be small imbalance indicating a slight retention of energy by the earth, which we might expect given some current knowledge about the composition of the atmosphere.
George
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface. It does, however, reduce the rate radiative cooling of the surface.
The black body temp of earth is 255 K, so how does slowing this cooling get us to 288K.
I think you’ve touched on the crux of the debate. Proponents contend the cooler atmosphere adds the additional energy to achieve observed surface temps. Skeptics are not satisfied by adding apposing flux (vectors) to achieve 288 K.
scienceofdoom has distilled net radiant transfer equation to an elegant proximity formula showing additive contrary flux do not promote thermal runaway but rather a set equilibrium. The caveat being black body surfaces. When non-equal emissivities are plugged in the formula goes awry.
The debate continues.
J. Lanier wrote:
“The black body temp of earth is 255 K, so how does slowing this cooling get us to 288K.”
A common misconception is that GHW is caused primarily by the increased downwelling IR at the surface when indeed, the initial step is the additional absorption of the surface-leaving (or upwelling) IR by the atmosphere. More absorber – more warming. Heat is transferred from the warmer earth surface to the cooler atmosphere and it gets warmer than it was- no problem.
That heat does not necessarily need to get transferred back the surface earth since it is the warmer lower atmosphere that is at issue. The energy transfer between the lower atmosphere and the surface is a complex turbulent system of latent and sensible heat transfer in addition to a radiation component where the radiation component if not reducing the net upward IR from the surface can actually add heat to the surface as discussed next.
Of course there are times when the atmosphere is warmer than the surface, like a lot of the time over the tropical oceans. Then there is net IR energy transfer from the atmosphere to the surface – warmer to colder body – no problem, but in which case the energy is subject to being stored and at least temporarily otherwise removed from the radiation balance argument.
To specifically answer your question 288K is the mean surface air temperature (air or ground since when in contact with each other they are usually very similar, where as the 255K as seen from space sees a lot of upper atmosphere instead of the earth surface which is colder, or an equivalent black body temperature of about 255K.
George
Gord writes:
Answer: No, the colder body radiation cannot reach and be absorbed by the warmer solid body causing the warmer solid body to heat-up.
“causing the warmer solid body to heat-up” looks like it might be a dodge to me. Nobody’s asserting that the cooler body will cause the warmer body’s heat to increase; instead, it will cause it to lose heat more slowly. This is why everyone keeps telling you that you don’t understand the meaning of the word “net”.
If you’re seriously claiming that the radiation from the cooler body doesn’t even reach the warmer body, that’s far more fascinating. In your haste to fetishize the Second Law, you’re apparently willing to violate the First Law.
Jenn and Carrick
Be careful about using the everyday meaning of “heat”.
In thermodynamics the word “heat” is used to describe the transfer of energy from a higher temperature surface to a lower temperature surface.
Its not even correct to say an object contains “a lot of heat”.
A good test for checking if it is really “heat” then it should be capable of doing work.
I personally find this formulation acceptable.
A cold body can radiate to a hot body.
A hot body can radiate to a colder body
A cold body cannot radiate heat to a hot body.
A hot body can radiate heat to a colder body.
I must thank Gord however for the solar heater reference. It really is worth a look and the magnitude of the “backradiation” must surely be questioned after this.
Bryan, if you want to be technical, the correct thermodynamic word is “heat energy”. “Heat” is an throwback term to the days of caloric fluids and of course there really is no such thing.
Transfer of heat energy via radiation goes under the moniker “thermal radiation”. And while we’re at it, the other common mechanisms for transfer of heat energy are conduction and convection. The atmosphere does all three.
You can say that a cold body can in general transfer heat energy via radiation to a hotter body, as long as the medium is not opaque and as long as the hot body is not a perfectly reflector, etc etc.
What the colder body cannot do in general is transfer net heat energy to the hotter body, without work being done. Net heat energy refers to the sum over all types of heat energy and the sum over all outgoing and incoming heat energy.
It’s that summed quantity that to, not individual constituents, that Clausius’s formulation of the second law refers to. As I pointed out above the word net is often omitted, but it is the key qualifier here.
Carrick
…..You can say that a cold body can in general transfer heat energy via radiation to a hotter body, …….
No, that is the opposite to what happens.
Can you get this transfer to do work?
If you can its heat.
If you cant its simply infra red radiation.
Heat has the thermodynamic capacity to do work.
However I must emphasise again the important lead the Gord has given.
http://solarcooking.org/research/McGuire-Jones.mht
This single experiment in cooling mode means that all the numbers in the K-T diagram are totally fictitious.
If I were an adherent of the IPCC consensus I would find it imperative to test this paper and to falsify it if possible.
This single and (perhaps unintended) result means the magnitude of so called “backradiation” has been totally exagerated.
“This single experiment in cooling mode means that all the numbers in the K-T diagram are totally fictitious.”
Would you care to explain why that might be? I for one don’t see where the conflict is.
D.
diessoli
The solar heater when pointed to a cold part of the sky at night should have picked up “backradiation” according to IPCC theory.
For instance one metre area parabolic dish should have 300W/m2 of infra red radiation concentrated at its focal point.
I thought it would be much less.
However this paper shows that the heat energy is going the other way and cooling the surfaces connected to the dish even I did not expect this dramatic effect.
Proving the “backradiation” if it exists is very smal and certainly nothing remotely like 300W/m2.
As I say this paper should be tested rigorously but if it holds up the IPCC theory is grossly in error.
Bryan,
Trenberth et al’s value of 333 W/m^2 is the annual global mean value computed over about four years. That does not mean that you have this amount of back-radiation at all times. Notice that a “solar cooler” will not work well or not at all in the presence of clouds.
See below for a more quantitavive treatment where you can see that even with a back radiation of about 250 W/m^2 you can cool the dish down to 0 Cel when the ambient temperature is 10Cel.
http://docs.google.com/viewer?a=v&q=cache:P9iH255zXn4J:www.asterism.org/tutorials/tut37%2520Radiative%2520Cooling.pdf+clear+night+back+radiation&hl=en&gl=nz&pid=bl&srcid=ADGEESh02xx-V2vpqe15UjCRCgUkJ6cnnhcBCMZyBXfNLoXhBd2gfuK67Y7iRfmZesh5nkbdXASXVzAOSqw4-WmXVnxg2e5VIiSYLLpDZ_5c-y-9DePLYnKDZQI8ebAI91sMNEi59Iib&sig=AHIEtbQqu3–bLKqS1QwMhhTOzwOTbnpBA
D.
Dr.George
You said…
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
Correct.
However, all the AGW’er “scientific papers” including Trenberth’s Earth Energy Budget Diagram (that is used by the IPCC) clearly show otherwise.
—
With reference to Kiehl and Trenberth’s Earth Energy Balance Diagram
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/Earthebal.html#c1
Trenberth clearly shows 324 w/m^2 Back-Radiation being ABSORBED by the Earth’s surface, causing heating to produce the +15 deg C Earth surface temperature.
The Sun’s contibution (the ONLY energy source in the diagram) only contributes 168 w/m^2 toward the 390 w/m^2 (+15 deg C) temperature of the Earth.
I repeat, the SUN is the ONLY energy source in the diagram, so 168 w/m^2 is the TOTAL amount of energy available!!!
Let’s look at Trenberth’s balance of energy at the Earth’s surface.
Incoming energy at the Earth’s surface:
1) Solar Energy = 168 w/m^2 (the ONLY energy source)
2) Back-radiation = 324 w/m^2 (comes from the atmosphere that is NOT an Energy source and gets ALL it’s energy ultimately from the SUN!)
Total = 492/w^2
Outgoing energy at the Earth’s surface:
1) Thermals = 24 w/m^2
2) Evapo-transpiration = 78 w/m^2
3) Surface Radiation = 390 w/m^2
ALL the above MUST get ALL their energy from the only energy source, the SUN!
Total = 492 w/m^2
So we can easily see that the Back-Radiation (not an energy source) is HEATING THE EARTH.
Which is IMPOSSIBLE!
Just like you said…”Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
——-
Here are some “Greenhouse Effect” links:
1)Greenhouse effect
“The greenhouse effect is a process by which radiative energy leaving a planetary surface is absorbed by some atmospheric gases, called greenhouse gases. They transfer this energy to other components of the atmosphere, and it is re-radiated in all directions, including back down towards the surface. This transfers energy to the surface and lower atmosphere, so the temperature there is higher than it would be if direct heating by solar radiation were the only warming mechanism.”
“The Earth receives energy from the sun in the form of visible light. This light is absorbed at the Earth’s surface, and re-radiated as thermal radiation. Some of this thermal
radiation is absorbed by the atmosphere, and re-radiated both upwards and downwards; that radiated downwards is absorbed by the Earth’s surface. Thus the presence of the atmosphere results in the surface receiving more radiation than it would were the atmosphere absent; and it is thus warmer than it would otherwise be.”
“If an ideal thermally conductive blackbody was the same distance from the Sun as the Earth, it would have an expected blackbody temperature of 5.3 °C. However, since the Earth reflects about 30% (or 28%[5]) of the incoming sunlight, the planet’s actual blackbody temperature is about -18 or -19 °C [6][7], about 33°C below the actual surface temperature of about 14 °C or 15 °C.[8] The mechanism that produces this difference between the actual temperature and the blackbody temperature is due to the atmosphere and is known as the greenhouse effect.”
http://en.wikipedia.org/wiki/Greenhouse_effect
—
2)Tutorial on the Greenhouse Effect- University of Arizona
“In this case, the Earth still gains 240 Watts/meter2 from the sun. It still loses 240 Watts/meter2 to space. However, because the atmosphere is opaque to infrared
light, the surface cannot radiate directly to space as it can on a planet without greenhouse gases. Instead, this radiation to space comes from the atmosphere.
However, atmospheres radiate both up and down (just like a fire radiates heat in all directions). So although the atmosphere radiates 240 Watts/meter2 to space, it
also radiates 240 Watts/meter2 toward the ground! Therefore, the surface receives more energy than it would without an atmosphere: it gets 240 Watts/meter2 from
sunlight and it gets another 240 Watts/meter2 from the atmosphere — for a total of 480 Watts/meter2 in this simple model.”
http://www.lpl.arizona.edu/~showman/greenhouse.html
—
3)The Greenhouse Effect
“The heating of the ground by sunlight causes the Earth’s surface to become a radiator of energy in the longwave band (sometimes called infrared radiation). This emission of energy is generally directed to space (see Figure 7h-2). However, only a small portion of this energy actually makes it back to space. The majority of the outgoing infrared radiation is absorbed by the greenhouse gases (see Figure 7h-3 below).
Absorption of longwave radiation by the atmosphere causes additional heat energy to be added to the Earth’s atmospheric system. The now warmer atmospheric greenhouse gas molecules begin radiating longwave energy in all directions. Over 90% of this emission of longwave energy is directed back to the Earth’s surface where it once again is absorbed by the surface.
The heating of the ground by the longwave radiation causes the ground surface to once again radiate, repeating the cycle described above, again and again, until no more
longwave is available for absorption.”
http://www.physicalgeography.net/fundamentals/7h.html
———-
Notice that they all have the Sun as the only real energy source….just like Trenberth.
Notice that they all have energy from the atmosphere (Back-Radiation) heating the Earth….just like Trenberth.
The Sun heats the Earth and the Earth heats the Atmosphere.
All the energy came from the Sun, the only energy source, and the Earth and atmosphere are merely passive receivers of the Sun’s energy.
This can be comared to a battery providing all the energy to an electronic circuit.
Remove the battery and the circuit does not function.
Remove the Sun and the Earth and Atmosphere will quickly drop in temperature to near absolute zero.
The electronic circuit may contain all sorts of components, negative feedback loops and postive feedback loops like Oscillators.
The energy all comes from the Battery and none of the circuitry including the postive feedback oscillators can release energy that exceeds what the Battery supplies.
If the energy exceeded what the Battery supplied it would mean that energy was Created, and violate the Law of Conservation of Energy by producing a Perpetual Motion Machine.
Now look at the Greenhouse Effect links above, they all have energy being Created by the Earth-Atmosphere that exceeds the energy of the only energy source, the SUN.
Link (2) and especially (3) involve creation of energy producing a Perpetual Motion Machine in Positive Feedback Loop between the Earth and Atmosphere that can only result in an Infinite Creation of Energy!
——–
The Back-Radiation cannot EVER heat a warmer Earth and ALL measurements absolutely PROVE THIS!
The “Greenhouse Effect” is very clearly a fantasy.
Please provide your comments on my post.
PS: Time prevents me from dealing with the rest of your post at this time, but I will address the rest soon.
Gord,
You need to consider the atmosphere’s storage of energy since it has mass and it has temperature greater than 0 K. It therefore has thermal capacity and becomes an effective secondary source of radiant energy deriving its available energy from a combination of absorbed upward IR, released latent upon water condensation, and sensible heat through convection and conduction from the surface. Yes, the only ultimate source of energy is the sun but remove it instantaneously (without blowing it up) and the atmosphere and earth’s surface will continue radiating at each other for some time before giving it all up.
In any an all global mean cases, net IR contributes to a loss of energy from the surface – no problem.
One thing that concerns a lot of people is the magnitude of the downwelling IR at the surface, a bit of a surprise during the learning process. Of course, the temperature and density profile of the atmosphere is not shown in the Trenberth-type diagram, which readily accounts for the greater downward IR from the atmosphere that that going to space. There absolutely is no energy created in the Trenberth, or about a couple other dozen similar diagrams used over the last 60 years to explain the mean earth energy balance. They are called Earth Energy Budget (or Balance) diagrams but all the numbers represented are not well known because of the extent and complexity of the observation effort required to gather all the necessary information, but they do balance energy because the authors require it.
You can save your efforts in providing me with references to GHW information but I will try to answer other questions or misrepresentations.
Byran, you missed my point. “Heat” isn’t a physical quantity. It’s a made up 19th concept that has no basis in any real world thermodynamics discussion.
“Heat energy” is defined as the “kinetic energy associated with molecular motion”. There is nothing in this definition that makes a proviso whether the “heat energy” is available to do work or not.
Thus, if you have radiation from any source impinging on a surface and being absorbed, thereby increasing the “kinetic energy associated with molecular motion,” you are increasing its total heat energy. And that is the end of the story regardless of the fact that the net direction of heat energy exchange is from the warmer to the cooler, in the absence of net work done on the system.
Bryan
Re: Solar Ovens
There are at least hundreds of thousands of Solar Ovens in operation around the world.
There are major Power Generating facilities using Parabolic Mirrors that use large arrays to produce Mega-Watts of power.
They ALL only work during the Day and ONLY when directed at the Sun.
If Back-Radiation actually reached ANY of these hundreds of thousands of Solar Ovens, they would ALL be producing Energy at Night.
Including the Mega-Watt Power Generating stations.
Gord:
Have you ever looked at the absorption spectrum of aluminum and other metals used for silvering mirrors?
You should do this, then compare the radiation spectrum for the sun, and where it peaks against the radiation spectrum for the sky.
Now that Bryan has finally provided an answer as to what happens to the radiation emitted by a colder body and received at a hotter body there is a new post on these “insights”:
Intelligent Materials and the Imaginary Second Law of Thermodynamics
What might be interesting to newcomers who have some concern that the 2nd law of thermodynamics (the real one) prevents the inappropriately-named “greenhouse” effect from working is the different approaches we can see:
1. Bryan claims that radiation from a colder body “reaches” the hotter body and then is “mostly” reflected. This is a problematic claim for 2 reasons, which you can read about in the new post.
2. Gord claims that radiation from a colder body never makes it to the hotter body because radiation is a vector and is therefore “cancelled out” before it gets to the hotter body.
Evidence such as measurements with various proven instruments like Fourier-transform infrared (FTIR) spectrometers is dismissed as fraudulent.
Brief digression.. By the way, the duo of Gerlich and Tscheuschner, not that I
endorse their work in any way, also disagree with Gord about radiation cancelling out and note:
Something all physicists agree with. End of digression.
Clearly a massive con job has been run for a long time as medical research, the military and many commercial organizations buy this FT-IR equipment and use it for valuable work. Concerned citizens who agree with Gord might want to Google this instrument type to find the suppliers and notify the justice department in their respective state about this fraud.
Anyone else who wants to know more about FT-IR’s can also google – here’s a simple article about how they work. I’m sure there are thousands of other resources.
3. The many other believers in the imaginary second law of thermodynamics have yet to explain what happens to radiation from the colder body, why it doesn’t reach the hotter body, or if it does, why the hotter body doesn’t absorb it.
The other believers are content to argue about definitions of heat and energy and repeat the mantra “this violates the 2nd law of thermodynamics” without ever explaining how.
Hopefully the responses by the supporters of this movement should help newcomers and interested parties to see that it isn’t actually science they are promoting, and the supporters – well-meaning though I’m sure they are – don’t actually understand the subject matter.
Carrick
……“Heat energy” is defined as the “kinetic energy associated with molecular motion”. There is nothing in this definition that makes a proviso whether the “heat energy” is available to do work or not……..
Sorry but I must repeat my point about the definition of heat.
Any good physics or thermodynamics textbook will confirm my definition.
diessoli
Thank you for your interesting post.
It contained some equations and emissivity values that I had not come across before.
The radiation frost example did not cover situations where occasionally the air temperature is greater than the ground.
This surely is when backradiation should have a chance to prove itself.
Alas the thermal capacity of the air is not sufficient to overcome the radiative losses of the surface and ground frost is observed.
Gords solar oven is a simple and very useful bit of kit.
If the cooling/heating box can be thermally isolated from the Earth surface then the IPCC endorsed theory of the atmosphere can be unambiguously tested.
Carrick
Did you not read the my post about the Physics Dept. of Brigham Young University measurements?
“The second area of solar cookers I looked at was their potential use for cooling. I tested to see how effective they are at cooling both at night and during the day. During both times, the solar cooker needs to be aimed away from buildings, and trees.
These objects have thermal radiation and will reduce the cooling effects. At night the solar cooker needs to also be aimed straight up towards the cold sky.”
http://solarcooking.org/research/McGuire-Jones.mht
Obviously, the thermal IR radiation from buildings and trees are concentrated at the focal point of Parabolic Solar Ovens and affects their measurements.
ALL EM fields from Visible light, the Infrared spectrum, Satellite microwave signals (Satellite dishes), UHF TV dish antennas, and even SOUND WAVES (parabolic micophones) will be concentated at a focal point of a Parabolic dish.
So, what is the point of your post???
—————
Do you really think that the Mega-Watt installations of Parabolic Mirror Solar Ovens and the hundreds of thousands of Solar Ovens in constant operation over the entire Earth would have failed to notice that ALL of them do not to generate HEAT at night?
The Back-Radiation that is fundamental to heating the entire Earth from -18 deg C to +15 deg C by the fantasy “Greenhouse Effect” does not even reach the Earth.
The “Greenhouse Effect” is a pure fantasy on par with the Tooth Fairy.
Gord Said
Reluctant as I am to support Gord, he has a point – but not the one he thinks he’s making.
This ties back to some comments I have made to Science Of Doom about the need to explore and explain the reason why the earth’s surface cools rapidly at night, while the atmosphere stays at roughly the same temperature.
My personal explanation is that the atmosphere acts as a ‘blanket’ at specific frequencies – those that are well absorbed by CO2 and H2O
The ground acts as a much better black body and radiates over a range of frequencies. Those that do not interact with the CO2 & H20 in the atmosphere merrily radiate straight out to space. Those in the required frequency bands interact strongly and it is though a blanket has been placed over them.
The net observation is that the atmosphere is a ‘grey body’ while the ground is much closer to a ‘black body’
The result is cooling of the ground at night while the atmosphere above stays at the same warm temperature – or slightly diminished due to conduction,
J. Lanier
You said…
“scienceofdoom has distilled net radiant transfer equation to an elegant proximity formula showing additive contrary flux do not promote thermal runaway but rather a set equilibrium. The caveat being black body surfaces. When non-equal emissivities are plugged in the formula goes awry.”
I repeat my previous post to Scienceofdoom:
“In case you have not realized it, your example of the two identical stars has CREATED energy.
E1 = E2 that you started with became 1.00000025 E1 = 1.00000025 E2
Where did the extra energy come from???
If you repeat your analysis again using 1.00000025 E1 = 1.00000025 E2 the energy will increase AGAIN.
Repeat the cycle a multitude of times and eventually each star will have infinite energy.
By any definition, that is called THERMAL RUNAWAY!”
——–
Have you not noticed that “Scienceofdoom” has failed to address this issue???
Jenn
You posted…
—
“Gord writes:
Answer: No, the colder body radiation cannot reach and be absorbed by the warmer solid body causing the warmer solid body to heat-up.
“causing the warmer solid body to heat-up” looks like it might be a dodge to me. Nobody’s asserting that the cooler body will cause the warmer body’s heat to increase; instead, it will cause it to lose heat more slowly. This is why everyone keeps telling you that you don’t understand the meaning of the word “net”.
If you’re seriously claiming that the radiation from the cooler body doesn’t even reach the warmer body, that’s far more fascinating. In your haste to fetishize the Second Law, you’re apparently willing to violate the First Law.”
———————-
In case you have not seen the 2nd Law here it is AGAIN:
“Second Law of Thermodynamics: It is NOT POSSIBLE for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow. Energy will not flow spontaneously from a low temperature object to a higher temperature object.”
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/seclaw.html#c3
DO YOU SEE the word “NET” anywhere????
DO YOU SEE THE WORDS “NOT POSSIBLE”?
It is there for a REASON.
——-
The 2nd Law is no more a “NET” Law of Science (allowing “some” heat to flow from cold to hot objects) than the force of Gravity will allow some people to be blasted into space as long as most people remain Earth bound.
——-
Electromagnetic force
“The electromagnetic force is one of the four fundamental forces. The other fundamental forces are: the strong nuclear force (which holds quarks together, along with its residual strong force effect that holds atomic nuclei together to form the nucleus), the weak nuclear force (which causes certain forms of radioactive decay), and the gravitational force. All other forces are ultimately derived from these fundamental forces.”
“In physics, the electromagnetic force is the force that the electromagnetic field exerts on electrically charged particles. It is the electromagnetic force that holds electrons and protons together in atoms, and which hold atoms together to make molecules. The electromagnetic force operates via the exchange of messenger particles called photons and virtual photons.”
NOTE!!!
“The electromagnetic force is the one responsible for practically all the phenomena one encounters in daily life, with the exception of gravity.”
http://en.wikipedia.org/wiki/Electromagnetic_force
————————
The Electomagnetic FORCE moves ZERO MASS Photons in the DIRECTION of the STRONGER FORCE (Hot to Cold)!!
It is NOT POSSIBLE for the ZERO MASS Photons to move in the opposite direction, (Cold to Hot)!!
Just like the Force of GRAVITY CANNOT REVERSE DIRECTION!!!
Jerry
I repeat:
Did you not read the my post about the Physics Dept. of Brigham Young University measurements?
“The second area of solar cookers I looked at was their potential use for cooling. I tested to see how effective they are at cooling both at night and during the day. During both
times, the solar cooker needs to be aimed away from buildings, and trees.
These objects have thermal radiation and will reduce the cooling effects. At night the solar cooker needs to also be aimed straight up towards the cold sky.”
http://solarcooking.org/research/McGuire-Jones.mht
Obviously, the thermal IR radiation from buildings and trees are concentrated at the focal point of Parabolic Solar Ovens and affects their measurements.
ALL EM fields from Visible light, the Infrared spectrum, Satellite microwave signals (Satellite dishes), UHF TV dish antennas, and even SOUND WAVES (parabolic micophones) will be concentated at a focal point of a Parabolic dish.
So, what is the point of your post???
Gord,
You don’t need fancy parabolic reflectors to get cold at night.
Simply putting a bowl or water out in the desert on a still night can get it down to zero C. In some cases it will form ice. And this is while the air above is relatively warm.
This is what inversions are all about.
The point I was making is that ground radiation spectrum and air radiation spectrum are different. It’s not just a matter of temperature but also substance.
My take is that the soil cools at night because it radiates on a lot of frequencies, some of which are just not absorbed by the air. The air radiates efficiently to itself and to some extent to the soil.
The spectral difference means the soil cools while the air stays warmer.
This effect is know world-over and shows that it’s not just temperature, but spectral response that is important.
I’d like to hear your take on why the soil gets cold at night while the air remains warm – despite them both starting at the same temperature.
Gord,
In particular, I’d like to hear why you think the ground, which is measurably colder than the air above it, can still radiate energy and get colder still.
If you look at it objectively, there is no way that a photon from the ground can get into outer space without hitting a hotter gas molecule on the way. The mean free path for photons in the air is measured in centimeters.
Jerry
Did you not read the my post about the Physics Dept. of Brigham Young University measurements?
The water will COOL even during DAYLIGHT, when the Solar Oven is pointed away from the Sun!!
ALL EM fields from Visible light, the Infrared spectrum, Satellite microwave signals (Satellite dishes), UHF TV dish antennas, and even SOUND WAVES (parabolic micophones) will be concentated at a focal point of a Parabolic dish.
Are you saying that IR Radiation cannot be CONCENTRATED at the focal point of a Parabolic Mirror?
If so, please provide your evidence.
————
You asked…
“In particular, I’d like to hear why you think the ground, which is measurably colder than the air above it, can still radiate energy and get colder still.”
Are you serious?
The average ground temperature is +15 deg C and the average atmospheric temperature is -20 deg C!!!
Where do you get your information from?
Do you just “imagine” these little gems of wisdom?
————–
You said..
“If you look at it objectively, there is no way that a photon from the ground can get into outer space without hitting a hotter gas molecule on the way. The mean free path for photons in the air is measured in centimeters.”
Are you serious?
ALL EM fields CARRY Photon Energy.
How do you think we can communicate with the Space Shuttle?
Where do you get your information from?
Do you just “imagine” these little gems of wisdom?
Gord,
Unlike almost everybody involved in this discussion I have worked for a long time in the direct measurement of temperature and lapse rates for earth and atmosphere.
I’ve frozen my arse (ass) off innumerable times recording temperatures all through the day a night using thermometers attached to balloons and sondes.
Your generalities are both amusing and insulting.
Your reference such as
appears to me to be an exercise in picking some textbook without any practical knowledge of what goes on in reality.
Do some research on ‘atmospheric inversion’ and have a look at lapse-rate.
When you have done, come and ask me about my direct measurements of soil temperature of 10C with air temperature of 30C not a few 10’s of metres above. Are these your hypothetical +15C to -20C?
The point I am making is that within a very few metres – as measured personally by me – the ground is getting colder and the local air is staying very much hotter despite all the heat from the cold ground passing through the hot air.
Now Gord, If you have any practical experience, any real measurements, please put them forward. If on the other hand you are just recycling some text-book, please address the following issues
Emission spectrum of soil
Absorbtion spectrum of air
Rate of energy loss by soil *through* air to the sky
When you have done, have a thought and report back on whether cold bodies can radiate energy to or via hot bodies
Jerry
I think you have the right approach to the day/night temperature change.
Even for the radiant frequencies there seems an asymmetry.
If CO2 or H2O absorb say a 15um photon from the surface the increase in their energy is quite large.
E=hf gives about double the average KE to the molecule.
So this lucky molecule now has three times the average molecular KE.
But not for long, at 10 to the power 10 collisions per second the KE is shared out and the molecule returns to average.
How likely will it send a 15um photon back to the surface?
Here the emission requires the molecule to get up to a minimum of just over twice the average KE or a speed of 1.46 V(rms).
Taken altogether, emission seems less likely than absorption.
Further there is the fact that the emission can go in any direction in a x,y,z three dimensional space then we would expect “backradiation” to be a lot less than surface up radiation.
Bryan,
you said
I understand that emission and absorption are not totally equivalent, but in general if you can absorb something you can emit it ‘later’
There are issues of absorbing X and emitting – say – 2 x X/2, but in general I expect that absorbtion and emission are mostly related by a delta-T (time) function.
If your comment is take literally then you would see a net absorbtion of energy which would eventually see an emission – perhaps – at a higher energy. I’m not sure this happens in practice.
Jerry
Well, I guess all the AGW’er so called “scientists” are wrong about the Earth and Atmospheric average temperatures.
See Trenberth’s Earth Energy Balance Diagram (also used by the IPCC).
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/Earthebal.html#c1
Maybe you should Email Trenberth and the IPCC and point out their “mistakes”?
Gord,
No need to email those guys because I don’t disagree with them.
What I would like to see is you addressing real questions rather than resorting to some form of authority that has a dubious relationship to any question asked.
What I am asking you GORD, you, yourself, no-one other than you is how can the ground get cold while the air above stays warm? Why does ‘cold’; radiation pass through ‘hot’ air.
So GORD. please answer. Don’t just quote someone else. Please explain to me using your knowledge, to someone who works in the field, why the ground gets cold while the air above stays warm and all the radiation is passing through it.
I don’t need random references to texts. I’d like you GORD, to explain this in simple terms.
“Why did I measure 10C on the ground, yet measured 30C 100m above during a night sonde experiment”
Textbooks, URL, nah. You GORD should explain this or forever remain quiet.
Jerry
You said…
“The point I am making is that within a very few metres – as measured personally by me – the ground is getting colder and the local air is staying very much hotter despite all the heat from the cold ground passing through the hot air.”
Looks like you have dis-proven AGW.
Thanks.
Gord,
I doubt it I’ve disproved AGW.
What I do see is you ignoring the hard questions I posed.
My previous question
Please answer the hard question rather than resorting to the trite comment. If nothing else your authority on these matters will be enhanced.
Jerry
You said..
“No need to email those guys because I don’t disagree with them.”
Well, “those guys” say the average temperature of the Earth is +15 deg C and the average temperature of the atmosphere is -20 deg C.
How can you agree with them and disagree with them at the same time???
Gord,
I’m not disagreeing with them, They are probably totally correct on a global scale but obviously the local scale measurements will vary with time and location.
What I’m trying to do is get you to commit to answering some perfectly valid questions. What see so far is dissembling on a grand scale.
If you were serious I’d expect to see a clear answer to such questions as
Now I don’t really expect a straight answer as you seem to be pretty slippery when cornered, but I hope that your failure to answer a reasonable question this time – the third time I think – will expose the possibility that you may be a troll and furthermore a troll with very little knowledge in the area.
A straight answer by you would help alleviate these fears.
Jerry
If a warm layer of air resides above a colder Earth surface, the heat radiation will flow from the warmer atmosphere to the colder ground.
It’s called the 2nd Law of Thermodynamics.
Gord.
You are wrong. I can state without any doubt whatsoever. My comment about freezing my arse off was totally based on practical experience.
Not only did I measure the warm air temperature above using sondes I was simultaneously putting on my cold-weather gear.
You are wrong, wrong, wrongity wrong.
At night it gets colder on the ground while it stay a lot warmer up in the air. Check out ‘inversion’ in Wikipedia.
Then come back and explain it using your present theory of the 2nd law.
Also, For the fourth time, please give an answer to:
Jerry
It’s called the 2nd Law of Thermodynamics, you know, A LAW OF SCIENCE.
Submit a paper disputing it and YOU will be FAMOUS!
Good Luck.
Gord,
Your final non-answer to a simple question says you are not a player, just a troll.
FAIL.
Jerry
Gee, I completely answered your question using a LAW OF SCIENCE.
What more do you want?
If you disagree with the 2nd Law, prove that you are right, submit a paper and become FAMOUS!
What could be more simple?….Right?
You could become very, very wealthy too!
Gord,
The answer you need for Jerry is that that the atmosphere is a semi-transparent medium, often treated as a gray body. The warmer clear air over Jerry when he is getting cold still has a lower “effective radiating” temperature and allows radiant heat from the surface, emitting as almost entirely as a black body with a higher, radiating temperature. Much of the energy being transferred away from the surface is at the wavelengths where the atmosphere is completely transparent.
This is really basic stuff and has been very well covered by SoD
My earlier discussion about net radiative transfer between warmer and cooler surfaces and air masses was in reference to the effective radiating temperature of the air, which I had not made clear in my first explanation. In any event, there is no violation of the 2nd Law, or even a reasonable formulation where that could even be proposed.
The atmosphere is not a specific radiating body per se, just a collection of molecules, each type with their own radiative identity. Those that help block the wavelength gaps that the earth’s surface uses to cool to space (even if the actual temperature of the air above it is warmer) will contribute an accumulation of heat in the atmosphere, which can then be further transferred to (or help prevent loss from) the oceans, not necessary by radiative processes, which seems to be your hang-up. Hence the earth gains heat energy as those gaps in the IR spectrum close while there is no impingement to incoming solar.
Note to SoD — I hope this is not bordering on an essay, there just seemed to be a need for some closure.
George
Dr George, you go right ahead, I appreciate your comments. Relevant essays are always welcome.
Jerry
Oh, Remember to include “The mean free path for photons in the air is measured in centimeters.” in your paper.
That will really impress them.
And your reference to the contrary for photons that interact with CO2 and H2O at seal-level is???
Just for reference, the thermal decay time for the whole atmosphere is measured in months, not microseconds
Jerry
……”I understand that emission and absorption are not totally equivalent, but in general if you can absorb something you can emit it ‘later’……
The energy of the 15um photon is turned into translational,vibrational and rotational Kinetic energy of the molecule.
i.e. the energy is” thermalized”.
This excess energy is quickly shared out by collisions.
To get enough energy to emit photon the molecule needs a minimum of twice the average KE.
This means that it is still possible but less likely than absorption.
Bryan,
I’m guessing that the 2x KE will statistically arise reasonably often, hence the re-emission of the photon.
If it didn’t there would be a continual rise in temperature until such time as the probability of emission was very likely.
I don’t know a lot about the process, but my hunch is that there is some form of equilibrium between incoming and outgoing radiation at a specific temperature and frequency. Perhaps this is ‘neutral’ over a wide range of common temperatures?
Jerry
..”If it didn’t there would be a continual rise in temperature until such time as the probability of emission was very likely.”
Remember we are talking about nighttime, and as you so aptly put it , the other frequencies;
…”Those that do not interact with the CO2 & H20 in the atmosphere merrily radiate straight out to space. “
Bryan:
Where did you actually give a thermodynamic definition of heat (n)?
Any modern textbook is going to explain that used as a noun it refers to “heat energy”. It might be a property of heat energy that you can do work with it, for example, but that isn’t its definition.
Carrick
Definition of Heat
Page 470 University Physics -Young and Freedman.
Energy transfer that takes place solely as a result of a difference in temperature is called heat flow or heat transfer and energy transferred in this way is called HEAT.
My note of caution was not because of the word “heat energy” if you understand it in terms of above definition.
What I was referring to was the use of the word net
…. the meaning of the word “net”…..
If you think that there is any “heat” going from lower temperature to a higher temperature without the input of work then you are implying a violation of the second Law of Thermodynamics.
Somehow, I suspect your sense of ‘work’ in “without the addition of work” is too narrow. This, I think, is why MY favored physics text book on thermo chooses somewhat different wording, namely:
“The Principle of Clausius … IOW, heat cannot SPONTANEOUSLY go from the colder body to the warmer, WITHOUT SOME KIND OF CHANGE IN THE SYSTEM.”
[Kubo, “Thermodynamics” p. 74].
That “some kind of change” might not be a change one would normally call ‘work’: it could be, for example, raising the internal energy of the working substance of the heat engine, by running a current through it rather than by doing mechanical work on it.
And of course, this is the clause people forget when they claim the 2nd law forbids any motion of heat from colder to hotter: with a change to the system, it IS possible, as refrigerators show very commonly.
Dr George,
I agree with you totally that the atmosphere is semi-transparent.
The distinction I make is that semi-transparent relates to frequency, rather than a simple gray filter.
If you like, the atmosphere has a color filter rather than simple gray filter.
What I say is happening is that the air, specifically CO2 and H2O molecules, allow some frequencies through without any effect, while the frequencies they interact with strongly are absorbed and re-emitted.
In effect, the sky is transparent for some frequencies, but is a dark fog for other – specific – thermal frequencies.
What the observer sees is the ground getting cold at night because much of the energy just radiates away without being filtered. What is also seen is the lower atmosphere doesn’t get cold at all because of selective frequency absorbtion and re-emission of ground radiation.
Science Of Doom,
A very serious question for you.
I have stated on this blog that radiative processes at ‘thermal’ frequencies takes a very long time.
In particular I have stated that terrestrial atmospheric energy transfer at thermal frequencies takes minutes/hours/days to travel over relatively small distances. By analogy, it takes thousands of years for thermal energy to travel from the core of the sun to the surface.
I would like you, if possible, to independently state how long it takes energy to travel at thermal frequencies in the lower atmosphere. I believe this is key to the night-time ground cooling phenomenon..
Jerry, That’s what I said, or at least that is what I tried to do.
The graybody analogy is a transition to the spectral window explanation, about which you are right of course.
Not to preempt SoD’s response but indeed thermal radiation travels at the speed of light, its all the same thing when it comes to that. Thermal processes overall in the atmosphere do take longer because of the various mechanisms involved other than radiation.
George
Dr George,
Photons including thermal photons travel at the speed of light. They travel in gas a certain distance which averages out as the mean-free-path. This is different for different gas densities, light frequencies, and molecular type.
When they interact with matter they get absorbed and they stay put for a while – a dwell time. Then get re-emitted in some random direction.
The combination of mean-free-path, dwell time, and random emission direction means that energy – not individual photons – may travel very slowly by radiative processes in the atmosphere.
In the end, it is just the mean-free-path gradient that determines net energy flow.
What I was hoping in these concepts were discussed by Science Of Doom and actual numbers introduced so that we can see the effect of radiation compared to convective and conductive processes.
Matt J.
……”raising the internal energy of the working substance of the heat engine, by running a current through it rather than by doing mechanical work on it.”……..
Heating up the working substance electrically is equivalent to work added to the system.
Work does not need to be of a mechanical nature.
Dr George
You said…
“Yes, the only ultimate source of energy is the sun but remove it instantaneously (without blowing it up) and the atmosphere and earth’s surface will continue radiating at each other for some time before giving it all up.”
Correct.
The major point being the Sun is the “only ultimate source of energy”.
This means that nothing in the Earth-Atmosphere system can ever exceed the energy provided by the Sun without CREATING energy, an IMPOSSIBLE outcome.
The Trenberth Earth Energy Budget clearly has the Earth radiating 390 w/m^2 and the atmosphere Back-Radiation of 324 w/m^2, both exceed the Solar Energy of 168 w/m^2!!
This is CLEARLY an IMPOSSIBILITY.
——-
You said…
“You need to consider the atmosphere’s storage of energy since it has mass and it has temperature greater than 0 K. It therefore has thermal capacity and becomes an effective
secondary source of radiant energy deriving its available energy from a combination of absorbed upward IR, released latent upon water condensation, and sensible heat through convection and conduction from the surface.”
The atmosphere’s ability to store energy in no way makes it secondary source of useable radiant energy!
A capacitor in a electric circuit powered by a battery can store energy but the capacitor is cannot store more energy than the battery provides.
Further, the capacitor stored energy cannot be ADDED to the energy of the Battery to CREATE energy since ALL the Capacitor energy came from the Battery!
Remember what Trenberth’s energy budget did with the Energy incoming to the Earth’s surface:
Incoming energy at the Earth’s surface:
1) Solar Energy = 168 w/m^2 (the ONLY energy source)
2) Back-radiation = 324 w/m^2 (comes from the atmosphere that is NOT an Energy source and gets ALL it’s energy ultimately from the SUN!)
Total = 492/w^2
This is CLEARLY CONTINUOUS ENERGY CREATION.
The Equivalent Battery-Capacitor energy balance would be:
1) Energy Provided by Battery = 168 watts.
2) Energy stored by the Capacitor = 168 watts (this is the absolute MAXIMUM POSSIBLE)
Total = 336 watts…an IMPOSSIBILITY since ENERGY would have to be CONTINUOUSLY CREATED!
You simply CANNOT get 336 watts from a 168 watt source of energy!!!
—————–
You said…
“There absolutely is no energy created in the Trenberth, or about a couple other dozen similar diagrams used over the last 60 years to explain the mean earth energy balance.”
Wrong, as demontrated above!
—————–
Your previous “correct” statement was:
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
I demonstated that Trenberth’s Energy Budget Diagram and the other “Greenhouse Effect” links ALL USE BACK RADIATION TO INCREASE THE TEMPERATURE OF THE EARTH.
This is an absolute FACT that you have failed to acknowlege!
Why is that??
Further, ALL MEASUREMENTS show that Back Radiation CANNOT heat the Earth.
See my posts on the Solar Oven measurements at the Physics Dept. at Brigham Young University and NASA instruments that use CRYOGENICALLY COOLED IR DETECTORS far below the -20 deg C
atmosphere temperature to make the direct measurement POSSIBLE, and then say that this Back-Radation MUST cause the Earth to warm in response.
——————
The “Greenhouse Effect” is very clearly a fantasy.
Dr George
You said…
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface. It does, however, reduce the rate radiative cooling of the surface.”
I have already dealt with the first part “Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
—-
Now let’s talk about “It does, however, reduce the rate radiative cooling of the surface.”
The most significant part of this is that you are talking about COOLING not HEATING.
It is not possible for an object that is Cooling to heat-up in temperature.
A blanket will keep a human body warmer than it would be if no blanket were used but the human will still COOL.
Radiation emitted by a human body
“The total surface area of an adult is about 2 m^2, and the mid- and far-infrared emissivity of skin and most clothing is near unity, as it is for most nonmetallic surfaces.Skin
temperature is about 33 deg C, but clothing reduces the surface temperature to about 28 deg C when the ambient temperature is 20 deg C. Hence, the net radiative heat loss is about Pnet = 100 W.”
http://en.wikipedia.org/wiki/Black_body
Blankets can’t increase a human body’s surface temperature of +33 deg C because the body has to radiate heat energy to the colder blanket to increase it’s temperature.
The result is a DROP in the human body’s surface temperature down to +28 deg C.
It is IMPOSSIBLE for the Blanket to heat-up the human body past the +33 deg C since that would require CREATION OF ENERGY.
I’m sure you have heard the FANTASTIC claims that the Earth would only be -18 deg C if there was no “Greenhouse Effect”.
Clearly, a “blanket” cannot produce a warming of 33 deg from -18 deg C to the +15 deg C average temperature that the Earth is at.
The Earth and a “insulating” Atmosphere means that the Atmosphere will ALWAYS COOL the Earth.
————————————————–
Here is another example of why the Earth MUST cool with the addition of an atmosphere.
Why would you even think that the colder atmosphere would somehow cause the Earth’s surface radiation to slow down and cause warming?
How can a cooler atmosphere impede the Earth’s radiation when the Earth provides the energy to heat the colder atmosphere?
The Sun HEATED the Earth, and the Earth’s radiation HEATED the the atmosphere and all the Radiation is then transferred to cold space.
Let’s examine a common device known as a Heat Sink.
It is used to cool electronic devices like the Microprocessor in your computer.
The electrical power (Sun) heats the Microprocessor (Earth) just like the Sun heats the Earth.
Lets say the Microprocessor operates with (and has to dissipate) 10 watts of power (Sun) and it’s surface area is 0.01 m^2.
The Electromagnetic Field radiated by the Microprocessor (Earth) is 10 Watts/0.01 m^2 = 1000 w/m^2.
The surface temp of the Microprocessor can be calculated by the Stefan – Boltzmann Law.
Power/Area = Boltzmann’s Constant X Temp^4
Temp^4 = 1000 w/m^2 / 5.67 X 10^-8 = 1.76 X 10^10
Temp = 364 K or 91 deg C
We want the Microprocessor to drop in temp by placing a Heat Sink on it that will only be heated to 25 deg C or 298 K
A Heat Sink (atmosphere) is initially at room temperature (20 deg C) and is placed on the Microprocessor (Earth).
The Heat Sink (atmosphere) has to have a larger surface area.
Power/Area = 5.67 X 10^-8 X 298^4 = 447 w/m^2
Area = 10 Watts/447 = 0.022 m^2
The Microprocessor and the Heat Sink will now operate at a temperature of 25 deg C.
So what happened here?
The Electrical power provided is 10 Watts (the Sun).
The Microprocessor (Earth) that was operating at 91 deg C transferred heat energy to the cooler Heat Sink (atmosphere)(initially at 20 deg C) raising it’s temp to 25 deg C.
The Microprocessor (Earth) and Heat Sink (atmosphere) stabilize at a temperature of 25 deg C and 10 watts of power is still dissipated to the suroundings (analagous to cold space).
Did the Heat Sink (atmosphere)impede the Microprocessor (Earth) radiation?….Of course NOT!
Did the Heat Sink (atmosphere) cause the Microprocessor (Earth) radiation to “slow down” and increase in temperature?….Of course NOT!…EXACTLY THE OPPOSITE OCCURED!
———-
Heat Sink materials are chosen for their high emissivity.
A material that has a high emissivity will be efficient absorbers and emitters heat energy.
The largest possible value for an emissivity is 1.
If the Heat Sink were a bubble of CO2 covering the Microprocessor the effect would be the same.
The Heat Sink will reduce the temperature of the Microprocessor as long as the Heat Sinks surface areas is greater than the Microprocessors surface area.
Just like the atmosphere produces a larger radiating surface area for the warmer Earth surface and MUST COOL THE EARTH.
———————————
Summary:
– A Blanket CANNOT heat-up a body, it can prevent a larger drop in temperature but it will still COOL it.
– A Heat Sink cools by increasing the radiating surface area of the Microprocessor.
– The greater the emissivity of Heat Sink, the more COOLING it will produce.
– The atmosphere increases the radiating surface area of the Earth, so it HAS TO COOL IT.
– Adding more CO2 into the atmosphere will increase the emissivity of the atmosphere, improving the Heat Sink efficiency of the atmosphere and produce MORE COOLING of the Earth Surface.
——————
The “Greenhouse Effect” is very clearly a fantasy.
——————-
Dr George
1) You said with regard to warmer air above a colder Earth surface:
“The answer you need for Jerry is that that the atmosphere is a semi-transparent medium, often treated as a gray body. The warmer clear air over Jerry when he is getting cold still has a lower “effective radiating” temperature and allows radiant heat from the surface, emitting as almost entirely as a black body with a higher, radiating temperature. Much of the energy being transferred away from the surface is at the wavelengths where the atmosphere is completely transparent.”
This implies NO HEATING of the Earth from a warmer atmosphere.
2) Yet, a couple of paragraphs later, in the same post, you said:
“The atmosphere is not a specific radiating body per se, just a collection of molecules, each type with their own radiative identity. Those that help block the wavelength gaps that the earth’s surface uses to cool to space (even if the actual temperature of the air above it is warmer) will contribute an accumulation of heat in the atmosphere, which can then be further transferred to (or help prevent loss from) the oceans, not necessary by radiative processes, which seems to be your hang-up. Hence the earth gains heat energy as those gaps in the IR spectrum close while there is no impingement to incoming solar.”
This implies HEATING of the Earth from a warmer OR colder atmosphere.
Which is it?….Does the atmosphere heat the Earth or does it not?
——————
What you describe as “…not necessary by radiative processes, which seems to be your hang-up” is NOT MY HANG-UP.
I am describing the radiative process “HANG-UP” described by Trenberth’s Earth Energy Balance used by the IPCC:
– Where 324 w/m^2 of BACK RADIATON from a COLDER ATMOSPHERE is ABSORBED BY AND HEATS THE WARMER EARTH SURFACE!
—
I am describing the radiative processes “HANG-UP” described by the GREEN HOUSE EFFECT LINKS “HANG-UP”:
– Where the BACK RADIATION HEATING OF THE EARTH is used as a PERPETUAL MOTION MACHINE in a POSITIVE FEEDBACK LOOP.
——
And, YOU SAID….”Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
Yet, YOU JUST SAID…”Hence the earth gains heat energy as those gaps in the IR spectrum close while there is no impingement to incoming solar.”
—————————-
Just how does “…the earth gains heat energy as those gaps in the IR spectrum close..” IF “Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.” ?????
I look forward to your answer.
Gord and others who may be interested,
This is in response to your (Gord’s) last three, at latest count, comments.
I do not have the time to respond to each of Gord’s statements but the following should clear a few things up.
Among other things, there may be a terminology issue when we talk about the “earth” where we need to be clear that we are talking about the earth system as a whole or just the surface and sub-surface and whether it is in the annual mean or a specific case.. I suspect I am guilty of that slip on occasion.
Also, there is a little problem with the terms heating and cooling. Heating is the process of adding energy to a system, cooling is removing it. Temperature change occurs with the net addition or removal, given the mass (type and quantity) or constant
We, or rather those who need and can afford to, heat our homes in the winter in order to keep them at about the same temperature as in the summer when the same group of people may use cooling to accomplish the same thing. Of course while the house dweller is doing one of those the outdoor environment is doing the other. Heating and cooling occurring at the same time to maintain the same temperature. Sound familiar?
Earth does the same thing, both as viewed from space and near the surface where we live. We understand (or have formulated) that the earth maintains about a constant heat content and hence temperature, since mass is constant, that stays about the same because net heating from the sun and net loss of energy though IR emission to space. At the surface where there is also an excess of solar energy received, it too must simultaneously cool while being heated for the mean temperature to remain the same (no warming), but the options are four: conduction, convection, evaporation/condensation, and radiation, all of which work to add heat to the atmosphere, perhaps to keep it at the same temperature, sort of because it gets in the way of more efficient direct radiative loss on space bodies without atmospheres.
What needs to be understood (or admitted) is that it is not about the atmosphere trying to heat the earth’s surface, what we are really concerned about is the warming (temperature rise), of the atmosphere and the reduced efficiency of the loss of heat (cooling) from the surface (that normally balances solar heat gain), primarily from the oceans.
It is not just a radiative problem and it is not about if the atmosphere does or doesn’t heat the earth’s surface. As I see it, the main concerns are air temperature and the amount of heat stored in the ocean.
George
Dr George
You said…
“What needs to be understood (or admitted) is that it is not about the atmosphere trying to heat the earth’s surface, what we are really concerned about is the warming (temperature rise), of the atmosphere and the reduced efficiency of the loss of heat (cooling) from the surface (that normally balances solar heat gain), primarily from the oceans.
It is not just a radiative problem and it is not about if the atmosphere does or doesn’t heat the earth’s surface. As I see it, the main concerns are air temperature and the amount of heat stored in the ocean.”
I don’t understand why you would be concerned with a warming of the atmosphere.
The average temperature of the atmosphere is -20 deg C and the Earth’s average temperature is +15 deg C.
If the average temperature of the atmosphere increased by a whopping one deg C, due to all Greenhouse gas emmissions, it would still only be -19 deg C.
The heat energy flow can easily be calculated using the Stefan-Boltzmann Law and EM field Vector addition and ignoring any Earth cooling by the atmosphere due to increased area of radiation gives:
Earth temperature of +15 deg C (288 K) = 390 w/m^2
Atmospheric temp of -20 deg C (253 K) = 232.32 w/m^2 radiated up and down
The resultant EM field between the Earth and Atmosphere = 390-232.32 = 157.68 w/m^2 with a direction of propagation Up towards the atmosphere.
Past the atmosphere the resultant EM field will be the SUM of the Fields 157.68 + 232.32 = 390 w/m^2 towards Cold Space.
Note that this is exactly the same amount that the Earth would radiate to Cold Space without an atmosphere.
This complies with the 2nd Law and The Law of Conservation of Energy.
The Earth has simply heated, or rather maintained, the atmospheric temperature of -20 deg C.
If the Atmospheric temp of -20 deg C increased to -19 deg C (254 K) = 236.02 w/m^2 radiated up and down.
The resultant EM field between the Earth and Atmosphere = 390-236.02 = 153.98 w/m^2 with a direction of propagation Up towards the atmosphere.
Past the atmosphere the resultant EM field will be the SUM of the Fields 153.98 + 236.02 = 390 w/m^2 towards Cold Space….exactly the SAME AS BEFORE!
The Earth DOES NOT HEAT UP due to atmospheric heating!
—-
This is similar to heating a pot of water on a stove.
The water will heat up and heat the air above the water.
The heated air DOES NOT CAUSE the water to heat-up even more!
—————–
The Sun heating of the Oceans is an entirely different matter.
The Sun’s heating potential at the Equator is much greater than at Polar latitudes.
Even using Trenberth’s very low Sun temperature of 5778 K will produce an Earth (with atmosphere and albedo = 0.3) temperature at the Equator of 87.34 deg C!!!
The Earth’s Coriolis acceleration (rotation of the Earth and the cause of the Gulf Stream and Kuroshio currents) will ensure the warmer Equator water passes heat to the Polar regions of the Oceans.
The result is warmer Ocean temperatures, world-wide, producing increased land temperatures and an overall increase of the Earth’s average temperature.
No, Stefan-Boltzmann only gives you internal energy of the photon gas at equilibrium with the black-body at a given temperature. You can then calculate the heat flux from that, but it has NOTHING to do with any “EM field vector addition”.
Doing vector addition of the EM field just gives you the resultant EM field. But for dealing with radiant heat/energy flux, you need to average the Poynting vector; computing EM field vector sums is not a useful step to this end.
I see that my post May 24, 2010 at 2:00 am is still awaiting moderation while my previous posts and posts afterwards have been displayed.
I will therefore re-post this post in two parts:
Here is part #1 with part #2 to follow:
The “Greenhouse Effect” links I posted CREATE energy by the fantasy build up of energy through a postive feedback loop.
The “Greenhouse Effect” is a Perpetual Motion Machine in a positive feedback loop.
Heat flow from Cold to Hot or even between objects with the same temperature will CREATE ENERGY as is evident from Scienceofdooom’s own Two Identical Star calculation.
I have shown that the Trenberth Earth Energy Budget links CREATE energy because the Earth-Atmosphere system radiation exceeds the the Sun’s energy as given by Trenberth.
Trenberth has Back-Radiation being absorbed by the warmer Earth, something even Dr. George says cannot happen.
Dr. George said…
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
The depiction of the two surface IR components, both readily measurable at a given time and place but practically difficult to get global annual means, indicate net IR radiation which is one mechanism to cool the earth’s surface, necessary to remove the excess heat provided by the sun. This has all been stated several times now. The downward arrow there is not a statement of GHW, it just one of the players.
George
The “downward arrow” is Back Radiation being absorbed by the warmer Earth and heating it, something you have already said cannot happen.
Dr. George said…
“Back IR radiation from the atmosphere does not increase the temperature of (add heat to) the surface.”
Did you forget that?
But since these fallacies have been repeated so often, and not just by you, you may find that they get deleted because of that repetition. The ‘Etiquette’ tab on this website does warn quite explicitly about such repetition.
And yes, it is a fallacy. There is no violation of the 1st or 2nd Law of Thermodynamics in the standard theory of “global warming” and the “greenhouse effect”: just poor explanations.
But I am glad to see that many on this site, and S of D himself in particular, have put forth a lot of effort to address the weaknesses of the usual presentation of the standard theory.
While doing that, they have refuted your fallacious arguments even before you post them. The rest is mere repetition and tedious detail.
Funny, Part #1 was displayed but Part #2 is still awaiting moderation.
This post#2 deals with the Sun and it’s temperature as used by Trenberth.
Essentially, Trenberth has used a far lower temperature Sun temperature compared with links that I have provided.
I did several calculations that shows that the Sun temperatures easily account for the Earth’s +15 deg C temperature.
Scienceofdoom:
Did these calculations violate any posting rules on this site?
Why is my Post#2 delayed for posting?
Jerry
I realize that you have asked this question before and sorry that I haven’t answered it previously.
The question/answer seems simple conceptually but I can see that you have a particular issue with it, although I don’t understand what that is.
Like any heating question it is just the net energy loss divided by the heat capacity.
That’s not necessarily simple to work out because we need to know the radiation (and convection) from each part of the surface and atmosphere (but it doesn’t imply any mystery or any long delays in changing temperatures).
Take one meter square.. if a body with a heat capacity, mc = 10,000 J/K is radiating at a net (up – down) of 100W then:
dT=100/10000=0.01K/sec = 0.6K/min..
But of course the temperature change will reduce the upwards radiation and the temperature change will diminish. And consideration has to be given to where the heat is absorbed and the temperature changes that occur there.
The point is the limiting factor isn’t the process of radiation because radiation travels at the speed of light, it’s just the many interacting processes along with the heat capacity of all of these elements.
Having said that, I understand that stratospheric adjustment to instantaneous doubling of CO2 (in models) takes 2-3 months and I don’t fully understand why that is. I assumed that this was simply the fact that reaching an equilibrium takes infinite time (when does e^-t reach 0? Never, unless you define it as “close to zero” with a number).
What do you think is going on?
Here is a link that readers may find interesting:
http://www.topix.com/forum/news/global-warming/TDM0OT6N8UV60H9OP/p14#c269
No, not really interesting. Except, perhaps, to those of us who love to see you get your comeupppance. But such schadenfreude is really inappropriate for this forum.
For the silent ones with questions:
I started posting about this subject because many people had questions or were confused about the basics.
Gord and Bryan have written voluminously, so the reason for this comment is to see if any silent readers have questions that they don’t feel have been addressed.
From my own side, I don’t feel that there is much point in “answering” the long repetitive comments / claims / statements from Gord primarily because someone who believes that radiation measurements are fraudulent isn’t going to be convinced by more difficult science questions.
This blog isn’t about convincing “the world” about anything. It is an opportunity for people to delve into some climate science questions so we can all learn.
This doesn’t mean there aren’t questions or comments from Gord that shouldn’t be addressed – so if others, who have so far been silent, feel that particular issues need clarification or answering, here is your opportunity.
As for Bryan’s comments and claims, I have tried to pin him down over a number of posts (see start of next article for these links) with little success.
But again – if others, who have so far been silent, feel that particular issues need clarification or answering please go ahead and ask.
I will also be posting a similar comment on the Intelligent Materials article.
I see that Gord makes the claim on another blog that he is being “blocked”.
There are currently 217 responses on this article. Gord arrived and made his first comment about no 95.
Since then 43 comments from Gord, which makes him 43 out of 122 comments or 35%.
Looking at “realestate” there are 85 “screenfuls” (judged by my monitor) since Gord’s first comment and approximately 42 screens are consumed by Gord, say 50%.
Given:
– the amount of space Gord has been given on this blog
– the amount of time and effort many people have gone to in answering his comments
– the disrespect he has shown to others
..I am not inclined to look through the spam filter to find the comment(s) that have been trapped there.
If someone has 43 screens full of comments on one post and yet goes crying on other blogs about how they are unwelcome they probably don’t deserve any tissues.
And they don’t deserve time and effort from other people.
Thanks SoD. I believe we must have been dealing with “confusion by choice.” It’s hard to believe that someone could be that wrong and persistent by accident. But then again, I may be a little naive when it comes to this media.
George
Dr George,
I make no judgement about people’s motives. Some people have questions. Some are very confused yet convinced they are right.
It’s easy to see the genuine questions and these are always worth responding to.
For the “very confused yet convinced they are right“, the real value in responding is primarily for the many “watchers”. There is perhaps a 50:1 or 100:1 ratio of watchers to commenters.
This is the important point to remember, and hopefully many like yourself will continue to offer assistance despite the antics of a few.
SoD,
Yes, I did consider refraining from using the term “wrong” in the admirable spirit of this site. I do understand that being told a person is wrong will cause some to become more resolute out of overwhelming concern that being wrong is not acceptable or there is a mission to accomplish. I’ve been wrong a lot and that is one way I learn. I came to this site to learn and one of the things I need to learn is how to better communicate and convey things I think I understand, so, maybe first lesson learned. There are too many “I”s in this message so will be looking forward to future exchanges on the science. Right now much of my available time, which isn’t much, is being spent looking through existing posts to better come up to speed as to just where you are on this journey. Keep up the good work.
George
Re: Confused and convinced
Gems I’ve plucked from the ether…
“He lives in a logic-free zone”
“There are two types of people – those who try to win in life, and those who try to win arguments. They are never the same.”
Amen.
Scienceofdoom:
A couple week ago I made the following post:
Your additive energies, [equations 2 & 3] seem to be at odds with my understanding of radiant heat exchange. From A Heat Transfer Textbook v3 by Lienhard:
Radiant heat exchange between two finite black body is given by:
Q1=A1F1(eb1-eb2) Where Q1 = net loss of energy to surface 2 (if negative, gaining energy)
A1 = area of surface 1
F1 = view factor of surface 1 to 2
eb1 = back body flux from star1
eb2 = back body flux from star2
As you can see Q1 equals zero based upon your problem criteria. Applied to equations 2&3 E1′=E2′=E1=E2…no increase in temp.
To which you replied: “In my example also the net heat exchange is zero. “ and “Why not find the flaw in my calculations? If your equation demonstrates that my result is wrong, it’s just a matter of pointing out which step is incorrect. “
In order to test your equation and better understand the variables and corresponding relationships over a myriad conditions I “spreadsheet-ed” your formulas. I went so far as to make variable radii, distance between stars (d=2r) min, start temperatures and emissivities. My results confirm yours…and go further. I was most interested in T1‘ and T2‘ with contrary inputs. That is, make one star large and hot while the other is small at 1K. Keep them 1000 km apart or bring them cheek to cheek; regardless of the conditions the results seem to validate your supposition.
However, when I varied e1 and e2, outcomes became less comfortable. For instance, with parameters set as you outlined, reduce emissivities equally to any value greater then zero… T1‘ and T2‘ remain unchanged. Take it step further, as ε1 becomes >> ε2 T1‘ inches closer to T1 while T2‘ >> T1‘. Not a flaw in your calculations but highlights an inaccuracy formula conception. But maybe, the manipulation of ε1 and ε2 is beyond the purview of equations 2&3. After all the set up specifically outlines black body.
Not satisfied I moved on to the time relevant argument posed by both Edward and Gord. That is, without a known time component equilibrium/present energy level is forever unknown and always increasing. Well let’s work it out
As detailed in your set up, what exactly is happening? In the instant the stars become in proximity quantum E1 b is added to E2 similarly E2 b is added to E1. Next instance t1 quantum (E1 + E2 b)b is added to E2 followed t2 [E1 + (E2 + E1 b)b]b + E2 and so on. The expansion is as follows:
E1 ‘= E1 + E2 b E2 ‘ = E2+ E1 b
E1” = E1 + E2 ‘ b E2” = E2+ E1 ‘ b
E1” = E1 + (E2+ E1b)b E2” = E2+ (E1 + E2 b)b
E1”’ = E1 + E2 ” b E2”’ = E2+ E1”b
E1”’= E1 + [E2+ (E1 + E2 b)b]b
E2 ”’=E2 + [E1 + (E2+ E1 b)b] b
E1 ”’ = E1 + E2b + E1 b^2 + E2 b^3
E2 ”’ = E2 + E1b + E2b^2 + E1b^3
and so on until
E1 ”’n= E1+(1+ b^2 + b^4 … b^n+1) +E2(b + b^3 + b^5 … b^n)
E2 ”’n= E2+(1+ b^2 + b^4 … b^n+1) +E1(b + b^3 + b^5 … b^n)
if E1 = E2 then we get the Taylor series
E1 ”’n= E1+(1+ b^2 + b^3 +b^4…+b^n)
E2 ”’n= E2+(1+ b^2 + b^3 + b^4…+b^n)
E1 ‘= E1(1/1-b)
E2 ‘= E2(1/1-b)
This too seems to support your supposition. On what leg do I a skeptic have left to stand…the conceptual one:
Suppose a transparent sphere r=5000m encompasses both star. With the stars at a distance of 8000 m the flux derived temperature of the sphere 532.4 K. Now bring the stars together d=2r that same derivation results in a temperature of 541.1 K…really hard to believe.
Or if in your scenario, the 1000 K suns are instantly taken from infinite distance to 10% impacting contact. Since both instantly conduct heat, no hot spots, equilibrium temp is 1000 K. What if they were maintained at d=2r, then impacted, I would suggest equilibrium temp is again 1000 K.
Which will stay cooler longer, a gallon of ice cream at 255 K alone in a thermos (non reflective interior) or two gallons at 255 K at distance just shy of contact? What if I vacuumed sealed the thermos?
My point is not obstinateness it’s a nagging senses imbalance. Regardless the method of heat transfer the end state, the equilibrium should be the same. Nevertheless, thanks for forcing (pardon the pun) my understanding of proponents arguments.
Re-post of the expansion, top portion, formatting issue:
E1 ‘= E1 + E2 b
E2 ‘ = E2+ E1 b
E1” = E1 + E2 ‘ b
E1” = E1 + (E2+ E1b)b
E2” = E2+ E1 ‘ b
E2” = E2+ (E1 + E2 b)b
E1”’ = E1 + E2 ” b
E2”’ = E2+ E1”b
E1”’= E1 + [E2+ (E1 + E2 b)b]b
E2 ”’=E2 + [E1 + (E2+ E1 b)b] b
E1 ”’ = E1 + E2b + E1 b^2 + E2 b^3
E2 ”’ = E2 + E1b + E2b^2 + E1b^3
and so on until…
[…] imaginary second law movement isn’t a monolithic voting block. Others may not agree, as in Radiation Basics and the Imaginary Second Law of Thermodynamics where a new advocate championed the idea that the radiation from the colder body never reaches the […]
J. Lanier,
If you enclose the stars in a very large box and measure the integrated flux at the surface of the box, that flux will be constant for whatever the distance between the stars. But mash the stars together, or even get them very close, and the total surface area seen by the surface of the box will decrease because it’s blocked by one of the stars. But the flux to the enclosure must remain constant. Solution, the surface temperature increases.
“But the flux to the enclosure must remain constant. ”
Isn’t this the crux of the debate. Proponents, and the formulaic math outcomes seem to support, that flux emitted by both stars increases the closer the proximity? Therefor the flux to the box also increases…the 1-b portion of a larger flux?
Once in contact with one another, the superconductive shell will dictate temperature be based upon internal source….?
Let me try again;
Once in contact with one another, the superconductive shell will homogenize temperature, based upon internal sources i.e. 1000 K….?
There is an alternative formulation of the “Imaginary second law of thermodynamics”. According to this alternative formulation, as entropy must increase … living indivduals can not exist … I mean this as a joke, but this argumentation is similar to the one “radiation does not flow from a cold source to a hotter one”. Perhaps we can use this argumentation in order to try that people using the “Im2ndLaw” understand their error. Just my two cents.
I have read several good stuff here. Certainly price bookmarking for revisiting. I wonder how much attempt you set to make such a great informative website.
Scienceofdoom 18 months later
The new higher equilibrium temperature is a mirage. The two bodies emit radiation as well as absorb it. At the elevated new equilibrium, power output exceeds internal power input causing the temperature to fall back to the original. Nevertheless, the whole episode was a fascinating read. Thank you all.
[…] as what the textbooks say.. Check out ScienceofDoom.. Get this from another skeptic.. Quote: Radiation Basics and the Imaginary Second Law of Thermodynamics | The Science of Doom The equation for how much radiation is emitted by a body – εσT4 – does not […]
Dear, oh, dear
Blackbodies CANNOT be heated by radiation of the same frequencies they produce. Blackbodies absorb all radiation, but only convert high frequencies they can’t re-emit into heat energy. All other frequencies are re-radiated. And this must be so for the second law to hold.
Here is a thought experiment:
Imagine a 300K blackbody encased inside a perfect mirror, itself at 300K. The blackbody receives its own radiation reflected in the mirror. It also receives radiation emitted from the mirror, which is glowing at 300K. The blackbody thus receives more than twice the radiation it is putting out. HOWEVER IT WILL NOT HEAT UP.
A mirror that reflects all incoming radiation of some particular wavelength cannot emit anything at that wavelength. The sum of emissivity and reflectivity is never more than one. This is the reason for the fact that a mirror cannot heat the body when both are at the same temperature.
dearme,
Or to put it another way: A perfect mirror is a perfect insulator. Any object without an internal energy source inside a perfectly reflecting container can neither warm nor cool.
This is intense. I read somewhere, I believe jerry said, that 2 bodies in the artic would freeze being separated and wouldn’t if together. Poor example or not, they will still freeze, and neither will exceed the body heat of a normal person, unless somebody starts a fire. The point of 2 stars in close together cannot exceed the initial temperature unless we consider other heating properties beyond thermal radiation. That would be the correct math. If you come to a different number that’s higher it’s wrong. (there is a formula for that) Then you can get really on the edge thinking about when thermal heat and light reach an intense enough strength, they might become more like waves in a pool of water. How would I know, this makes my head hurt. Of course there is nuclear fusion and runaway lighting bolts. If any body has any idea of how the two are related. I’m sure you do. We can both all be right and be wrong at the same time.
But not here, time only flows in one direction. And much like energy of any sort, back radiation would occur where there is an impedance mismatch. For example, 4 lanes of traffic to 2.
rishrac,
Think of it like this.
No internal heating, i.e., not stars as we know them
In the case where 2 bodies have no internal heating. This is probably how most people confused about this subject are thinking – they are considering the classic cases of thermal equilibrium.
a) The bodies are at 1000K and there is no “cooling to space” because they are in an isolated (insulated) environment and at TE (thermal equilibrium), then they will not change temperature.
b) If the bodies are at 1000K and there is “cooling to space” because they are not in an isolated environment, ie, they are not at TE, then they will cool down to the background temperature of space (3K). At that point (after infinite time) they will be at TE and at 3K.
With internal source of energy, i.e., they are stars
c) The stars have internal source of energy inside them.
It are cooling through their surfaces to space which has a background temperature of X.
Therefore, they are not at TE.
Suppose you increase the background temperature to the point where X = 1000K. Would the surface temperature of the star increase?
The answer is yes.
If you surrounded the star with space with X=900K. Would the surface temperature of the star increase?
The answer is yes.
The simple reason why
It’s just the same as working out the external surface temperature of an oven which has fixed internal heating when the oven is placed in a cold room.
The surface temperature of the oven is 40’C and it is in “steady state” – that is, it has settled at 40’C. The cold room, with a much larger heat capacity than the oven, has a temperature of 0’C.
Now we increase the temperature of the room to 35’C.
There is no question that the surface temperature of the oven increases.
The reason it is at 40’C at the start is a balance between internal heating and external cooling rates.
That is, Energy in = Energy out. Therefore internal energy change = 0, therefore temperature change = 0.
Now we increase the surrounding temperature to 35’C – therefore, we have reduced the cooling rate (cooling rates are dependent on the temperature difference).
This means that less energy per second is removed. But we have the same heating rate.
In this second case, Energy in > Energy out. Therefore internal energy change >0, therefore temperature change is positive.
So, amazingly, with an oven with a surface temperature of 40’C at an external temperature of 0’C, increasing the external temperature to 35’C (less than 40’C) means that the surface temperature goes up and reaches a new “steady state” >40’C.
This is not amazing for anyone who studied their first undergraduate heat transfer problems (and got them right).
The simple reason why so many people are confused about this
Because they are thinking about TE problems not textbook heat transfer problems (which I doubt anyone who is confused ever studied and understood).
TE problems are bodies in equilibrium with their environment. Everything is at the same temperature. There is no internal source of heat and no cooling to a different temperature environment.
Clearly, if you put one body in an isolated system and let it reach thermal equilibrium which turns out to be 1000K, and then somehow add another body at 1000K into this isolated system they will finish up at the same 1000K.
If you put the second body in at 900K and both have the same heat capacity, then, once they reach thermal equilibrium they will be at 950K (assuming the isolated environment walls have zero heat capacity).
Please ask if this is not clear.
Clearly there is something wrong with the models or else the observational data would agree with the models. They don’t. They haven’t. Correlation between co2 levels and increases or decreases in temperature have yet to be established and to what degree. If anything, the last few years have thrown considerable doubt on that relationship.
One other thought, ever work with co2? Have you ever made snow with co2? It doesn’t go away when it should. I could see a case where just the opposite happens, more co2 leads to global cooling via a negative feedback. Water is really such strange substance.
I digress.
I am not confused at all by heat transfers or energy transfers or how energy gets reflected back or background noise, interference patterns, random standing waves and a whole host of strange things. I wouldn’t mind discussing some of the more unusual stuff, but the environment for sharing this information has become tainted.
You are approaching this issue with the knowledge and information that you have available to you. You may not be able to see what I see, or my failing to properly communicate it to you, or your unwillingness to accept any thing other than what you know.
rishrac,
Let’s take it as read that there is a lot wrong with the models, so we can focus on one or two items, like the claims of this article, or the points I made in the above post that were nothing to do with climate models and nothing to do with CO2. The article, your original comment, and my reply, are all about heat transfer basics and how the equilibrium temperature of a body is changed by the presence of another body or the background temperature changing (effectively the same thing).
So the right way to find out the answer to the specific problem is for you to:
a) explain what is wrong with the equations in the article, and/or
b) show a different solution
Or
c) an alternative approach for you is to set out a relevant equation (or set of equations) with appropriate definitions to demonstrate that the solution above contradicts another well known law – defined with equations.
Or
d) show what is wrong with my example of the oven.
In your original comment you stated:
“other heating properties” – The star is heated internally by nuclear fusion.
“If you come to a different number that’s higher it’s wrong. (there is a formula for that)” – please show this equation.
Since we’re best buds and all, p-l-e-a-s-e, pretty please with sugar on top, not theoretical but actual, when you achieve a number greater than 1. Don’t tell anybody else just me. It doesn’t even have to be a lot over, just a little tiny fraction. You can do it!!! I know you can, it would solve so many problems. But just tell me, I want to be rich beyond my wildest imagination. .. like either of those events would ever happen. It truly would be unity.
A long time ago there was a magazine called Chemtech. I read every issue cover to cover. I remember one of the articles, (I actual remember most of them) and in it the discussion turned away from the purely technical aspects of chemistry to the more philosophical aspect, since there wasn’t much else to discover about chemistry itself. Physics was an entirely different matter. Sometimes you have to lay down the slide rule and look at the problem in an entirely different way. Climate science has been a nightmare. Not because it is complex, but because of a whole host of issues that has little to do with science. I have not been treated with respect, but more like, “here let’s take you by the hand, your stupid and don’t know anything. ”
Never, never, ever, ever has any project been close to 1. Besides the promise of nuclear fusion. 2nd law stands in this case no matter how much you try to prove otherwise. You can call it what you will, won’t change anything.
And you don’t know the formula for this… hummmmm, makes me wonder. Well, back to some real work… cold fusion, (you’d be surprised at who is working on it still) and if I can figure out this Bendini motor. Work on the stupidest stuff, but hey, let no stone be unturned. All I need is one. (and just a little more)
The problem of giving you a formula has been that you will debate it to death. Done that, don’t want to go back. Your a smart person, look it up yourself. And as I stated earlier, this issue is a side issue that has taken up a lot of time. “A thirsty man digs his own well”.
Well, I’m not done yet. I think the final analysis is well founded that co2 is not the climate driver that they think it is. Or if it is, the explanation is bad, not to mention all the stuff that has been reported to support it. There is a difference between making a mistake and doing something that isn’t right But then, who in there right mind would throw away original data? we’ve descended into skeptic and not critic. A critic is your best friend. A family member will cheer you on, point out your good points, and make no mention that you sing like a cat on a hot night.
Throwing out the original data. At Princeton Uiniv, they have a sort of museum in the physics department, or did. Who knows what’s been done with all the building going on. But one of the things that struck me was an early battery. each cell was carved out of wood. I just thought wow, that is really interesting that they thought this up when there was nothing. I’m glad they didn’t throw it out.
Unlike words, full of precision and clarity.
Perhaps you can scour this blog to find examples where I have “debated commenters equations to death”.
You won’t find them.
The many many people like yourself who are sure there is “something obviously wrong” with the many simple examples never provide an equation.
You will find many many examples where I have hounded commenters to provide just one equation, please, just one!! All in vain.
Silent readers can draw their own conclusions once again.
I did.
If you aren’t interested in presenting anything scientific then no point me responding. No way for me to point out any flaws in your argument. No way for you to point out any flaws in my argument. Other than “you can’t get more than 1”, whatever that means. Or the fact that you read a series of journals. That’s fantastic, but doesn’t constitute a proof, a critique, or anything.
Even without equations you haven’t critiqued the examples I provided above, or explained why a heat transfer system in non-TE conditions should get the same result as a TE problem.
I invite you again to critique the simple example of the oven. How did the surface temperature reach 40’C? What is special about 40’C? Nothing. It’s just a steady state solution to the internal rate of heating and external rate of cooling. Reduce the rate of cooling and you increase the surface temperature. It’s just the first law of thermodynamics.
For new readers, it’s not about this specific problem, but take a look at “Blah blah blah” vs Equations .
Another example is provided in The Three Body Problem – a simple example with three bodies to demonstrate how a “with atmosphere” earth vs a “without atmosphere earth” will generate different equilibrium temperatures. This example includes entropy calculations to prove that the 2nd law of thermodynamics is not violated.
There’s a reason why no one ever provides entropy calculations to show “this can’t happen”.
One other thought, this topic was started when it became evident that the amount of heat transferred during a rainstorm was large enough to offset the warming provided by increases in co2. The heat became retained rather than released. This line of debate is a side discussion from the discussion that injecting co2 was a regulator of temps. The only way for runaway global warming to happen at this point was that the heat had to be retained rather than released. So without a impedance mismatch or an index reflective that would do that, there is not much left of the argument. And since a colder body cannot transfer heat to a warmer one, what would you call that?
To be sure technically, there is an impedance mismatch, but it doesn’t allow for back radiation. It’s like traffic on 2 lanes becoming 4 and then 8. Additionally, there are less molecules per cubic meter, the pressure is reduced, and the dimensions are also increased. Because the air is generally more transparent, telescopes are situated on mountains, there is less internal reflection from a gradient that works similar to fiber optics.
If you place two stars of equal temperature, size, and rate of energy production in proximity, they will both become hotter. You have to consider at the very least all three of these factors. This is not a result of violating the laws of conservation, but the simple result that you change the rate at which the sum of the energy generated by both planets leaves those planets when considered as a single system.
This should be very obvious and simple to any thinking scientist, and I’m just a highly informed amateur. The idea that either one not getting hotter or you’re are violating the law of conservation is absurd! It ignores that the energy is created by nuclear fusion and has to radiate into space at some rate, which is very much dependent on the effective surface areas of the system as a unit that is exposed to that portion of space that is not sending radiation to either star.
What is the run away temperature in a star? Is there a limit? Replace ‘star’ with a pan of water. Then will the water grow colder if not supplied with additional heat? Are we measuring the mass of 2 separate 1 kg of water at some temperature (either the same or different) at stp, then we can know the rate of cooling by the distance between the pans. In no case will the pans become hotter without additional heat. So now you are thinking, ah yes, if we insulate it that will change the rate of cooling and if everyday we add heat, then it will get hotter and hotter. So again the issue comes down to released or retained through water vapor. And then another theory, the death of the universe. Now if there is quantum physics involved or multi universes or some other dimensions that aren’t directly related, then for most everyday stuff 2 nd law comes in pretty handy.
Since this conversation is related to the science of climate, where is the exceptional heat we should be having? That’s what somebody is trying to prove. Isn’t it? Science of doom is missing something in his formulas.
Sorry SoD, but you are confusing the emittance of a radiating body with a real energy flux. Real energy transfer rate from body 1, T1 to body 2, T2 = ∆[emittance]. The single S-B equation gives emittance.
This is why climate alchemy has failed.
The Law of Conservation of Energy applied to matter and the EM aether is:
qdot = – ∇.Fv where Fv is the monochromatic radiative flux density and qdot is the monochromatic heat transfer rate per unit volume of matter. Integrate this and you get the above.
And you are making a distinction without a difference.
Qdot requires that there be two objects at different temperatures. The S-B equation is only equal to qdot if the other object has a temperature of absolute zero. Do you disagree that qdot for infinite parallel planes, to avoid view factor complications, at temperatures T1 and T2 is σ(T1^4-T2^4)? How is this different from subtracting the two single temperature S-B equations?
Alec
Don’t know what point you are trying to make.
Emission of radiation = emissivity x the Stefan-Boltzmann equation.
Emittance usually means emissivity.
Emissivity is well-defined. It is a function of wavelength and direction and is the proportion of radiation emitted compared with a blackbody at that wavelength and direction.
I didn’t use the term emittance in the article.
I did use flux, which is also a well-defined term.
Whatever that means.
This article, written a long time ago, tries to explain some basics without being really technical.
Take a look at Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations and explain what is wrong with the actual equations of radiative transfer.
Or explain what climate alchemy is.
Or which term in the above equations is actually wrong.
Climate basics agrees that net energy transfer by radiation is the radiation emitted minus the radiation absorbed. Everyone knows that radiative transfer is the term you cite.
So I think you need to be actually clearer about what point you are trying to make.
Your first statement, ‘Emission of radiation = emissivity x the Stefan-Boltzmann equation.’ is only correct if by ‘radiation’ you mean ‘Radiant Emittance’ or ‘Exitance’.
The latter is the Power per unit Area radiated by a surface at a given temperature to an enclosing sink at Absolute Zero, i.e. it is the Potential Radiant Power per unit Area from the emitter. When this impinges a plane in Space, the Potential Radiant Power per unit Area incident on the plane is called the Irradiance.
Power per unit Area is an Energy Flux. To get Net Real Radiant Flux at a plane, you calculate the vector sum of the opposing Radiant Emittances, the Potential Radiant Energy Fluxes from two or more emitters. The vector sum incorporates what we engineers call the View Factor.
The only time Net Real Radiant Flux equals Potential Radiant Flux is when there is zero Radiant Emittance from the second enclosing emitter, i.e. it is at Absolute Zero. Heat Flux from a Radiant Emitter to a second Emitter is the negative of the Net Real Radiant Energy Flux.
You are correct to state ‘net energy transfer by radiation is the radiation emitted minus the radiation absorbed’, which is why Climate Alchemy’s ‘Back Radiation’ or the Radiant Emittance of the Atmosphere to the Earth’s Surface, for a normal temperature gradient, cannot transfer any energy to the surface. The Real Net Radiant Energy Flux, Surface to Atmosphere, is [Surface Radiant Emittance minus ‘Back Radiation’], 63 W/m^2 according to the 2009 Trenberth et. al. ‘Energy Budget’.
Climate Alchemy refers to the desperate attempts using multiple runs of the climate models to predict global warming, as Alchemists of the past repeated the same experiment in the hope they would find the Philosopher’s Stone!
Glad to be of assistance!
PS to summarise, the single S-B equation does not give Radiant Flux but Radiant Emittance, the Potential Energy Flux to a sink at Absolute Zero. This failure to understand Radiant Emittance is not real has poisoned the well of Atmospheric Science for ~50 years.
The fundamental problem, easy for us engineers to detect, is that the boundary conditions of the heat transfer in the climate models are wrong.
The modellers (1) don’t understand you can only apply the two stream approximation to the gas phase and (2) by assuming a single -18 deg C OLR emitter operating over 360 degrees, falsely claim a negative Radiant Emittance approximately equal to |OLR| down from ToA. The net result is ~40% increase in energy, a Perpetual Motion Machine of the 2nd Kind. Others besides me have stated this point.
SoD: It’s hopeless. It’s been pointed out to AlecM many times. I tried here:
I got no response from him.
Read the above; you will find I am correct.
The formula proposed by AlecM is simply wrong. That it’s totally wrong can be seen by considering the case of two parallel planes with vacuum between the planes (assume also very large planes close to each other). The radiative flux is constant everywhere in the gap. Thus the divergence is zero, but heat flux is definitely not zero.
The equation is also meaningless as it implies that the heat transfer rate is a scalar quantity without any direction.
Formulas of that type apply to some other physical problems. It does not apply to this one.
The Net Real Radiative Energy Flux between two parallel plates at the same temperature in a vacuum with a short distance between them, View Factor ~1, is very nearly zero, as is the Divergence of the Monochromatic Radiative Flux Density integrated over all overlapping wavelengths that can be emitted and absorbed by each emitter.
In wave terms, you have on average for each wavelength a standing wave of amplitude twice the amplitude of the Monochromatic Radiant Emittance, i.e. for each wavelength, the net Poynting Vector is zero.
Thermal incoherence imposes on this standing wave a travelling wave of amplitude up to +/- 4x that monochromatic amplitude, oscillating about zero.
Glad to be of assistance!
Imprecise wording; it should be:
In wave terms, you have on average for each wavelength a standing wave of amplitude twice the amplitude of the Monochromatic Plane Wave in the Emittance, i.e. for each wavelength, the net Poynting Vector is zero.
AlecM,
What I was referring to is your expression qdot = – ∇.Fv. That expression is meaningless as it’s dimensions and scalar nature don’t make sense. The other point that I made on that equation is that according to it the net radiative energy transfer between two large planes in vacuum of different temperature would be zero, because Fv cannot vary in the vacuum that separated the planes. Thus the derivatives, including the divergence of your formula are zero.
Poynting vector is formed from electric and magnetic fields. When radiation is formed from IR photons, the Poynting vector must be calculated separately to each photon, because the photons are emitted and absorbed independently. Adding up the fluxes does not give results that would agree with any of your explanations.
You are inventing your own formulas that have nothing to do with real physics. You use also words that have well defined meanings in physics to mean something totally different from their standard usage. Often your concepts are so badly self-contradictory that they cannot really mean anything. The above formula I took from your comment is an example of that.
qdot = – ∇.Fv is from ‘Goody and Yung, ‘Atmospheric Physics, Ox. Ac. Press.
It is the expression of the Law of Conservation of Energy between the Material and Electromagnetic domains.
qdot is also a differential.
Heat transfer is scalar: ‘The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system.’
https://en.wikipedia.org/wiki/Heat
AlecM,
Now I understand, what the equation is about. That was difficult to see, because your concepts are so confused.
That equation tells, how much net energy is locally absorbed or emitted in the atmosphere (or other intervening material the radiation is going through) at each point. That has almost nothing to do with the amount of energy transferred between the two bodies separated by some layer of air.
Integrating that tells the total net absorption or emission of the intervening media. As energy is conserved this amount of energy must lead either to a change in the temperature of the intervening media or be balanced by other forms of energy transfer. In the atmosphere the other form of energy transfer is convection of sensible and latent heat.
That Law of Conservation of Energy when integrated at a plane and over all wavelengths gives the following: Qdot = – V.∆[Emittance], the same as you get from the S-B route. V is the View Factor.
This is what engineers use to predict radiative heat transfer. Emittance has in it the individual emissivities.
The real issue is that the Net Real Radiative Energy Flux is set by the Net radiation Field, not the difference of photons being blasted out of the two emitters, with the excess photons bouncing back!
Photons don’t exist except at the instant of energy transfer to or from the EM domain. Poor old Max Planck hated the concept. I was taught by a man who was a post-doc under Planck in the 1930s.
The underlying issue is Auguste Prévost who in 1791 devised his Theory of Exchanges, the idea that Radiated Energy was emitted from a body at a rate solely dependent on its temperature. This has confused people ever since!
AlecM,
Max Planck worked mainly before Quantum Mechanics was fully developed. (He lived long enough to see also most of non-relativistic QM, but not QED.) It may well be true that he was not satisfied with the original derivation of his radiation law, because the derivation was ad hoc, and not based on any full physical theory of his time. The situation is very different now, when first Quantum Mechanics came out and then Feynman and others developed Quantum Electrodynamics (QED) to fill the remaining gaps in the theory of electromagnetic radiation.
Pierre Prévost is really ancient history (1809) in the understanding of radiation. Why do skeptics so often refer to so outdated knowledge?
Presently we know very well that photons allow for the best description of radiative heat transfer, and that the theory based on them is one of the most precisely verified theories of physics, possibly the most accurately verified of all.
Photons do exist in the sense that they transfer energy from one location to another. What “exists” means in QM is somewhat different from what “exists” means in, what we observe directly, but the physics that has been verified as accurate depends essentially on the concept of photons that are created at one location and absorbed at another.
Looking at the “net radiative field” is a failing proposition, perhaps correct in principle, but hopelessly difficult to apply. It’s, however, known that when correctly applied it must produce the same results that the description based on photons produces much more easily.
In terms of the QED the “reality” of photons is linked to the quantitative dominance of the simplest Feynman diagrams, which include only one photon. If more complex diagrams would dominate, the situation would be different.
It’s Pierre Prevost, not Auguste.
And Einstein wasn’t thrilled with quantum mechanics: “God doesn’t play dice with the world.” So what. Not believing in photons will likely get you banned here.
Yup; got Pierre wrong. The date is 1791 though.
You are right that photon and net radiation field theories should match. However, Young’s double slit experiment with single photon capture proves wave particle duality AND that the photon is only evident when the energy quantum is transferred!
My real sticking point is that I too have imagined streams of photons being emitted from matter in isolation but when you have two emitters, what do the ‘excess’ photons do?
I thought about them bouncing off filed sites but you might as well have them not being produced at the emitter to get the same effect. In that case, you must invent a method by which the photon sink, the cooler body, communicates it has spare Planck dissipative harmonic oscillator sites of the right energy to be filled, and the warmer body duly obliges by firing a photon in the right direction!
To solve that i’m learning Quantum Field Theory; my current idea is that in a net radiation field, after the quantised energy transfer to the cooler body, quantum holes are produced which travel at the speed of light to the hotter body. Seems logical.
Alec,
I link here some Feynman diagrams to make my previous comment more clear
Electromagnetic interaction is so weak that the simplest diagram top left dominates. All more complex diagrams like the rest shown are much less important. The dominance of the simplest diagram tells that we get the right quantitative results by thinking that all IR and shorter wavelength radiation consists of independent photons created by one charged particle at one point and absorbed by another charged particle at another point. That charged particle transfers then the energy to other nearby particles. In case of CO2 in the atmosphere the charged particle may be the carbon nucleus that starts to vibrate relative to the nearby oxygen nuclei until the energy is later transferred in a molecular collision to translational thermal motion of molecules. More generally the charged particle is one of the electrons or nuclei of the matter that emits or absorbs radiation.
The dominance of the simplest diagram tells also that each photon behaves as if it would be the only photon, diagrams like to bottom right one involve several photons, but have a very small influence in situations like that of visible and IR radiation in the atmosphere (in lasers the situation is different).
Single photons form interference patterns. They have many properties of waves, but they are still practically independent of all other photons. Therefore it’s right to count separately photons that are going up and down or absorbed and emitted. Therefore it’s highly misleading to think that the temperature of a body would affect significantly which photons get absorbed and which not.
It turned out that the graphics was not shown on this site. It can be seen through this link
AlecM,
No, it doesn’t.
Hence, the concept of a quantum photon hole is a non-starter.
AlecM,
Assume we’re all a little thick here. Please provide either a citation or a link to the proof that the Laplacian (I’m assuming here that ∆ is the Laplace operator) of the emittance equals σ(T1^4 -T2^4) for infinite parallel planes at T1 and T2 separated by a vacuum.
Then you can show how the integral (dν only?) of the divergence of the monochromatic radiative flux density Fν becomes the Laplacian of the emittance. i’m also assuming that ν is meant to be nu or frequency, not the letter vee.
The Delta means difference of.
SoD agrees with my interpretation by agreeing that Net Real Radiative Energy Flux is the difference of the opposing Emittances but he mistakenly thinks these are also Real Fluxes.
Only the net Radiation Field transmits energy.
AlecM,
Again: That’s a distinction without a difference.
Consider two infinite parallel planes with emissivities of identically 1 separated by a vacuum at equal temperatures T. Qdot equals zero. But there’s still a photon gas with a blackbody spectrum of temperature T between the planes, unless like someone who won’t be named to avoid moderation but who’s initials are C and J, you don’t believe in photons. Those photons are constantly being absorbed and emitted. Now instantaneously reduce the temperature of one wall to absolute zero while maintaining the other wall at T.
Does the rate of photon emission of the wall at T change? No, it doesn’t. However, if I’ve reasoned correctly, the total energy of the photon gas is reduced by a factor of two, but the brightness temperature doesn’t change. My logic here is that effectively the emissivity of the system has been cut in half.
Now let’s set the temperature of wall 1 to 300K and wall 2 to 200K. What’s the spectrum of the photon gas? I can say some things for certain, It isn’t the difference of the two spectra and it’s not the spectrum of a blackbody at 250K either. In principle, then, the two stream approximation should be testable by measuring this spectrum. I have no idea if it’s ever been done. Probably not, as the result is likely uninteresting and it wouldn’t be trivial to do.
Sorry, photons only exist at the instant of conversion from heat to EM energy. EM energy obeys the Laws of Geometrical Optics. Energy transmission is by Poynting Vectors. Radiation Pressure is the time averaged PV/c.
If you suddenly reduce the temperature of one plate, the vector sum of PVs at the zero temperature plate suddenly increases from zero to the black body level, the maximum possible rate of energy and momentum transfer.
The idea of a photon gas can be considered to apply to the thermal incoherence. But that averages zero PV and momentum transfer. CJ is right; my ideas are identical but easier to comprehend.
Alec,
In QM and QED the existence of photons cannot be discussed in terms of classical physics. What’s important is that the description is chosen in the way that gives correct results in actual calculations needed to answer specific questions. It has been shown very thoroughly that a right approach (and in practice the only working approach) in questions of radiative energy transfer is to consider photons as particles that exist in the same way as other particles from the moment of emission to the moment of absorption.
You are free to discuss ontology of photons as long as you end up with formulas that give correct results, i.e. as long as you get the results you get considering photons as real particles emitted at one point and absorbed at another, when the issue is radiative energy transfer. Some other physical questions require different (or supplementary) considerations.
AlecM,
Two words: quantum entanglement.
Explain that with Poynting Vectors.
I should say: Explain that with Poynting Vectors that are not otherwise identical in all respects but name with photons.
SoD,
AlecM wrote:
Isn’t that a direct violation of the Basic Science is Accepted rule?
DeWitt,
I haven’t been following the discussion well enough.
As you know I have zero interest in discussing whether basic physics is correct.
It is entertaining that so many people convinced climate science has lost its way cite what CJ teaches as “support”. CJ teaches something completely opposite to basic physics and the many adherents who show up here don’t even realize that.
CJ believes photons don’t exist. However, I’m sure that not everything CJ believes is wrong. To take a trivial example, 2+2 =4 is something all of us accept. At some point there is a divergence.
AlecM believes photons do exist I think, although I’m not interested enough to reread the exchange. But these photons, according to AlecM, are different from standard physics accepted photons.
When I get a moment and sufficient interest I will review.
There’s nothing in atmospheric physics (“climate science”) that is in any way divergent from the physics in heat transfer textbooks.
The only novelty is the complication in the real atmosphere of the absorption and re-emission of radiation as it travels through the atmosphere. This subject is well covered by fundamental physics as explained by Novel prize winner Subrahmanyan Chandrasekhar in the 1950s.
SOD wrote: “The only novelty is the complication in the real atmosphere of the absorption and re-emission of radiation as it travels through the atmosphere.”
I hope the word “re-emission” isn’t misread to imply that GHGs excited by absorption of a photon, “re-emit” that energy as a photon faster than they are relaxed by collisions with their neighbors (“thermalized”). A clearer name for such a re-emission process is photoluminescence. However, more the 99% of the photons emitted from the troposphere come from molecules that were excited by collision, not by absorption. This is a prerequisite for LTE. The Schwarzschild eqn assumes a Boltzmann distribution of excited states and therefore can’t be used where LTE doesn’t exist.
There are many misleading descriptions of the GHE that imply that 50% of absorbed OLR is “re-radiated” or “re-emitted” back towards the surface. Where LTE exists, “re-radiation” or “re-emission” is negligible, and essentially all emission arises via the n*o*B(lamba,T) term of the Schwarzschild eqn.
Interference exists and fields cancel only when the interfering radiation is coherent. I suspect AlecM is treating the radiation passing through the atmosphere as if it were coherent, which it generally is not. Unfortunately I found coherence to be a complicated subject, which may approached differently for the emission of a photon by a single molecule and for a wave train emitted by a radio antenna or a radar. I presume that two red laser pointers won’t interfere with each other if they cross, but one can get interference with a beam-splitter or a double slit and red light from any source. Photons seem easier to understand, but I suspect there is a purely EM wave answer to the difficulties AlecM is raising.
I tried to indicate in some of my above comments that many quantitative questions related to the potential presence of coherence can be answered by QED. Equations derived from QED can tell, how large corrections result form taking into account mechanisms that involve more than one photon. When a Feynman diagram includes two or more photons, coherence plays always a role. Fortunately (at least for the ease of calculations) it turns out that all corrections from the more complex Feynman diagrams are small enough to be ignored in calculations of radiative energy transfer in the atmosphere.
The situation is different inside lasers, where coherence is crucial. In the theory of laser alternative approaches have been proposed and used. These alternative approaches I have in mind are not based on the concept of photons but quantify the equations in a different way. Such an approach is believed to be essentially equivalent to QED, but many issues important for lasers can be approached more easily in that way.
What I write above is based on a couple of seminars I was following decades ago at my home institute. The lectures where given by a Finnish theoretical physicist who built a successful career in the area of laser theory in Finland, U.K. and Sweden. This was not my field but some ideas stick and remain clear over decades.
The message that I try to give is that describing QM using classical concepts is seldom complete, and that several different approaches can often be used properly. It may be that the different approaches seem incompatible, but only because QM is so different from classical physics on fundamental level. This means also that it’s sometimes difficult to tell in simple terms why a particular faulty theory is so wrong when it’s justified by some less known physics, which is correct under some specific conditions. The the claims cannot be properly dismissed without showing that that the conditions are not right for the theory.
The most reliable way of knowing that a theory is wrong is using it to give concrete predictions, and noticing that the predictions are totally wrong. That’s the case for the claims of CJ, and that’s the case for all the theoretical constructs that imply high coherence is situations, where coherence is negligible.
Pekka: I doubt AlecM’s mistakes can’t be addressed classically. Imagine the electric field in the x-direction and the magnetic field in the y-direction and the EM waves traveling in the z-direction. When two such waves traveling in opposite directions meet, they simply pass through each other. The changing electric field creates the changing magnetic field and vice versa. To stop this self-propagation, one needs to prevent these fields from changing with time, not merely have them cancel each other at some location in space. On the other hand, the Poynting vectors for these waves cancel. The net energy flux is correctly calculated by vector addition, but the waves still pass right through each other with no loss in energy and are eventually absorbed (including their energy).
In a double-slit experiment, the Poynting vectors from each slit point in roughly the same direction. They don’t cancel. The electric and/or magnetic fields constructively and destructively interact in different location. Interference is created because the E and B fields cancel, not because Poynting vectors cancel.
As for CJ, any theory he may come up with will have to make the same predictions as QM for situations that have been carefully studied experimentally. Absorption by GHGs has been thoroughly studied in the lab; so has emission of thermal radiation. Chances are excellent that the GHE will survive any revolution in physics with minor modifications, so I need to speculate about whether or not CJ might create one.
Frank,
You are right in noting that classical theory of electromagnetism has many similarities with its QM replacement, the QED. It’s limitation is that it cannot handle properly emission and absorption of radiation, but given the source terms the outcome is similar.
Technically the reason for this is that Maxwell’s equations are linear and homogenous when charges and currents are counted equally to the fields. We can calculate separately the fields produced by several sources (charges and currents) and add up the fields. There’s no interaction between the component solutions.
The discussion of the double slit experiment is relevant only for radiation (or waves) coming from a single source. Reflection from a two-strip mirror and also scattering from a grid is essentially the same phenomenon. Thus the direction may change by an arbitrary amount but the source must be a single source.
Pekka,
I used to do x-ray emission spectrometry and other forms of atomic spectroscopy before I retired. It’s difficult to not believe in photons when you’re dealing with those energies. Not to mention that the intensity of the emitted x-rays is so low as to require using counting statistics for the results because you are counting the photons that hit the detector, or, for that matter, the the line emission spectra. In fact, even for atomic emission spectrometry in the visible and UV, with solid state detectors you’re still counting photons rather than measuring an analog current. Strictly speaking, you’re counting electrons in a well, but it amounts to the same thing.
DeWitt,
I fully agree. On the other hand photons are not a useful concept in most cases where radio waves are considered. The same fundamental equations apply, however, to both.
Pekka,
The approximately ten orders of magnitude difference in frequency and three orders of magnitude difference in energy between 100MHz radio waves and the Fe Kα (showing my age here, never did learn Siegbahn notation) emission line frequency might have something to do with that.
I’ve discovered why SoD and many commentators are wrong: these MIT physics’ notes are wrong, so i presume the same applies to many US University equivalent:
http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/node134.html
This claims that the single S-B equation predicts an energy flux; it does not. It predicts the Radiant Emittance, aka Exitance. Check this is standard textbooks. It is a potential energy flux to a sink at absolute zero. Only the vector sum of opposing Emittances dives the net EM energy flux; the difference of individual Poynting Vectors.
http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/node135.html
Claims that Emissivity is Emittance, a bad error.
So, it appears that even MIT teaches incorrect physics. No wonder climate Alchemy has failed. 18 year no warming and no missing heat proves it.
Quantum Entanglement. You have yet to explain how that works with Poynting Vectors. Until you can, go away. We’re completely uninterested in your completely unsupported assertion that physics has been wrong for 100 years.
AlecM
Time for you to do the heavy lifting.
Instead of assuming that because someone at MIT may have been lacking precision in their physics notes (I haven’t looked at your link), and “therefore” the whole of climate science may also have copied this same “mistake” – just take a look at the equations of radiative transfer and explain which step is wrong – and why:
Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations.
I will wait for your update.
AlecM: You may be confusing “emittance” (the dimensionless ratio of emitted radiation to blackbody radiation) with “radiant emittance” or exitance (an energy flux). As best I can tell, the term emissivity is used inconsistently – sometimes meaning emittance and sometimes meaning radiant emittance. Since I lack a reliable textbook on this subject, however, I can’t speak definitively on this subject. The following is offered in hopes that others will point out any major mistakes.
We normally think of emissivity as a dimensionless ratio (or intrinsic property). When referring to the greenhouse effect, however, our host teaches that the emissivity of a gas depends on how many GHG molecules are present. The emissivity of low-e glass depends on the thickness its coating. Here emissivity (like emission of photons) varies with quantity (extrinsic). Some of the confusion appears to arise from the basics of blackbody radiation itself. Semi-classical derivations of Planck’s Law ASSUME radiation in equilibrium with a medium (originally quantized oscillators). This assumption is appropriate for hohlraums and typical solid objects. When one deals with a thin layer of solid (or a gas), however, emission and absorption have not reached equilibrium before radiation reaches the surface. Planck’s Law can’t describe what is emitted when equilibrium isn’t present, nor can the “emissivity” correction applied to “graybodies”.
As best I can tell, radiation is traveling through all materials (solids, liquids and gases) and reaches blackbody intensity at a particular wavelength when absorption and emission have reached equilibrium. When the radiation reaches a surface, some is transmitted and some is reflected/scattered, producing “emissivity” less than 1. The same thing happens to radiation approaching the surface from the opposite direction, making emissivity equal to absorptivity at any wavelength. (If correct, emissivity is a surface phenomena that has nothing to do with the thermal emission of photons.) However, under situations where absorption and emission haven’t reached equilibrium, one needs to fall back on more fundamental physics, the Schwarzschild eqn,, which I assume is based on Einstein coefficients.
Frank,
Einstein coefficients are not directly related to the Swarzschild equation. That’s the differential equation describing how the intensity of a beam of light of a given wavelength varies with distance along a path where the absorptivity and emissivity at that wavelength are between zero and one.
The Einstein coefficients are the probabilities of the spontaneous (A) or stimulated (B) emission of a photon from an excited state. If you know the number of excited states, the volumetric spontaneous emission intensity is the product of the number of states and the corresponding Einstein coefficient for that state.
DeWitt: And the cross-section for emission/absorption used in the Schwarzschild equation presumably must arise from the Einstein coefficient for emission. You could look at the reference below (from a new open source journal and peer review of unknown quality).
http://www.hindawi.com/journals/ijas/2013/503727/
The same author has a more controversial article in the same journal
Click to access 3001-846.pdf
Frank,
The Einstein coefficients do not define the Swarzschild equation. It can be derived without any assumptions about the details of the absorptivity/emissivity as a function of wavelength. If instead of absorption, you include scattering from aerosols and call it extinction, the Einstein coefficients are not sufficient. Also, If you are not using a line-by-line program to calculate radiative transfer using the equation, you’ll never see them.
DeWitt: Thanks for the reply. I suspect we are in agreement here, but not communicating.
For the moment, I’m ignoring scattering. The Schwarzschild equation tells us how radiation traveling through a non-scattering atmosphere changes with incremental distance traveled. It is accurate enough to make reasonable estimates of how radiation behaves in the earth’s atmosphere.
At a more fundamental level, however, emission and absorption of photons are described by Einstein coefficients. How is the Schwarzschild equation derived from this perspective? It seems to me that their must be a mathematical relationship between Einstein coefficients (and excited state lifetime) and the absorption cross-section. As best I can remember. the physics presented at SOD hasn’t covered this relationship. Instead, absorption cross-sections are MEASURED in the laboratory and we apply that cross-section to both the absorption AND emission terms of Schwarzschild equation. This paper presumably addresses some of these questions. See equations 16 and 17, connecting Einstein coefficients to the Planck function. Also see the Schwarzschild eqn, which arises from eqns 47 and 48, which connect a single cross-section to the Einstein A coefficient.
http://www.hindawi.com/journals/ijas/2013/503727/
In this paper, a lot of physics (that I don’t understand in detail) is needed to connect the absorption coefficient we measure in the laboratory to the coefficient used in the emission term of the Schwarzschild equation. The connection between absorption and emission is always made by assuming radiation in equilibrium with its surroundings at some temperature, ie that absorption rate = emission rate. And a Boltzmann distribution of energy states is often postulated (LTE), though Section 4.3 of this paper doesn’t. Section 2.3.2 deals with stimulated or induced emission (which isn’t included the Schwarzschild eqn.) I’m surprised to see that stimulated emission rates for some of the strongest CO2 lines may be as big as 4% of the spontaneous emission rate.
Frank,
Read 2.3.2 again. Stimulated emission is only significant in the absence of collisions.
And it is included in the Swarzschild equation. At LTE, absorption must equal emission whether there is stimulated emission or not.
Einstein presented the result that the coefficient of stimulated emission is equal to the coefficient of absorption. Because both are due to the same incoming radiation, the relative strength of stimulated emission and absorption is given by the ratio of molecules in the excited state to that in the ground state. That applies to each pair of states, but there are two excited states of the same energy in the case of the 15µm level of CO2. The factor of two must be taken into account in some considerations.
At temperatures of the atmosphere 3 – 3.5% of CO2 molecules are in each of the two excited states. Thus stimulated emission is about 3% of absorption. Total emission is not exactly equal to total absorption, because the local temperature is not exactly the same as the effective average temperature at locations, where the incoming radiation is emitted. In the troposphere total emission is stronger than total absorption, but not much smaller. Thus spontaneous emission is around 97% of all emission, and stimulated emission about 3%.
The reference of that paper to the collision rate of several 10^9 1/s is misleading. The actual rate of collision induced vibrational state transitions is around 10000 per second as Eli taught me, when I made the error of referring to the billions of collisions per second. The total number of collisions is that high, but only a few out of million collisions lead to change in the vibrational state for the molecules initially in the excited state (and less in the opposite direction). The rest of collisions affect only the rotational state.
DeWitt: I did learn more from re-reading section 2.3.2. To see if I understand the situation correctly and if you may have oversimplified, let’s consider several cases:
The difference between rotational energy levels is small enough that they are almost equally populated at atmospheric temperature. Absorption of a microwave photon will change from one rotational state to another. Since these states are almost equally populated (and assuming equal degeneracy), the rate of induced emission and absorption should be similar (See equation 14). If collisional relaxation didn’t exist, we could deduce that the rate of spontaneous emission was small compared with induced emission.
The difference between vibrational energy levels is much larger, so they are not equally populated at atmospheric temperatures. Therefore, the rate of induced emission is much less than the rate of absorption. If emission and absorption were in equilibrium, most emission would be due to spontaneous emission (not induced emission). However, in the atmosphere, absorption and emission are NOT in equilibrium at many wavelengths – that is why we observe TOA radiation intensities appropriate for blackbody radiation with temperatures from 200 to 300 degK. At these wavelengths, we rely upon collisions (not radiation) to ensure a Boltzmann distribution of vibrational energy levels (LTE). If we go above the stratosphere, collisions aren’t frequent enough to maintain LTE.
The difference between electronic energy levels is even larger. Collisions rarely produce electronically excited states in the earth’s atmosphere. So the atmosphere is not in LTE with the visible and UV radiation passing through it.
(There is a gradual change from one regime to another that doesn’t depend on the nature of the excited state. By chance, the temperature and density of the earth’s atmosphere allows me to ascribe these differences in behavior to rotational, vibrational and electronic excited states. On a very cold planet, collisions won’t be energetic enough to create a Boltzmann distribution of vibrationally excited states.)
You said: “At LTE, absorption must equal emission whether there is stimulated emission or not.” As best I can tell, the troposphere is in LTE because of collisions, but emission and absorption are not in equilibrium. Furthermore, it is the relaxation rate, not simply the collision rate, that determines whether LTE exists.
What I still don’t fully understand is how we can use the absorption cross-section we measure in a laboratory in the emission term – n*o*B(lamba,T) – of the Schwarzschild eqn. When infrared emission is mostly spontaneous, it depends on A_21; whereas absorption in a laboratory spectrophotometer depends mostly on B_12. The reference I provided is pretty indirect on this subject, partially due to line width. And one might hope for a more direct path from the absorption coefficient to the lifetime of an excited state/emission rate.
Frank,
The coefficients of induced and spontaneous emission and absorption are closely related. Thus determining one of them or a combination of them determines all.
Frank,
On the relationship of the three coefficients, see here. As Pekka stated, the coefficients are not independent. If you know one, you know all three.
The simple form of Schwarzschild’s equation assumes thermal and radiative equilibrium. Radiative equilibrium in the troposphere is only a good assumption for the most strongly absorbing frequencies because then the temperature difference between emitting and absorbing molecules is small. All this is taken into account in a line-by-line radiative transfer program like LBLRTM.
DeWitt and Frank,
The derivation of your link is based on accepting some formulas and using consistency requirements to figure out, what the relationships between the coefficients must be. That’s the original approach of Einstein in 1917, when Quantum Mechanics was not available for understanding from the basics, what determines the constants and gives then values that satisfy those relationships.
The situation is not fully satisfactory without the QM derivation of the coefficients. This is not the place to go deep enough to QM, but some idea of that can be obtained from a href=”http://electron6.phys.utk.edu/qm2/modules/m10/einstein.htm”>this presentation.
There may be better explanations, but this the first one that I found.
Basically the result is based on the time reversal symmetry of QM and on the properties of electromagnetic radiation and its interaction with matter.
The formulas contain transition amplitudes that are unchanged, when the initial and final states are interchanged. The bosonic nature of photos plays also an important role in more advanced derivations.
Pekka,
Using that approach or something like it, it’s possible to calculate the frequencies and Einstein coefficients for the vibrational and rotational transitions of simple molecules like CO2 ab initio. In fact, I believe that’s been done for the majority of lines in the HITRAN database.
DeWitt,
It’s also my understanding that only a fraction of the lines has been measured, while the most by count have been only calculated. All the strongest lines have, however, been both measured and calculated as far as I have understood.
There are 7,400,447 spectral lines in the present database. Measuring most of those would be obvious waste of effort.
The HITRAN2012 database contains about 300.000 lines for CO2 (128,170 for the main isotopes). About 140,000 measured line positions and 44,000 measured intensities of various isotopologues were used. Thus only 15% of the intensities are based on measurements, while the share is close to 50% for the positions.
Frank,
We should start by including (at least) three alternatives, not two:
– transmission
– emission or absorption
– reflection or (back-)scattering
In case of gas transmission and emission/absorption dominate. There’s also some scattering, but to a good approximation it can often be ignored.
In case of typical solid surfaces we have emission/absorption and reflection/back-scattering.
For a layer of water (or glass) all three must be included. It’s, however, possible to consider oceans (etc.) in a way where transmission is excluded, when the depth of absorption is not of interest. In this case reflection/scattering occurs mostly at surface, but also as scattering from varying depth.
The sum of transmissivity, absorptivity, and reflectivity is always one, whether one of the three is negligible (as reflectivity is typically for gases) or all three are significant.
From this consideration, you can see that there’s nothing strange in the definition of the absorptivity of a layer of gas.
Defining emissivity is not as clear for a thick layer of gas, when the temperature is not constant throughout the layer. Comparing with a blackbody is not possible, when the temperature is not well defined. For layers thin enough to have a single temperature emissivity and absorptivity are both well defined and equal also for gases.
(On the terminology emittance seems to be the most problematic. I think that it’s better to avoid using it as a synonym for emissivity. On that point I agree with AlecM, but that must be almost the only issue related to physics on which I agree with him.)
Pekka: I agree – when one considers gases, liquids and thin solids, the complete description must include transmission. However, this doesn’t address what we mean by emissivity: Is it an intrinsic, dimensionless ratio that depends on what happens at a surface or an extrinsic quantity that sometimes depends on the number of emitting molecules? Neither you nor DeWitt commented on this issue. The MIT course AlecM complained about did not list “emissivity” in its index of terms, perhaps because it can be confusing. Using “emittance” for the dimensionless ratio and exitance or radiative emittance for the energy flux per unit of emitter would avoid the confusion which appears to be associated with emissivity.
Frank,
Emissivity is fully as well defined for a isothermal layer as it’s for a surface. It’s the ratio of the radiation flux that originates in the layer and exits the layer to the radiation flux of a black body. A thin highly transparent layer has a small emissivity, but that does not make the definition any less clear.
Emittance is much more confusing that emissivity. I would say that emissivity is not confusing at all, while emittance is, and it’s particularly confusing when it’s used as it is those lecture notes. Those notes are extremely concise, thus it’s not surprising that they don’t explain everything, but the second of the pages linked is poor even on the standards of very concise presentation, and not only because of the use of the word emittance. (The first page is OK).
Pekka: Thanks for the reply. As I read it, your reply suggests that emissivity is sometimes a property of a surface (bulk solids), sometimes a property of a layer without a surface (gases) that can vary with quantity, and sometimes a property of both (thin films). However, you are correct that one can define an emissivity relative to a black body at.the same temperature in all three cases. It just seems strange that there are two reasons for emissivity less that 1: scattering exiting the surface and too few emitters inside the surface (for emission and absorption to reach black body equilibrium).