In Part One we took a look at what data was available for “back radiation”, better known as Downward Longwave Radiation, or DLR. And we saw that around many locations the typical DLR was in the order of 300 W/m2, and it didn’t decrease very much at night.
In Part Two we looked at several measured spectra of DLR which clearly demonstrated that this radiation is emitted by the atmosphere.
In this article we will consider what happens when this radiation reaches the ground. The reason we want to consider it is because so many people are confused about “back radiation” and have become convinced that either it doesn’t exist – covered in the previous two parts – or it can’t actually have any effect on the temperature of the earth’s surface.
The major reason that people give for thinking that DLR can’t affect the temperature is (a mistaken understanding of) the second law of thermodynamics, and they might say something like:
A colder atmosphere can’t heat a warmer surface
There are semantics which can confuse those less familiar with thermal radiation.
If we consider the specific terminology of heat we can all agree and say that heat flows from the warmer to the colder. In the case of radiation, this means that more is emitted by the hotter surface (and absorbed by the colder surface) than the reverse.
However, what many people have come to believe is that the colder surface can have no effect at all on the hotter surface. This is clearly wrong. And just to try and avoid upsetting the purists but without making the terminology too obscure I will say that the radiation from the colder surface can have an effect on the warmer surface and can change the temperature of the warmer surface.
Here is an example from a standard thermodynamics textbook:
Probably this diagram should be enough, but as the false ideas have become so entrenched let’s press on..
Note that this topic has been covered before in: Intelligent Materials and the Imaginary Second Law of Thermodynamics and The First Law of Thermodynamics Meets the Imaginary Second Law
The First Law of Thermodynamics
This law says that energy is conserved – it can’t be created or destroyed. What this means is that if a surface absorbs radiation it must have an effect on the temperature – compared with the situation where radiation was not absorbed.
There’s no alternative – energy can’t be absorbed and just disappear. However, as a technical note, energy can be absorbed into chemical bonds or phase changes of materials. So you can put heat into ice without changing the temperature, while the ice turns into water. Of course, energy is still not lost..
Therefore, if your current belief is that radiation from a colder atmosphere cannot “change the temperature” of the hotter surface then you have to believe that all of the radiation from the atmosphere is reflected.
Or, alternatively, you can believe that the first law of thermodynamics is flawed. Prove this and your flight to Sweden beckons..
Bouncers at the Door – or Quantum Mechanics and the Second Law of Thermodynamics
One commenter on an earlier post asked this question:
But if at the surface the temperature is higher than in the atmospheric source then might the molecules which might have absorbed such a photon be in fact unavailable because they have already moved to a higher energy configuration due to thermal collisions in the material which contains them?
Many people have some vague idea that this kind of approach is how the second law of thermodynamics works down at the molecular level.
It (the flawed theory) goes like this:
- the atmosphere emits “a photon”
- the photon reaches the surface of the earth
- because the temperature of the surface of the earth is higher the photon cannot be absorbed – therefore it gets “bounced”.
Except it’s not physics in any shape or form – it just sounds like it might be.
Let’s review a few basics. It’s important to grasp these basics because they will ensure that you can easily find the flaw in the many explanations of the imaginary second law of thermodynamics.
“They all look the same to me” – The Energy of a Photon
This part is very simple. The energy of a photon, E:
E = hν = hc/λ
where ν = frequency, λ = wavelength, c = speed of light, h = 6.6 ×10−34 J.s (Planck’s constant).
You can find this in any basic textbook and even in Wikipedia. So, for example, the energy of a 10μm photon = 2 x 10−20 J.
Notice that there is no dependence on the temperature of the source. Think of individual photons as anonymous – a 10μm photon from a 2,000K source has exactly the same energy as a 10μm photon from a 200K source.
No one can tell them apart.
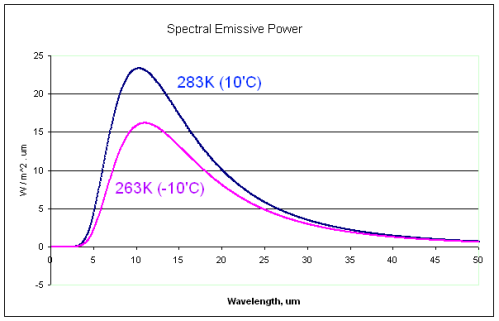
Wavelength Dependence on the Temperature of the Source
Of course, radiation from different temperature sources do have significant differences – in aggregate. What most, or all, believers in the imaginary second law of thermodynamics haven’t appreciated is how similar different temperature Planck curves can be:
Notice the similarity between the 10°C and the -10°C radiation curves.
Alert readers who have pieced together these basics will already be able to see why the imaginary second law is not the real second law.
If a 0°C surface can absorb radiation from 10°C radiation, it must be able to absorb radiation from -10°C radiation. And yet this would violate the imaginary second law of thermodynamics.
What determines the ability of a surface to absorb or reflect radiation?
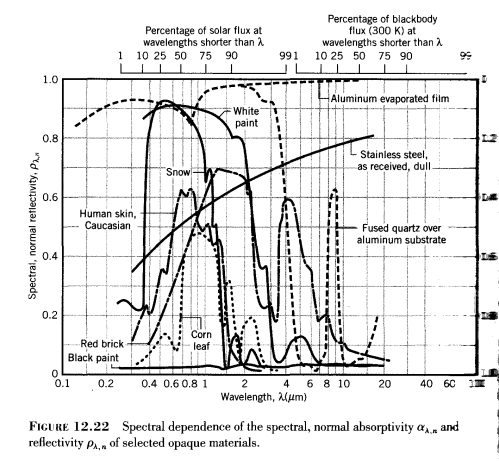
Absorptivity and Reflectivity of Surfaces
The reflectivity of a surface is a measurement of the fraction of incident radiation reflected. It’s very simple.
This material property has a wavelength dependence and (sometimes) a directional dependence. Here is a typical graph of a few materials:
As you can see, the variation of absorptivity/reflectivity with wavelength is very pronounced. Notice as well in this diagram from a standard textbook that there is no “source temperature” function. Of course, there can’t be, as we have already seen that the energy of a photon is only dependent on its wavelength.
Just to be clear – radiation incident on a surface (irradiation) can only be absorbed or reflected. (In the case of gases, or very thin surfaces, radiation can also be “transmitted” through to the other side of the material or gas).
Conclusion from Basic Physics
So from basic physics and basic material properties it should be clear that radiation from a colder surface cannot be all reflected while at the same time radiation from a warmer surface is absorbed.
And if any radiation is absorbed it must change the surface temperature and therefore violate the (imaginary) second law of thermodynamics.
You have to ditch something. I would recommend ditching the imaginary second law of thermodynamics. But you can choose – instead you could ditch the first law of thermodynamics, or the basic equation for the energy of a photon (make up your own), or invent some new surface properties.
While considering these choices, here’s another way to think about it..
If All the “Back Radiation” Was Reflected..
So let’s suppose you still think that all of the radiation from the atmosphere, all 300W/m2 of it, gets reflected.
That presents a problem even bigger than the tedious physics principles articulated above. Why is that?
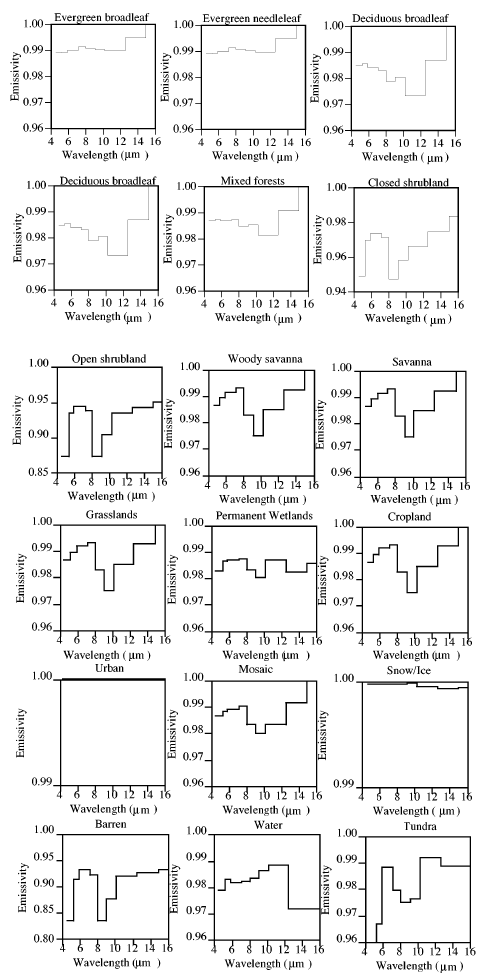
Well, let’s take the earth’s average surface temperature of around 15°C (288K) and the typical emissivity of various surface types:
As you can see, the emissivity is pretty close to 1. So for a temperature of 15°C the Planck curve will be pretty close to a blackbody, and the total surface emitted radiation (“flux”) is given by the Stefan-Boltzmann equation of σT4 – so in the typical surface case:
j = 390 W/m2
Now we have to add the reflected surface radiation of 300W/m2. So the upward radiation from the surface will be around 690 W/m2.
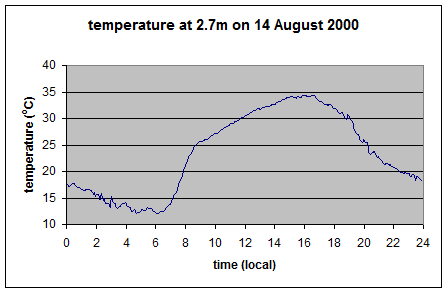
Here is one result from a very thorough experiment, The Energy Balance Experiment, EBEX 2000 (reference below):
The location was a cotton field of 800m × 1600m at coordinates 36°06’ N, 119°56’ W, approximately 20 km south-south-west of the town of Lemoore CA, USA. In this experiment, radiation measurements were taken at nine sites across the field along with wind and humidity measurements in an attempt to “close the energy budget” at the surface. Downward measurements were taken at a few of the sites (because the values wouldn’t change over a small distance) while upward measurements of both shortwave and longwave were taken at every site. Some sites measured the same value with two instruments from different manufacturers.
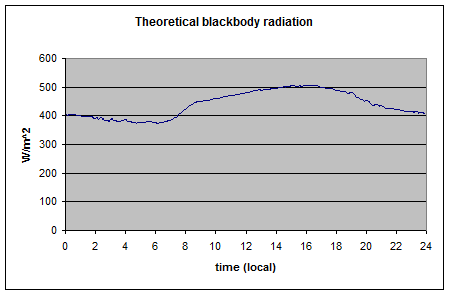
As you can see the upward longwave measurement is around 400-500 W/m2. The paper itself doesn’t record the temperature on that day, but typical August temperatures in that region peak above 35°C, leading to surface radiation values above 500 W/m2 – which is consistent with the measurements. [Update – the peak temperature measured at this location was 35°C on this day – thanks to Wim Kohsiek for providing this data along with the temperature graph for the day]
And so here it is the theoretical upward longwave radiation from the temperature graph (=σT4):
As you can see, this upward radiation calculation matches what was measured.
If the surface reflects all of the downward longwave radiation then the upward longwave measurement for these temperatures should be in the region of 700-800 W/m2.
There is a great opportunity for some enterprising people who still think that DLR is all reflected and not absorbed – buy a decent pyrgeometer and take some upward surface measurements to demonstrate that the whole science community is wrong and upward surface measurements really are 80% higher than everyone thinks. If you can afford an FT-IR to do a spectral analysis you will be able to prove your theory beyond a shadow of doubt – as the spectrum will have those characteristic CO2, O3 and water vapor peaks that were shown in DLR spectra in Part Two.
Conclusion
DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.
The physics behind this are very basic and have been known for around 100 years.
Proving that the surface doesn’t absorb DLR should be a walk in the park for anyone with a small amount of cash. But only if it’s true.
The world we live in does absorb DLR and adding 300W/m2 to the surface energy budget is the reason why the surface temperatures are like they are.
Further reading – Do Trenberth and Kiehl understand the First Law of Thermodynamics?
Darwinian Selection – “Back Radiation”
References
The Energy Balance Experiment EBEX-2000. Part III: Behaviour and quality of the radiation measurements, Kohsiek et al, Boundary Layer Meteorology (2007)


“Therefore, if your current belief is that radiation from a colder atmosphere cannot “change the temperature” of the hotter surface then you have to believe that all of the radiation from the atmosphere is reflected.”
What if the surface was emitting as much radiation as it was absorbing? Isn’t it energy imbalance that causes temperature change?
Wha? Before: radiation coming in. Now: radiation coming in plus backradiation.
Unless the sun has noticed the blanket of air around the earth and turned itself down, there’s a change in the radiation balance to the positive.
A surface can’t emit more radiation than defined by the Stefan-Boltzmann equation for balckbodies. Which means that the emitted radiation at a point in time is dependent on the temperature of the body not incident absorbed radiation. Therefore if you increase the incident radiation you need to raise the temperature to even out the difference, unless you are able to achieve cooling by other means like convection and conduction. Convection and conduction are most likely to increase as well with increased temperature, so most probably all of the increase in incident radiation wouldn’t be balanced out by an equal amount of temperature increase.
Well, except for lasing etc.
Think of the absorption spectra overlaid over the blackbody radiation.
But because of all the nearby N2 molecules with kinetic energies about right for absorption for any unexcited CO2 molecule, the CO2 molecules are filled far more quickly than if they were in the atmosphere alone.
Therefore the *power output* is higher (same energy photons, just more per unit time) per wavelength than the power output of that segment of the BB radiation, but the total power output added together is that which SB explains.
John Millett:
I was trying not to write every sentence with long caveats.
Is there a difference in the temperature for the two cases:
a) all of the DLR is reflected
b) all of the DLR is absorbed?
Yes, the temperature will change between these two cases. Therefore, DLR has an effect on the temperature of the surface.
Of course, reflected DLR cannot affect surface temperature whereas when absorbed it may. Whether and how it does so depends on its strength relative to that of surface emission. If the strengths are equal, there will be no energy imbalance and no temperature change. If absorption exceeds emission there will be an imbalance and temperature will rise until emission increases to equal absorption when energy imbalance reduces to zero and temperature stays constant; and vice versa. The effect DLR has on surface temperature depends not only on whether it is absorbed or reflected but also on ULW from the surface.
John Millet,
I suspect you are still thinking that radiative energy leaving a body has an effect on radiative energy incident on a body. I’m thinking it does not. It is not like two fire hoses pushing water at each other. The first working analogy that comes to mind is two armies firing bullets at each other, but I’m thinking even that doesn’t quite capture it because of the wave aspect of radiative energy. It’s like two boys shining flashlights in each others eyes. They both see the other’s flashlight just fine, even if they hold the flashlights so that the beams overlap for most of the journey.
A surface can interact with radiation in only three ways: absorption, transmission and reflection. When expressed as fractions as absorptivity, transmissivity and reflectivity, they must sum to exactly 1. Transmissivity can be ignored in the LW IR for most solids. So radiation is either reflected or absorbed. But then there’s Kirchhoff’s Law that for objects in local thermal equilibrium, emissivity equals absorptivity. So any highly reflective surface also has low emissivity.
You don’t have to buy a pyrgeometer to show that the ground surface has high emissivity in the IR. A simple IR thermometer available in auto parts and kitchen supply stores for ~$50 will do it. Put a regular thermometer on the ground at night and let it equilibrate, then use the IR thermometer on the same area. If the temperatures are the same then the emissivity of the ground is very close to 1. You can also take the temperature of the sky at the same time. For a clear sky shortly after the sun has gone down, if it’s not too humid, the sky temperature will be quite a bit lower than the ground temperature. If it’s too cold, the sky temperature might be below the lower limit of the IR thermometer. But it still means that the radiation must be emitted from the surface rather than reflected from the sky.
hmm… btw do you have any comments in this?
http://mindeavour.blogspot.com/2010/07/comments-on-backradiation.html
Nobody else was bothered to comment, gnbatt.
But the earth is heavy. And momentum changes from photons won’t move it to any measureable effect.
The earth is also not a thin flat plane.
There’s a lot of errors in there and this may be why nobody has commented: NWOR.
Instead, do YOU have any comments on this page?
While I understand what is probably meant to be said, I must point out that there is no need for radiation balance on the surface of the earth. There are plenty of other methods for it to get rid of excess energy.
……”there is no need for radiation balance on the surface of the earth”……
I agree, for instance, in the photosynthesis process, the incident em radiation can be converted into chemical energy by a plant.(production of starch)
But the biggest cause is “getting hotter”.
Can I use mirrors and lenses to focus the radiation from a colder source into a beam of photons and heat a hotter source with it?
Yes. As long as you don’t break the energy totals.
Take a black body and focus radiation from it onto a photovoltaic cell. Use that to power a small motor. The motor returns its movement as heat. Which must be re-absorbed by the black body. Put the whole assembly in a vacuum flask. No heat in or out. I have a perpetual motion machine. Or do I? What am I missing?
“Or do I? What am I missing?”
You do. It just requires that no work be done which your system is set to do.
What you’re missing is that your setup is impossible in a real world unless you have
a) perfect conversion from heat to energy
b) perfect reflection
c) no friction losses
But, yes, if you have those then you will have a perpetual motion machine. To a large extent, the motion of pluto around the sun is just such a perpetual motion machine. It will orbit the sun for billions of years with no new energy input.
It doesn’t require no work be done. Just use the motor to lift a weight or something. Anything you do is eventually returned as heat to the system anyway. It doesn’t require perfect conversion from heat to energy. Any loss would return as heat to the system. It doesn’t require no friction as that would return as heat also.
It would require a perfect mirror to remain isolated from the rest of the universe. But I suppose you could instead treat the universe as your ultimate source of black body radiation. Such a machine would continue to operate even in an otherwise heat dead universe. As perpetual motion machines go, that would be fairly perpetual. Unless there’s some law to prevent such a thing.
“It doesn’t require no work be done.”
It does.
Your set up does no work. Therefore it would be a perpetual motion machine.
This is no problem because no work is being done.
“It doesn’t require no friction as that would return as heat also.”
Except you get friction from breaking the bonds on the frictional surface. Breaking those bonds removes energy that isn’t captured by your system.
Why else do you think you get little bits of metal or whatever in your lubricant (or, if you can’t see them, why you have to change your lube)?
“Such a machine would continue to operate even in an otherwise heat dead universe.”
This is not a problem since you’ve set this up never to reach heat death.
“As perpetual motion machines go, that would be fairly perpetual.”
Yes, just like pluto orbiting a dead white dwarf once known as “The Sun”.
I fail to see where you’re going to spring the “gotcha”, because your situation is set so as not to have any losses, therefore perpetual motion doesn’t break the laws of thermodynamics (entropy can remain the same or increase, but you’ve set a scenario where it remains the same).
“Unless there’s some law to prevent such a thing.”
No, the second law allows it.
Your system is not trying to extract any energy and entropy is held even.
There’s no problem here, except the practicalities of making a system with zero losses.
If I have a motor and it’s being powered by a non zero amount of watts, why can’t I use that to do work?
There is a rule that I’m missing: the conservation of phase space. According to this write up, this rule prevents the concentration of radiation to a point that the target is hotter than the source. Which prevents me from breaking the second law like that:
http://www.av8n.com/physics/phase-space-thin-lens.htm#sec-discussion
“DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.”
The second sentence does not follow from the first.
The temperatures of the atmosphere and the surface are convectively coupled. The two are held in a fixed relationship to one another. If the surface temperature rises by absorption, then the atmosphere’s temperature must drop (it is emitting energy, after all) and so this dual changing of temperature implies an increase in the lapse rate.
But the lapse rate is fixed by the convective coupling. Any change is cancelled out by a corresponding change in convection.
You can’t just ignore convection throughout most of the presentation, and then tack it on as a minor adjustment to the purely radiative mechanism at the end. You can’t address the mechanism here piecemeal.
It’s like me arguing that I can lift myself off the ground by my bootstraps. The force my arms apply to my boots is real. It’s quantifiable, it’s measurable, and its upwards. Saying that because DLR is absorbed by the ground, the ground’s temperature must rise is analogous to saying that because my arms apply an upward force on my boots, the boots must rise. This is, after all, basic mechanics. An upward force must induce an upward acceleration, yes?
The flaw in the argument is that my boots and my arms are mechanically coupled. My body holds them a fixed distance apart. It’s not something that you can tack on at the end, as a minor correction.
I do not doubt that there is a surface warming effect, but I do not believe that this is the mechanism by which it works. I look forward to your next post.
“The temperatures of the atmosphere and the surface are convectively coupled. The two are held in a fixed relationship to one another.”
The second does not follow on from the first.
“If the surface temperature rises by absorption, then the atmosphere’s temperature must drop (it is emitting energy, after all)”
There’s no “must” to it.
“But the lapse rate is fixed by the convective coupling.”
No, it’s fixed by the gravitational strength and the heat capacity of the gas.
“You can’t just ignore convection throughout most of the presentation”
It isn’t.
But compared to 390W/m^2, the 24 from convection is peanuts.
“It’s like me arguing that I can lift myself off the ground by my bootstraps”
No, it’s like arguing you can’t be pulled up by your bootstraps by someone else.
It only works if you forget the “someone else” bit.
“The force my arms apply to my boots is real. It’s quantifiable, it’s measurable, and its upwards.”
And so your toes will be pulled up. Your heels will be pulled down and if not careful you’ll fall over.
“The flaw in the argument is that my boots and my arms are mechanically coupled.”
The argument has no boots or arms in it. Yours does.
“I do not doubt that there is a surface warming effect, but I do not believe that this is the mechanism by which it works.”
What do you believe it is, then? Fairies?
Your first two comments I didn’t understand. The third is just a restatement of what I said. The fourth ignores the fact that convection varies under a form of feedback control, it’s not a constant. And the fifth again assumes that the atmosphere is not convectively coupled. Your next two don’t seem to add anything useful, and as for your final point, I hate to break it to you like this, but Fairies don’t exist.
But thanks for the reply.
Convection happens in the troposphere because the atmosphere radiates heat to space faster than it absorbs radiation from the surface and directly from the sun. This heat loss would cause the lapse rate to exceed the adiabatic rate so convection makes up the difference. The convective heat loss causes the surface to be cooler than it would be in the absence of convection. Convection isn’t ignored or tacked on at the end. They’re called radiative/convective models for a reason.
You would be correct if the system were closed. It’s not. There is a continuous energy flow from the sun to the earth and from the earth to space. So when the surface warms, the atmosphere also warms. The other thing you’re ignoring is that if CO2 were instantaneously doubled, the surface would see higher radiation from the atmosphere and the atmosphere would radiate less to space, so the atmosphere, to a first approximation, wouldn’t change temperature.
Thanks for your reply, too.
I agree about the radiative/convective models, but I was talking about SoD’s explanation. I’m quite happy with the way the models deal with it, but the explanation given here is, I believe, mis-characterising the way they work.
I agree that the system is not closed, but this is precisely what I’m objecting to when it is said that increased absorption of DLR leads to increased temperature – I’m arguing by analogy with that argument. Energy is transferred from atmosphere to surface. *If* this inevitably raises surface temperature, then by the same logic, it *must* lower atmospheric temperature.
If the surface is warmed by the sun, then the atmosphere can be warmed by the surface. But what you seem to be saying here is that if the surface is warmed by the *atmosphere* then the atmosphere will be likewise warmed by the surface.
I wasn’t sure what you meant about CO2 doubling – are you talking about a transient effect? I would expect that if CO2 was instantaneously doubled, radiation to space would come from a higher, cooler altitude, and temporarily reduce. This would warm both atmosphere and surface together, until the emission to space returned to normal.
Excellent series of posts SOD. Your patience and thoroughness in explaining this information to those interested is very admirable.
I find it interesting, in a way, that what was a relatively simple scientific curiosity from over 70 years ago as to, “What might the climatic affects be of steadily increasing atmos CO2 that will absorb a small portion of the outgoing surface thermal irradiance (because all CO2 spectral bands not overlapping H2O aren’t saturated) and then re-radiate both upward and downwards (like the existing CO2, water vapor and a few other things have been doing for the last few billion years) after having consequently warmed the atmosphere, ever so slightly in the initial absorption, and further increasing the atmosphere emitted irradiance received by the surface?”, now must turn into discussion of the mere existence of atmospheric “back” radiation and/or what happens to radiative energy encountering a surface, quite amazing!
The knowledge is out there. Many kudos to SOD for putting it together in a manner to address even the most remedial of issues and ardent of inquisitors. Out there also are people of power and greed that know how to take pieces of knowledge to twist and confuse to promote their personal selfish and otherwise misdirected desires and goals. Then again there are also those that just have a hard time fitting it all together – it can become complicated. SOD appears to “Stand tall” amongst that crowd and those observing it all.
I have just updated the article with some data received from Wim Kohsiek, the EBEX paper’s lead author.
He kindly emailed me the temperature graph at that location along with the data. I used the Stefan-Boltzmann equation to calculate the upward surface radiation.
No surprise to most of us that it matches the measured upward surface radiation..
Nullius in Verba:
On my comment:
“DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.”
Said:
To calculate the temperature and heat flow in any system you have to consider conduction, convection and radiation.
What this post is about is ensuring that everyone can get past first base and understand that radiation doesn’t have magic vanishing properties.
We can’t begin a sensible debate about heat flows if people believe DLR doesn’t exist, isn’t caused by the atmosphere, or isn’t absorbed by the surface.
My first question is just to check you agree with these points. I.e., the 3 points about DLR.
The second question is to clarify something. If the 300 W/m^2 of DLR just vanished – to use a thought experiment – are you saying that the surface temperature would still be the same?
So if we take the example of the Californian cotton field in this article, if we remove the 300W/m^2 DLR the daytime peak would still have been 35’C and the night time low about 12’C?
The third question is to confirm that with a temperature of 35’C in a cotton field, do you believe that it radiates about 500W/m^2 – regardless of any other heat transfer mechanisms?
Thank you.
Your first question – I agree with all three points.
To the second question – I’d say that it was tricky to answer, because one unphysical effect potentially leads by necessity to other unphysical effects. I’ll try to answer as honestly as I can, but it’s a bit hypothetical…
If you stopped the upper atmosphere emitting DLR by magic, there would be a power imbalance there which would start to heat it rapidly, until downward convection carried the heat down to the surface. Assuming convection would continue under such circumstances, the net heat flow due to convection would reverse, and yes, the surface would maintain approximately the same average temperature. The horizontal distribution of temperatures would probably be a bit different though, which makes that conclusion a bit fuzzy.
To the third question, yes, I agree.
“until downward convection carried the heat down to the surface.”
Rather odd that you talk about this unphysical “downward convection” right after you go on about
“because one unphysical effect potentially leads by necessity to other unphysical effects.”
“Rather odd that you talk about this unphysical “downward convection” right after you go on about…”
Is it?
Downward convection is perfectly physical. Otherwise we’d run out of air at the surface!
What goes up must come down.
The question is whether the equator-pole temperature difference would be enough to drive the present level of circulation throughout the troposphere. I’m following the usual convention in unphysical thought experiments of trying to keep as much the same as possible – but it would be possible for someone to argue that the atmosphere would no longer be fully convective – at which point everything I say about the consequences of the atmosphere being convectively coupled would be moot. I’m not sure exactly what would happen, though.
“Energy is transferred from atmosphere to surface. *If* this inevitably raises surface temperature, then by the same logic, it *must* lower atmospheric temperature.”
No, that would be by illogic it “*must* lower atmospheric temperature”.
Just because I turn up the electric bar heater doesn’t mean that the air in the room gets colder.
All it MUST mean is that the atmosphere is losing energy.
But it’s gaining it too, from the earth (and 67W/m^2 from the sun).
Radiation cools the bar of the heater.
An electric bar heater has a separate energy input from the electricity supply. Does the atmosphere have a separate energy input, besides the surface?
And the sun is not in the earth’s atmosphere.
It’s outside it.
as to this nonsequitor:
“Does the atmosphere have a separate energy input, besides the surface?”
You have the earth’s surface, the atmosphere and the sun.
And, yes, the sun heats the atmosphere too.
But this doesn’t have ANYTHING to do with your earlier question “then by the same logic, it *must* lower atmospheric temperature.” because the surface is hotter, and that heats the atmosphere.
So if the atmosphere loses energy to the ground by radiation, it gains it from the ground by radiation. At equilibrium, there’s no temperature change. That’s what “thermal equilibrium” means.
I think you need to look at this graph:
“I think you need to look at this graph:”
Seen it. It’s a simplification.
“Radiation cools the bar of the heater.”
No, radiation stops the bar increasing in heat to infinity.
“Your first two comments I didn’t understand”
No surprise there.
“The third is just a restatement of what I said.”
The third is not.
“The fourth ignores the fact that convection varies under a form of feedback control, it’s not a constant.”
It is a constant in balance and average. The sun’s output is not constant. But the average is. And that it is not a constant doesn’t make your spurious claim true: it doesn’t follow on.
“And the fifth again assumes that the atmosphere is not convectively coupled.”
No, it just doesn’t insist that the coupling is the only effect.
“Your next two don’t seem to add anything useful,”
Well it was a lot more useful to people who want the truth, but there you go. It was also more useful than your bewailing the bootstrap scenario.
When you pull on your bootstraps, your bootlaces will pull your feet up. The force applied from your arms will have to progress through to the soles of the feet., thereby requiring the feet to be pushed down.
” and as for your final point, I hate to break it to you like this, but Fairies don’t exist.”
Neither does your assertion I was responding to.
Neither does there exist a point in your diatribe against back radiation.
What would happen if you put a glass plate above the instruments – stopping the back-radiation from above. Would the temperature go up or go down.
And why would putting an IR block above an instrument reading IR fluxes relate to the temperature of something?
You mean like create a greenhouse?
Well, most greenhouses increase the temperature underneath the glass.
Perhaps some will consider the following to be useful:
Consider the mechanisms that cool the (naked) human body (with initial skin T = +35C) — all are in action, but some are more effective than others depending on the environment.
Standing naked in still air, radiation is often (but not always…) one of the more effective (why is that?).
Now, ask yourself in which environment would my body cool most rapidly? Standing naked in -20C (still) air on cloudy day in a dry desert (**) or standing naked at +34C (still) air on a cloudy day in a dry desert? Or if you were to determine the mechanism most efficient in cooling the human body, what would you find at the two temperatures? Why is radiation so effective in cooling the human body at -20C, while ineffective at +34C? Does the temperature of the cooler environment have an effect on the human’s body to cool via radiation?
(** Of course the air’s water vapor content affects the body’s ability to cool via evaporation. For the sake of simplicity I’ve assumed this content is the same in both environments.)
““I think you need to look at this graph:”
Seen it. It’s a simplification.”
And “the earth is a sphere” is a simplification.
This doesn’t make the earth flat.
“Downward convection is perfectly physical. Otherwise we’d run out of air at the surface!”
This is not going to move any heat toward the ground.
“The question is whether the equator-pole temperature difference would be enough to drive the present level of circulation throughout the troposphere.”
The answer is “no”.
And neither does it mean that backradiation doesn’t happen.
However much you want it to.
Why wouldn’t it move any heat toward the ground?
And I’ve said, several times, that back radiation does happen. Where did you get the idea that I thought (or wanted to think) otherwise?
Because if it were hotter than the air already at the ground, it would find itself more buoyant and stop falling.
Duh.
Here:
“Because if it were hotter than the air already at the ground, it would find itself more buoyant and stop falling.”
Unless it had sufficient momentum, or pressure behind it, to force it against the temperature gradient.
The question would be whether the buoyancy force upwards at the equator was greater than the buoyant resistance to its descent at temperate and polar latitudes.
Hadley cell circulation is one of the ways that heat is transferred from equator towards the poles.
“Duh.”
Well, if you can’t argue politely and with respect, I don’t think I’ll bother any more.
Nullius in Verba:
What’s your equation for convective transfer of heat from the atmosphere to the surface?
“What’s your equation for convective transfer of heat from the atmosphere to the surface?”
Not sure how that helps, but here you go:
MALR = g*(1+H_v*r/R_sd*T)/(c_pd+H_v^2*r*e/R_sd*T^2)
g = gravitational acceleration at surface;
H_v = heat of vaporisation of water;
r = ratio of mass of water vapour to dry air;
R_sd = specific gas constant of dry air;
R_sw = specific gas constant of water vapour;
T = temperature;
c_pd = specific heat capacity of dry air;
e = R_sd / R_sw;
Or did you want the Navier-Stokes equation?
H_v would be not convection but condensation.
Please try again.
I notice you haven’t tried again.
H_v would make this about condensation, not convection.
And that would require that the air cools as it drops down.
“Well, if you can’t argue politely and with respect, I don’t think I’ll bother any more.”
You haven’t bothered yet. What’s this “any more”?
“Hadley cell circulation is one of the ways that heat is transferred from equator towards the poles.”
And that has nothing to do with the vertical profile of the earth, it only moves the energy around the surface, not out of the system, and the rate at which polar transfer moves northward is limited by pressure differences against the Coriolis forces and doesn’t manage to get the flow rate required to equilibriate temperatures. We have a drop of ~40C in ~12,000 miles. You get that in 10 miles of atmosphere.
“Unless it had sufficient momentum, or pressure behind it, to force it against the temperature gradient.”
So unless there’s a confluence of difficult factors, your effect doesn’t exist. How can it explain the temperature of the earth surface, then?
It doesn’t, but you seem to be clutching at straws.
I note that all your work here has been, to use your dismissive phrase, a simplification.
Make a proper model and write to a journal, get it published.
“The question would be whether the buoyancy force upwards at the equator was greater than the buoyant resistance to its descent at temperate and polar latitudes.”
No, the question is, do you think that the reason why the poles are cold is because the cold upper air over the tropics comes down over the poles?
“No, the question is, do you think that the reason why the poles are cold is because the cold upper air over the tropics comes down over the poles?”
That’s an easy one! No I don’t.
Foehn winds that blow down the sides of mountains are notably warm. And yet the air at the tops of mountains where they come from are cold. Where do you think the heat comes from? How can it be descending?
Why, for that matter, do you think the tops of mountains are cold? Everyone knows that hot air rises…
“And yet the air at the tops of mountains where they come from are cold. Where do you think the heat comes from?”
Gravity.
“How can it be descending?”
Not by downward convection.
It’s done by pressure differences occasioned by the fact that the air has been forced over higher orography.
But PV=nRT.
If P is lower, V has to either be higher or T lower.
Which happens is dependent on the specifics.
You’re flailing, trying to find a reason why the downward radiation from the atmosphere is having no effect on the earth’s temperature.
God knows why you think hadley cells are it.
“You’re flailing, trying to find a reason why the downward radiation from the atmosphere is having no effect on the earth’s temperature.”
The question is why the downward radiation has so little effect.
“Gravity.”
Tch! And I was expecting you to say “back radiation”! That is why you think the surface is warmer, isn’t it? Presumably you think the mountains are colder because they don’t receive as much back-radiation, or something, what with being nearer to the source. Or I guess maybe you’ve changed your mind?
Actually, I’d have said “latent heat”, but I’ll accept gravitational potential energy. Well done.
“It’s done by pressure differences occasioned by the fact that the air has been forced over higher orography.”
Forced by the prevailing wind, which is the result of temperature differentials between different areas – i.e. the horizontal component of… yes! convection!
I notice you didn’t answer the question. You said earlier that convection-driven hot air could not descend to the ground “Because if it were hotter than the air already at the ground, it would find itself more buoyant and stop falling.”
So the foehn is descending, is hotter than the air already at the ground, and yet does not stop falling because it is more buoyant. But you said such an effect did not exist.
So how can it happen?
30 C surface temperature rises over a matter of a few hours have been reported, as the wind changes direction. 30 C!!! That’s a pretty dramatic warming effect. And it runs downhill from higher altitudes! Impossible, yes?
The reason I mention Hadley cells is that they form a loop in which hot air rises, moves polewards, and then descends. That descent is driven by the rising air at the equator. It warms those sub-tropical areas above the temperature they would have without the circulation. It’s a good example of heat-carrying downward convection in action.
Believe me, I’m not flailing. I’m just having a bit of fun while I wait for SoD to come back with his carefully considered answer.
“The question is why the downward radiation has so little effect.”
33C warming isn’t a little effect.
“33C warming isn’t a little effect.” I should have said: why the extra downward radiation supposedly caused by extra CO2 has so little effect.
Why should you have said that? It’s not true either.
CO2 contributes about 11C to that 33C.
I think the little effect is you on reasoned discourse.
“Reasoned discourse”; very good Mark, you’re obviously setting the bench[mark]. But I think you are a bit off with CO2’s contribution to the 33C; the greenhouse ratio of water to CO2 is about 2.5:1; so that would make CO2’s contribution = 33/7 x 2 = 9.43. Plus I said EXTRA, not the already established 33C; according to AGW that 33C is now ~ 33.7C; on the basis of the above ratio, CO2’s contribution to that is ~ 0.7/7 x 2 = 0.2C. Is that correct Mark?
“But I think you are a bit off with CO2′s contribution to the 33C; ”
No, it’s at the top end, but if it isn’t responsible for anywhere near 11C of that 33C warming, then the sensitivity of the system to CO2 increases is much higher than 3.
“33C warming isn’t a little effect.”
You mean 68 C, don’t you?
The top of the troposphere is at -54 C, and the bottom at +14 C, all in one straight line, which means the temperature difference created by the atmosphere you need to explain is 68 C.
“You mean 68 C, don’t you?”
No.
“The top of the troposphere is at -54 C, and the bottom at +14 C”
Aye.
“which means the temperature difference created by the atmosphere you need to explain is 68 C.”
No.
All that needs to be explained is how an earth at ~288K actual measured average temperature pertains when the solar radiation out here 1AU distant manages to account for only 255K.
That is all that needs to be explained.
And it has.
I think YOU need to explain why you think 68C needs explaining…
Has it occured to anyone that the 300 watts/cm^2 DLR is caused by absobtion of lwr from the sun and that the greenhouse gasses are already saturated or energized and releasing that absorbed energy down to the earth. Then the outgoing raadiation can pass through because the GHG were saturated by the solar energy.
Nullius in Verba,
Emission and absorption are inextricably linked (Kirchhoff’s Law, local thermal equilibrium, Boltzmann distribution, etc.). If the atmosphere stopped emitting in any direction, it must stop emitting in all directions. But at the same time, it must stop absorbing too and become perfectly transparent. So if it stopped emitting, it couldn’t warm. The surface would then emit directly to space and with only incoming sunlight to warm it, the surface temperature would drop. The resulting heat loss would eventually cool the atmosphere as well.
DeWitt,
Thankyou. I don’t disagree. (Although I would note in passing that what you say is only true at each particular wavelength.)
I agree that with a transparent atmosphere, the surface would cool. I think perhaps that some people are misunderstanding what I’m trying to argue. Because I argue and object to SoD’s DLR argument, some of you are assuming that I fall into the general category of people who don’t believe there is any greenhouse effect, who think that GHGs have no effect on the surface temperature, or that I either don’t know or don’t believe conventional meteorology. Or for that matter, that what I’m arguing is a crank theory and not already extant in the orthodox literature. I can certainly understand that, in the context, and I don’t mind.
I took SoD’s question as a sensible attempt to try to understand exactly what I was trying to say, and I truly appreciate that he makes the effort – unlike a lot of other people elsewhere. (His responses on previous occasions have clarified things for me, and led me to change my mind on a point or two.) In this case, the question of what would happen if DLR was stopped, but absorption and upward emission continued is very relevant, since it is my contention that these last two are essential to the greenhouse effect, but that DLR has no net effect in a convective atmosphere. It certainly exists, but it is cancelled out.
It is an excellent thought-experiment, but I wanted to make clear that in trying to answer it, I didn’t want to commit to any particular unphysical consequences of the unphysical assumption.
Yes, if you assume DLR stopping implies all radiative interaction stops, then it changes things. If you allow a bit of ‘thought-experiment’ magic, then one can arrange for it not to.
The point I am trying to make is that in a convectively-coupled atmosphere, the temperatures of the parts are held in rigid relationship, and radiation internal to such an atmosphere, like the internal forces in a rigid body, make no difference. Either you have to claim that the troposphere and surface are not convectively coupled, or that the lapse rate in a convective troposphere is radiation-dependent – being increased by DLR.
I don’t see any other way of doing it – although I am open to new options – and I don’t see anyone saying either of the above.
Thanks for being so patient.
“I don’t see any other way of doing it – although I am open to new options –”
No you’re not.
One option you’re not open to is the one in the title.
“Either you have to claim that the troposphere and surface are not convectively coupled”
OK, it isn’t.
http://en.wikipedia.org/wiki/Hydrostatic_equilibrium#Atmospherics
http://farside.ph.utexas.edu/teaching/sm1/lectures/node54.html
But local excesses outside equilibrium are a mechanism to remove these small deviations.
Since nobody jumped on me yet, let me expand on that concept. At night the sun is no longer exciting the GFGs so you are left with a straightforward energy flow from warm to cooler.
Nobody jumped on you because you don’t make any sense.
No, the GHG are excited by the Earth’s radiation and all gases contribute to the storage of energy hence when CO2 lets go, N2 bumps it back up until the energy is again equipartitioned.
And even during the day, you have straightforward energy flow from warm to cooler. Night time doesn’t change that.
Mark; What happens to all that lWR from the sun comming through all those greenhouse gasses?
What “all”?
microwatts from the sun >4um.
It is a completely negligible sum.
Various absorption (as in clouds where about to rain they become more absorptive water rather than reflective droplets) totals 67W/m^2, throughout the volume. But that absorption is more at the short wave lengths, not LWR.
Compared to the 492W/m^2 it gets from underneath, this is peanuts.
““Gravity.”
Tch! And I was expecting you to say”
Why? Do you think that back radiation explains the lapse rate?
No, it doesn’t (see Venus pt2).
“That is why you think the surface is warmer, isn’t it? ”
It IS why the surface is warmer than the absence of GHG would have it.
“So the foehn is descending, is hotter than the air already at the ground,”
It isn’t. It’s falling adiabatically and therefore, though warmer than it was up above, is not producing extra energy, it’s just realising gravitational potential energy.
This is not “reverse convection”.
“30 C surface temperature rises over a matter of a few hours have been reported”
Citation needed.
I bet you mean air temperatures.
And we don’t get fohn winds everywhere (this is why they have such a funny name: they’re uncommon).
“The reason I mention Hadley cells is that they form a loop in which hot air rises, moves polewards, and then descends.”
And they aren’t reverse convection and they don’t GIVE heat to the tropical region.
They take it away (because the bottom of the Hadley Cell draws the tropical air toward the equator because the loft of the air causes a pressure drop there, drawing air in.
Funny how you don’t seem to know how Hadley Cells work and you’ve complained about K&T’s “simplification” yet you have proposed an even grosser simplification to avoid K&T.
Odd.
“Believe me, I’m not flailing.”
Yes. Yes you are.
“Why? Do you think that back radiation explains the lapse rate?”
No, I think you do.
“It IS why the surface is warmer than the absence of GHG would have it.”
It can’t be, in a convectively coupled atmosphere.
“It isn’t. It’s falling adiabatically and therefore, though warmer than it was up above, is not producing extra energy, it’s just realising gravitational potential energy.”
I didn’t say “producing extra energy”, I said “hotter than the air already at the ground”. The two are not the same. It would help, I think, if you didn’t keep answering points I didn’t make.
“This is not “reverse convection”.”
I didn’t say it was. I’m not even sure what “reverse convection” is.
The wind is a consequence of perfectly normal, “forward” convection. ‘Convection’ consists of a cycle. The air goes up in one place. The air comes down in another. The air quite frequently travels horizontally to get from one to the other, which is where we get wind from. You can’t just cut out one tiny piece of a global phenomenon and totally ignore what happens to the air in the rest of the cycle.
“I bet you mean air temperatures.”
It reportedly melts the snow, being given the name “snow-eater”.
“And we don’t get fohn winds everywhere”
Now where on Earth did I say we got them “everywhere”? Who is it you are refuting?
And it’s a perfectly unfunny German word.
“And they aren’t reverse convection and they don’t GIVE heat to the tropical region.”
Again, I didn’t say they were “reverse convection”, and I didn’t say they give heat to a tropical region. I said they transported heat polewards to sub-tropical regions.
“They take it away (because the bottom of the Hadley Cell draws the tropical air toward the equator because the loft of the air causes a pressure drop there, drawing air in.”
Absolutely correct! And what happens to it then?
“Funny how you don’t seem to know how Hadley Cells work”
But you’ve just agreed with everything I said on how Hadley cells work. So if I don’t understand, then you evidently can’t, either.
Without wishing to be rude, I think the problem is reading comprehension. I say one thing, and you respond as if I had said something entirely different. It’s not getting us anywhere.
I must say, I find it mildly entertaining, but I don’t think SoD would approve, so I think I’ll skip the next couple of rounds; let things cool down a little.
“The wind is a consequence of perfectly normal, “forward” convection. ‘Convection’ consists of a cycle”
But that cycle doesn’t bring energy down to the ground. Therefore it cannot cause heating.
Mark; You’re right. I didn’t make any sense. Sorry.
That’s OK, being wrong is accepted.
An idea that comes to you often only makes sense while you aren’t thinking it through.
Humans do it all the time.
And that’s why so many problems disappear when talking to someone else: it gets you thinking about it all over again.
““Funny how you don’t seem to know how Hadley Cells work”
But you’ve just agreed with everything I said on how Hadley cells work.”
No, you’ve also said they cause the temperature difference between the air and the ground.
This I disagree with and nothing I HAVE agreed with enables this potty hypothesis of yours.
Nullius in Verba,
You are obviously suggesting that SOD’s post is misleading because it is an oversimplification of the actual relationship between the atmosphere and surface. What do you think is the consequence of SOD’s simplified explanation?
My two cents: The normal backradiation argument seems to be unnecessarily confusing (and can only be illustrated by oversimplification of the physics). It seems that the more straightforward explanation is to point out the following:
1) The surface is heated by solar energy.
2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.
3) Energy travels from surface through troposphere mainly by convection (since gas concentrations are high enough to prevent large net flow of radiation). ie. Air is heated by surface causing it to expand. Expansion causes air to simultaneously rise to higher altitude and also cool. The net effect is energy travelling from surface to higher altitude as the warmed air simultaneously rises and cools (relative to atmosphere/surface interface).
4) Thermal energy is transfered from upper atmosphere to space via radiation where it is cooler and more transparent to the outgoing radiation.
This is my understanding. I think more closely resembles the actual physics and is less confusing with regard to 2nd law. What am I missing?
Mike,
Thanks for helping out.
Here’s my version.
1) A known and fixed quantity of energy enters the system, and it must all leave by radiation to outer space. The two must balance on average, to a very close approximation.
2) The temperature of the radiating surface adjusts to emit precisely this amount of energy.
3) Because of GHGs in the atmosphere, this radiating surface is not the solid ground, but a fuzzy layer that averages 4-6 km up in the air.
4) The layer 4-6 km up adjusts in temperature to emit precisely the required amount of radiation to space.
5) The temperature of the rest of the atmosphere, both above and below, is locked in a fixed relationship to that of this middle layer by convective coupling. As air mixes vertically, the change in pressure with height compresses and expands the air, changing its temperature. The rate of change of temperature with altitude is a fixed constant (called the adiabatic lapse rate), that does not involve radiation in setting its value.
6) The temperature of the surface depends only on the altitude at which the effective radiative temperature is achieved (i.e. 4-6 km) and the lapse rate between there and the surface. The surface temperature is T_eff + LR*h.
7) As more GHGs are added to the atmosphere, the opacity of the atmosphere at LW increases and the radiating surface gets higher. As h increases, so does surface temperature. Global warming.
The only bits of real physics you need are that it is the visible emitting surface that approaches the Stefan-Boltzman calculated equilibrium, which is obviously above the ground with a semi-opaque atmosphere, and the adiabatic lapse rate, which is just the idea that gases get hot when you compress them. These in combination imply surface warming above T_eff.
And while SoD is perfectly correct about DLR being real and physically important, it’s a complete red herring in terms of explaining why the global temperature is changing, or is what it is. All this argument about whether back-radiation complies with the laws of thermodynamics (which it does) is a waste of time. Except to the extent that learning about physics is never a waste of time.
I’d like to point out that I didn’t invent any of this myself. It was the result of reading Soden and Held 2000 in the peer-reviewed and quite orthodox literature, where you will find the gist my explanation in the text just below figure 1 on page 447.
It’s even been cited by the IPCC.
So if anyone wants to prove me and the IPCC wrong, I am, as I said, open to new ideas. But it’s going to take something a bit better than making up bizarre stuff I never said and then calling it “potty”. 🙂
As I said above, Mike, my thanks.
“My two cents: The normal backradiation argument seems to be unnecessarily confusing (and can only be illustrated by oversimplification of the physics)”
No, it can be illustrated by the actual processes taking place. Managing without backradiation would require a simplification of the physics.
“2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.”
Like this. HOW does the atmosphere insulate the system and how does that reduction make the system hotter?
Backradiation is the actual process. What’s your description?
“3) Energy travels from surface through troposphere mainly by convection”
No.
“I think more closely resembles the actual physics and is less confusing with regard to 2nd law.”
Only because you ignore the actual processes.
Backradiation doesn’t get confusing with the second law, no matter how much you try to make it confusing.
“What am I missing?”
How this:
“2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.”
happens.
What physical processes take place to do this?
Mark,
People intuitively understand the effects of insulation. You point out that these concepts of insulation/backradiation are not contradictory. I thought that was obvious.
You corrected me on one point though. I suggested that convection was primary means of transporting heat through troposphere. I guess that means that the greenhouse gases in troposphere are better conductor (ie. weaker insulator) of radiation than I thought. Either that or you’re mistaken.
Mark, might I suggest that you work on adding more tools to your commenting repertoire.
‘When all you have is a hammer, every problem [or alternative argument] looks like a nail’.
“I guess that means that the greenhouse gases in troposphere are better conductor (ie. weaker insulator) of radiation than I thought.”
No, you guess wrong.
Radiation goes as T^4.
Conduction goes as T.
Convection goes as a lower power of T (buoyancy).
“Mark, might I suggest that you work on adding more tools to your commenting repertoire. ”
Mike, may I make a suggestion that you add more physical tools before propounding on physics.
Nullius in Verba:
I’m guessing that MALR is the moist adiabatic lapse rate? That isn’t the equation of heat transfer from the atmosphere to the surface.
Generating around 300W/m^2 of conduction/convection seems challenging.
Remember that the surface is radiating at a high level, so the difference between the direct solar absorption and the surface radiation has to be supplied by convection.
There is a 2nd point related to your original comment from July 31, 2010 at 9:15 pm:
I guess there are a number of ways to view the DLR not being absorbed by the surface. What I was thinking about – although I didn’t explain it – was that the DLR would be radiated to the earth and reflected.
But if the atmosphere instead stops radiating then there is a huge heat transfer problem – how does heat leave the planet?
Back to your original concept – without DLR being absorbed and “changing the temperature” of the surface, the exact same conditions would still be recreated by the miracle of convection.
It’s perhaps a similar discussion to what took place in Venusian Mysteries – Part Two
However, what I’m looking for here is something more than “the lapse rate does it”. That might take some thinking about.
The first obvious place where there was a weakness was how the heat transfer to the surface took place.
How can you demonstrate your hypothesis?
And perhaps you need to explain the thought experiment conditions more precisely.
Nullius in Verba:
I see that you have already partly commented on the experiment at August 1, 2010 at 7:07 pm. I only read that after writing my comment above.
What it suggests to me is that your thought experiment should simply be one of decreasing the quantity of GHG’s in the atmosphere, rather than just removing absorption of DLR.
Clearly there is a point at which “the miracle of convection” no longer “makes up” for the loss of GHGs – as you are in agreement that with no GHGs the surface would be – on average – radiating only 239W/m^2.
This also hints at the idea that finding this point might expose the weakness in your thought experiment. (Like, if you can’t find this point there might be something wrong with the argument in the first place).
SoD,
Well, I wasn’t exactly sure what you meant by “the equation”. The main point of interest in the equation I gave is that it doesn’t involve any radiative physics. The temperature gradient with altitude depends only on the properties of gases. But other than that, since the mechanism doesn’t work by fixing the quantity of heat transfer, an equation to specify that wouldn’t be very meaningful.
I agree that in an atmosphere where the radiative lapse rate never reaches the size of the adiabatic one, you wouldn’t get any convection, and your radiative model would result in an exponential temperature profile. It’s an interesting and useful thought experiment. But is it how it works in the real atmosphere?
Anyway, see my comment above, which crossed with yours.
I tend to agree with Nullius; there is a greenhouse effect, there is backradiation but that convection is the dominant heat transfer mechanism; Lindzen looks at this here and considers the scenario of purely radiative transfers [which would result in a much hotter atmosphere] and CO2 contribution to the greenhouse:
http://blogs.news.com.au/dailytelegraph/timblair/index.php/dailytelegraph/comments/green_themes/
Chilingar, while getting bad press, also considers the dominance of convection over radiative transfer:
http://www.informaworld.com/smpp/content~db=all?content=10.1080/15567030701568727
I don’t have access to the full paper but it says:
“According to our estimates, convection accounts for 67%, water vapor condensation in troposphere accounts for 25%, and radiation accounts for about 8% of the total heat transfer from the Earth’s surface to troposphere.”
Found a nice site that provides near-real-time measurements of upwelling and downwelling radiation for several sites – the SurfRad Network.
All kinds of measurements are available and there are easy-to-use plotting and data download functions.
http://www.srrb.noaa.gov/surfrad/index.html
For example, the monthly means net solar, downwelling IR and upwelling IR radiation for Table Mountain Co. is interesting (daily and other measurements are also available).
http://www.srrb.noaa.gov/cgi-bin/ave_check?site=tble&year=2009&p7=dpir&p8=upir&p9=rns&ptype=gif
Upwelling IR provides a good indication of surface air temperature using the SB equation.
Surface temperature, not surface air temperature.
Under sunlight, there’s a large temperature drop and conduction only occurs in the bottom few mm.
Almost the entire flux upward is shortwave from the ground, not the air near the surface.
Surface temp, not surface air temp (generally 1.5m or 10m above surface).
Reading a few comments wondering why the focus on “back radiation” and how this focus is simply making understanding atmospheric physics more difficult..
The reason for the three articles about “back radiation” is because so many people say:
1. It doesn’t exist
2. It doesn’t come from “greenhouse” gases
3. It doesn’t have any effect on the surface of the earth
Armed with one, or more, of these three points of view, atmospheric physics will forever be a mystery.
If you have safely grasped that DLR exists and doesn’t violate any thermodynamics laws – as most commenters have – then that’s wonderful.
Probably there will be many readers who are not commenting who do have a problem understanding DLR.
These articles are written for them.
Nullius in Verba:
I think you have stated a few different ideas, some of which I agree with (at least in principle) but one that I can’t see being correct.
The one that I can’t see being correct is the idea that with no absorption of atmospheric radiation by the surface that convection can supply this back to the surface.
Typically, equations for convective transfer from a fluid/gas to a surface require a temperature differential and some kind of convection coefficient. These equations are of the form: q=h(T2-T1), where h is the coefficient, but not a constant.
These convection coefficients are usually empirically derived because solving the fundamental physics is so difficult/impossible.
There probably are some empirical equations covering heat transfer from the atmosphere to the surface – but I’m guessing that to transfer 300W/m^2 you need a very high temperature differential.
Now, if that doesn’t happen the surface temperature starts to fall, less circulation and heat and finally you have a much lower temperature for everything.
However – and this is why I am confused – you also seem to agree through your later statements, e.g.,
And on your question at the end, perhaps I might write an article on that one..
A point of clarification: when you say “The one that I can’t see being correct is the idea […] that convection can supply this back to the surface”, do you mean can convection move it from high altitude to low altitude, or can it move from the air (at low altitude) to the solid surface?
I am thinking of it as like a fan heater. You have a 50 W fan blowing past a 3kW heater element. It delivers 3.05 kW of heat. Convection only has to provide the 50 W to move the air. So looking at the total heat to be delivered as being the same as the amount of power driving the convection might not be the easiest way to go. The question would be, is the 50 W sufficient to push the hot air down to the surface?
Since the Earth is heated differentially, from equator to poles, I don’t see how it would be possible for there to be no convection. The temperature differential will have some effect on the air. But I can’t be sure what it would be. Maybe the tropopause is lowered in altitude or something.
One possibility might be that the upper atmosphere over the tropics is warmed far more than the air over the poles, a large temperature differential builds up at the top of the atmosphere, and this drives extremely rapid circulation. You can transfer the same amount of heat at a lower temperature if you move the air faster. It would be a very different situation from the normal atmosphere, though, so I’m not sure how illuminating it is regarding the actual mechanism.
But interesting thought-experiment aside, it still doesn’t answer my original point. If the atmosphere actually is convectively coupled, so that temperature differences between its parts are held fixed, how is it possible for an internal heat transfer between the parts to result in a temperature change at one end of the transfer? Without having the convective coupling immediately erase it?
Mark:
You have a comment in the pending queue. The spam / moderation filter is now tuned into various uncomplimentary words that can be used.
If you want to help someone see your point of view this isn’t the way.
It would be very easy for Science of Doom to become like so many other blogs but I would really like to avoid it.
Please help me in this endeavor.
You are assuming that there are people here asking questions in good faith.
They aren’t.
Please help me in this endeavor by acting as if everyone is asking questions in good faith.
I believe they are.
Can you say that of Nassif?
No.
How about Bill who refuses to say whether he will accept the line model for absorption?
Some are.
Some aren’t.
“Lindzen looks at this here and considers the scenario of purely radiative transfers ”
And here are several other scientists who consider back radiation to be absolutely correct:
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
The point is: is convection a bigger heat mover than radiation; it is.
I’ve also moved this exchange down here for convenience:
“on August 1, 2010 at 10:32 pm | Reply cohenite
“Reasoned discourse”; very good Mark, you’re obviously setting the bench[mark]. But I think you are a bit off with CO2′s contribution to the 33C; the greenhouse ratio of water to CO2 is about 2.5:1; so that would make CO2′s contribution = 33/7 x 2 = 9.43. Plus I said EXTRA, not the already established 33C; according to AGW that 33C is now ~ 33.7C; on the basis of the above ratio, CO2′s contribution to that is ~ 0.7/7 x 2 = 0.2C. Is that correct Mark?”
“on August 2, 2010 at 7:32 am Mark
“But I think you are a bit off with CO2′s contribution to the 33C; ”
No, it’s at the top end, but if it isn’t responsible for anywhere near 11C of that 33C warming, then the sensitivity of the system to CO2 increases is much higher than 3.”
I don’t know what you mean by “at the top end” and how you conclude that if CO2 contributes < 11C to the 33C that CS to CO2 increase is greater; could you explain please.
“The point is: is convection a bigger heat mover than radiation; it is.”
No it isn’t.
When something rises, it loses energy to the gravitational potential field.
Look up CAPE (Convectively Available Potential Energy). That is available to pass up the atmosphere, but it isn’t a common effect and it is a small fraction of the energy flux.
“and how you conclude that if CO2 contributes < 11C to the 33C that CS to CO2 increase is greater; "
Because CO2 (e) is a driver, H2O isn't.
If 3 degrees was produced by CO2 and the feedbacks were 11x the effect, then we would have 33C. That is a high sensitivity to CO2, though.
If the feedbacks multiplied by 3x, we would only have 9C total effect, too small to explain the earth's temperature.
“Plus I said EXTRA, not the already established 33C; according to AGW that 33C is now ~ 33.7C; ”
And we are not at equilibrium yet.
Null, you may have missed the question. I repeat:
How this:
“2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.”
happens.
What physical processes take place to do this?
1- Chart the Delta T of water as it evaporates.
2- Chart the Delta E of water as it evaporates.
3- Chart the black body spectrum of water as it evaporates.
Repeat for the phase transition of water to ice.
Assess the value of radiation physics in comprehending phase change on a planet that is covered with an ocean.
???
Profit!!!!
CAPE is where an ascending air parcel is warmer than the ambient air. At the surface the parcel is warmed by either conduction or radiation; that radiation is either back-radiated to the surface [from a low height] or maintained in the parcel which takes on LTE properties until it reaches the CEL where the parcel is no longer warmer than the ambient air; that ascent is at a rate of metres per second which is much faster than diffusion at the surface which is dependent on the specific heats of the gases in the air parcel, as is the convective uplift. You can’t have it both ways; the same properties of the air parcel which produces slow diffusion also produces the rapid convection.
“Because CO2 (e) is a driver, H2O isn’t.” This is not true:
http://www.agu.org/pubs/crossref/2007/2007JD008431.shtml
CO2 is a feedback naturally:
http://www.nature.com/nature/journal/v463/n7280/abs/nature08769.html
The IPCC argument, which I presume you are making, is that ACO2 is not natural and is therefore a forcing or driver; but as the Franks paper shows the sensitivity of the ACO2 to the climate change it produces is low; which is why IPCC estimates that 2xCO2=1C.
I don’t follow your arguments about CO2’s role in establishing the greenhouse temperature of 33C, however this is problematic: “And we are not at equilibrium yet.” ES, as a concept seems to be one of the most dubious of the AGW lexicon, dependent on the notion of a long residency rate for CO2 which is debatable:
http://c3headlines.typepad.com/.a/6a010536b58035970c0120a5e507c9970c-pi
ES [equilibrium sensitivity] also relies on sinks or reservoirs for the energy and the deep ocean does not appear to be doing that; generally climate response is less than a year and mostly much less;
Click to access 2000JD000298.pdf
I’ll post this response to Mark again as I obviously overdid the links the first time and got caught in the moderator; so I’ll try again leaving out the final link to the Trenberth paper:
CAPE is where an ascending air parcel is warmer than the ambient air. At the surface the parcel is warmed by either conduction or radiation; that radiation is either back-radiated to the surface [from a low height] or maintained in the parcel which takes on LTE properties until it reaches the CEL where the parcel is no longer warmer than the ambient air; that ascent is at a rate of metres per second which is much faster than diffusion at the surface which is dependent on the specific heats of the gases in the air parcel, as is the convective uplift. You can’t have it both ways; the same properties of the air parcel which produces slow diffusion also produces the rapid convection.
“Because CO2 (e) is a driver, H2O isn’t.” This is not true:
http://www.agu.org/pubs/crossref/2007/2007JD008431.shtml
CO2 is a feedback naturally:
http://www.nature.com/nature/journal/v463/n7280/abs/nature08769.html
The IPCC argument, which I presume you are making, is that ACO2 is not natural and is therefore a forcing or driver; but as the Franks paper shows the sensitivity of the ACO2 to the climate change it produces is low; which is why IPCC estimates that 2xCO2=1C.
I don’t follow your arguments about CO2’s role in establishing the greenhouse temperature of 33C, however this is problematic: “And we are not at equilibrium yet.” ES, as a concept seems to be one of the most dubious of the AGW lexicon, dependent on the notion of a long residency rate for CO2 which is debatable:
http://c3headlines.typepad.com/.a/6a010536b58035970c0120a5e507c9970c-pi
ES [equilibrium sensitivity] also relies on sinks or reservoirs for the energy and the deep ocean does not appear to be doing that; generally climate response is less than a year and mostly much less;
http:www.cgd.ucar.edu/cas/papers/2000JD000298.pdf [// excluded]
“CAPE is where an ascending air parcel is warmer than the ambient air.”
Indeed.
Which will be over-endowed in the energy department.
“that ascent is at a rate of metres per second ”
More normally cm/sec or less. Meters per second is the large uplift you get in big convective storms.
““Because CO2 (e) is a driver, H2O isn’t.” This is not true:”
I’m afraid it IS true.
http://www.ipcc.ch
“CO2 is a feedback naturally:”
And takes a long time to outgas. Of the order of a thousand years.
“The IPCC argument, which I presume you are making, is that ACO2 is not natural”
If ACO2 is meant to be “Anthropogenic CO2”, then it isn’t natural. Petrol doesn’t naturally refine itself and then combust in a refined steel alloy chamber…
“but as the Franks paper shows the sensitivity of the ACO2 to the climate change it produces is low”
It shows no such thing.
Franks paper shows that his calculations give a low sensitivity (Franks paper is wrong).
But if that is the case, then the proportion of CO2’s contribution to the 33C warming from GHG must be higher.
“I don’t follow your arguments about CO2′s role in establishing the greenhouse temperature of 33C,”
Then read up on it:
http://www.aip.org/history/climate/co2.htm
And we’re not at equilibrium because it takes ~800 years for the deep oceans to overturn and reach equilibrium.
PS, that’s Franks *letter* not paper.
It doesn’t have to go through any peer review.
PPS that paper on CO2 residency doesn’t say what you want it to mean.
You want it to mean that a 10Gt leaves the atmosphere in 8 years.
‘fraid not.
All it means is that that specific CO2 molecule will have passed in to the biosphere in that time (on average). The excess is not annulled. Just moved.
Thanks for the blog. It helps people like me who studied physics at high school forty years ago, and are still interested. Asking questions can be difficult. Partly through fear of appearing stupid, but often because half way through writing the question I realise there are two (then four, etc) other things I need to ask too before I can finish my original question.
You said above, “Many people have some vague idea that this kind of approach is how the second law of thermodynamics works down at the molecular level.”
I guess I fell into that group, but I don’t fully understand your explanation. I think you are saying that the options are that a photon is absorbed or reflected, but it can’t be “bounced”. I’m wondering about the option that the photon is not emitted in the first place. I gather from previous articles that this idea is seen as absurd because it implies intelligence or pre-knowledge on behalf of the photon or the emitting molecule. I.e. knowledge at a distance. But from my understanding of relativity, from the photon’s point of view, there is no distance to travel. It merely transfers it itself to an immediately adjacent target. When seen like that, “intelligence” seems less of a requirement.
I’m not suggesting this is what happens, or trying to re-instate imaginary laws. I’m just questioning how absurd the idea is.
DaveC; That would be Young’s experiment and superposition.
So Dave can use “s t u p i d” but I can’t?
Here again (though much shortened because frankly I can’t be arsed doing it all again):
It’s OK being s t u p i d because that’s what happens when you don’t know. If you only ask questions you already know then you’ll appear clever but won’t learn much. It’s when you ask questions you don’t know you learn. And since you don’t know them, you may appear s t u p i d.
Can’t see why that was such a horrible thing to say.
Davec:
I appreciate you asking the question. I hope that your question will encourage others with questions not to feel intimidated.
Well, that’s not really the case for relativity. You might be thinking more about quantum mechanics.
In any case, let’s look at emission. What is the mechanism behind the emission of a photon?
The mechanism is simply about a quantum of energy – meaning that only distinct levels of energy can exist rather than continuous changes in energy – from a molecule which is in “an excited state”. This excited state might be from vibration or rotation from energy acquired earlier.
When emission takes place the energy is “given up” and so a photon is released. The photon has that distinct energy “quantum”. So, for example, a 10um photon is released if the energy change from a rotational or vibrational state to a ground state is 2×10^-20 J. (Or between states).
The photon will in fact be released in any direction. This is what is observed.
It would be easy to prove (if it was true) that photons only went in the direction of lower temperature, but no one has ever observed this.
In the strange world of quantum mechanics it seems like anything could be possible – but this one doesn’t actually take place.
The main thrust of the argument theoretically is simply that the emission of a photon depends on the energy states that exist for that molecule – and the far surroundings don’t come into the equation.
And just to clarify a less important point:
Well, in the vernacular I was using, “bounced” is “reflected”. Photons have to be absorbed or reflected from the surface of the earth, so there isn’t a third choice.
S.o.D.
Thanks for the reply. In my mind at least, I still see my question as one of relativity. I.e. not the mechanism of emission, but the path between the source and destination. My view is something like this “go-splat” description found on another site:
‘The photon lives in a “go-splat” world. The clock of a photon completely stops the instant it is emitted and stays stopped throughout its journey. The distance traveled by a photon becomes zero as compared to the distance measured by the stationary observer. ‘
I can accept the idea that a photon can be released in any direction, and also that the temperature of the destination has no bearing on which path is taken. However, I’m still not sure why the idea that some destinations may be preferred over others due to their current state, while possibly wrong, is necessarily absurd.
Cohenite,
I’m not sure why you mentioned Young’s experiment. But oddly enough, it was the way the experiment was described to me at school and that has bugged me ever since that probably triggered my question. I couldn’t accept that a photon ‘travelled’ in the same way as a thrown ball. I still view it as a go-splat sequence, and while we can try to measure the path it took, it’s impossible to to say anything about its path before it arrives at its destination. While the teacher was asking us to consider “which slit”, I had an image of the photon asking “what slits?”
From the photon’s POV, there’s neither distance nor time.
But from the POV of the absorber or emitter, there’s definitely both.
And therefore the absorber has to know the emitter is there instantaneously.
cohenite,
I just wanted to comment on a point that you made:
“I tend to agree with Nullius; there is a greenhouse effect, there is backradiation but that convection is the dominant heat transfer mechanism; Lindzen looks at this here and considers the scenario of purely radiative transfers [which would result in a much hotter atmosphere] and CO2 contribution to the greenhouse:”..
This is fairly consistent with my understanding (which may or may not be correct). I’m trying to figure out whether or not this is the same thing that Nullius is arguing. This was why I asked Nullius about what he thought the consequence was of describing only radiative energy transfer.
When we say that convection is the dominant heat transfer mechanism I assume we mean to say as a mechanism of drawing heat away from surface through troposphere. Otherwise if heat could only travel as radiation or through conduction you would presumably need a larger temperature differential across the troposphere (and higher surface temperature) to transfer enough thermal energy to match absorbed solar at the surface.
If, on the other hand, there are no greenhouse gases then there would be very little impedance to radiation and this would be the dominant heat transfer mechanism away from the surface (and presumably the surface would be much colder – unless there is some argument about how temperature of upper atmosphere and mechanism for downward convection would establish a similar temperature profile, but I’ve not been convinced of this point).
“Otherwise if heat could only travel as radiation or through conduction you would presumably need a larger temperature differential across the troposphere”
Please show your calculations.
If, for example, the radiation were not in an absorption band in the atmosphere, it would require NO temperature difference to radiate out of the system.
Mark,
I think what he means is what would happen if the atmosphere had infinite viscosity so that convection was non-existent. In that case, the surface temperature, all other things unchanged, would have to be enough higher to account for the 102 W/m2 that is normally lost from the surface by convection. specifically ~305 K instead of ~288 K.
That doesn’t make any sense either.
If the atmosphere was purely N2/O2 then there would be 100% transmission (give or take a whisker) and so radiative losses would be 100% even if the atmosphere was isothermal throughout its depth.
Even if it was 100% viscous.
Therefore there’s no “obviously” about it.
Mark,
Good grief. Read other people’s comments and think for a moment before replying. You seem to be replying to an argument that you invented in the straw-man area of your brain.
Dewitt Payne,
You are correct that this is the scenario that I was describing. Thanks also for mentioning the magnitude of the effect (~100W/m2). I thought it (ie. the average value) would be higher than that.
Blackadder
If, for example, the radiation were not in an absorption band in the atmosphere, it would require NO temperature difference to radiate out of the system.
This shows that IN THIS CASE, you don’t *need* (read that again: NEED) *any* temperature difference in the atmosphere to have radiation.
Since your statement was:
“Otherwise if heat could only travel as radiation or through conduction you would presumably need a larger temperature differential across the troposphere”
This is shown for the case of radiation in a transparent medium to be ACTUALLY INCORRECT.
Don’t you read responses, or do you just assume you don’t have to?
When I describe convection as being “dominant”, I mean that it is the one that controls the outcome, not necessarily the one that is largest in magnitude. It is causally dominant.
The convection acts like a thermostat – turning the cooling on if the lapse rate gets too high, and turning off as the correct setting is reached. If you want to know why the temperature is as it is, or why it’s changed, the thermostat is more “explanatory”.
I don’t know, but my belief was that the magnitude of the adjustments required to keep the temperature at the right setting were comparatively small.
If there were no greenhouse gases, then the surface would be cold (T_eff) and the convecting upper atmosphere even colder. We can see some of this already in the fact that the top of the troposphere is cooled to -54 C, far below the Stefan-Boltzman temperature. With no GHGs, the surface would be about -20 C and the upper atmosphere around -90 C. (Again, assuming the same degree of convection, which is by no means assured.) The adiabatic lapse rate only sets the gradient of the line, not the intercept.
“When I describe convection as being “dominant”, I mean that it is the one that controls the outcome”
That would be TOA radiation then.
“If there were no greenhouse gases, then the surface would be cold (T_eff) ”
About 255K. Why couldn’t you manage that?
“and the convecting upper atmosphere even colder.”
No, probably warmer than it is at the moment.
The atmosphere would be colder and the height of the tropopause lower. Since the adiabatic lapse rate would be extant, that would warm the TOA by 10C each km.
“We can see some of this already in the fact that the top of the troposphere is cooled to -54 C,”
See some of what?
That isn’t cooling because we don’t have GHG.
“With no GHGs, the surface would be about -20 C and the upper atmosphere around -90 C. ”
Nope, would be ~10C warmer (the TOA would be ~3km lower, 30C warmer, the ground it hangs off 20C colder, difference 10C warmer)
” (Again, assuming the same degree of convection, which is by no means assured.) ”
What does convection have to do with it?
Gravity.
Not convection.
“If you want to know why the temperature is as it is, or why it’s changed, the thermostat is more “explanatory”.”
The lapse rate doesn’t tell you the temperature any more than knowing that this hill is a 10% gradient tells you the height I am standing on it.
The better way of explaining it is this:
http://en.wikipedia.org/wiki/Hydrostatic_equilibrium#Atmospherics
http://farside.ph.utexas.edu/teaching/sm1/lectures/node54.html
And then finding the ground temperature from this:
https://scienceofdoom.files.wordpress.com/2010/06/energy-budget-trenberth-kiehl-1997.png?w=499&h=352
“The adiabatic lapse rate only sets the gradient of the line, not the intercept.”
Then why do you say this (in the same post):
“If you want to know why the temperature is as it is, or why it’s changed, the thermostat is more “explanatory”
?
That thermostat you describe is only about the lapse rate:
“The convection acts like a thermostat – turning the cooling on if the lapse rate gets too high, and turning off as the correct setting is reached”
But you’re using this lapse rate to determine the actual temperature. OR at least insisting you can.
“That thermostat you describe is only about the lapse rate:”
A sensible question.
The intercept is set by the temperature at the average altitude of radiation to space being stabilised at T_eff. The temperature at the surface is T_eff + LR*h.
Think of h as being like the dial on the thermostat, and the lapse rate as the bit that turns the cooling on and off to hold the temperature everywhere else to the set value.
“The intercept is set by the temperature at the average altitude of radiation to space being stabilised at T_eff. The temperature at the surface is T_eff + LR*h.”
Well now you’re getting somewhere.
Yes.
“Think of h as being like the dial on the thermostat,”
Oh dear. Snatching defeat from the jaws of victory.
No, you already sorted that one out just earlier: The intercept is set by the temperature at the average altitude of radiation to space being stabilised at T_eff.
The thermostat is TOA balance.
NOT h.
“and the lapse rate as the bit that turns the cooling on and off to hold the temperature everywhere else to the set value.”
Darn it. See you had it right right there at the beginning, then you drop it in favour of your pet hypothesis.
NO.
The lapse rate doesn’t care about the temperature of the earth. Beyond 1 optical depth, it doesn’t know what the temperature of the earth IS, yet the lapse rate still holds.
It holds even when there IS no surface.
You were right at the beginning:
TOA is the thermostat.
Lapse rate depends on gravity and the specific heat capacity.
Convection has nothing to do with either.
Convection only comes into play as a faster mechanism to restore the lapse rate to the adiabatic one.
But compared to the fluxes already in effect, the fluxes that convection equalise is small potatoes.
390W/m^2 from the earth surface in radiation.
24W/m^2 in convection.
Nullius in Verba,
Sorry, but I cannot accept allowing absorption but not emission as a valid thought experiment. Since the assumption requires amassive violation of the Second Law, no conclusions relating to the real world can be drawn.
DeWitt,
Absorption and emission are only coupled at a given wavelength. I absorb shortwave from the sun but do not emit it, because I am at a different temperature.
Suppose I were to float some balloons in the upper atmosphere, which were black on top, and silvered on the bottom – but only over the emission band at the temperature of the upper atmosphere. At the shorter wavelength emission band of the much warmer surface, the silvery bottoms are in fact transparent.
(I have no idea if such a material exists – but I don’t have a problem with impossible thought experiments, so I’m not going to worry about it. Certainly, there are substances that reflect some frequencies while being transparent to others.)
So the upwelling IR from the ground passes through the lower surfaces and is absorbed by the upper surface. The upper surface radiates this heat away, and because of the balloon’s asymmetry, preferentially upwards.
Does this violate the second law?
It doesn’t matter really for what we were discussing. It’s just an interesting side-topic.
I repeat:
How is it this:
“2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.”
happens.
What physical processes take place to do this?
“So the upwelling IR from the ground passes through the lower surfaces”
?
There is no surface above the ground. It’s the definition of the ground.
” and is absorbed by the upper surface. ”
Ditto, but more so.
“Does this violate the second law?”
If you’re going on about absorption in the atmosphere, that is done at specific wavelengths, where the emission and absorption are the same.
And the inter-collisional time much shorted than the relaxation time of the excited molecule.
But this still doesn’t make your convection hypothesis correct.
“I absorb shortwave from the sun but do not emit it, because I am at a different temperature.”
No, you can still emit that photon because you absorbed it.
You’ll just be left at the original temperature.
Mark,
You keep asking:
”
‘2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happens.”
The original point was from one of my comments, and I replied to this question when you first asked. I’m pretty sure that I said it had something to do with fairies…
Null also thinks the same.
You didn’t reply.
No, it had nothing to do with fairies.
But if you want to reply now, do so.
I haven’t figured out how to link to other comments yet so I’ll just repost my reply here (you’ll see that it follows soon after my original comment ~ 7 hours ago):
====
Mark,
People intuitively understand the effects of insulation. You point out that these concepts of insulation/backradiation are not contradictory. I thought that was obvious.
You corrected me on one point though. I suggested that convection was primary means of transporting heat through troposphere. I guess that means that the greenhouse gases in troposphere are better conductor (ie. weaker insulator) of radiation than I thought. Either that or you’re mistaken.
Mark, might I suggest that you work on adding more tools to your commenting repertoire.
‘When all you have is a hammer, every problem [or alternative argument] looks like a nail’.
====
Let me know if anything about that is unclear.
“====
Let me know if anything about that is unclear.”
Yes, what is unclear is how
“People intuitively understand the effects of insulation.”
is an explanation of the physical process that causes that insulation.
E.g. “Everyone understands a light bulb” doesn’t explain how a lightbulb works. If you say “an incandescent light bulb uses a high current through a resistor that heats up in an evacuated tube to a temperature where significant radiation output is in the visible range, about 4000K”, then THAT explains how a lightbulb works.
But “Everyone understands what a lightbulb is” doesn’t.
So please explain what the physical process is that causes the insulation you allude to here:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
“Mark,
People intuitively understand the effects of insulation. ”
blah blah blah
No, as I said before you didn’t answer the question:
How does this:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happen?
What is the physical mechanism that makes the atmosphere insulate the surface against outgoing radiation?
“The effects of insulation is well understood” isn’t saying how it happens, just that people understand the effect.
So answer the question.
What is the physical mechanism that causes the atmosphere to insulate the surface?
PS it wasn’t that anything was unclear except how you “answered that question before” since you didn’t.
You clearly show that there is a difference between reflectance and absorption of 200-300 w/m2.
Since you claim the pyrgeometer is accurate, what happens to this 200-300 w/m2?? It wouldn’t appear to be getting stored by the surface, or is it?? Dang that moon paper is hard to get away from innit??
It would be nice if you would show a shortened energy balance showing what you think the surface is absorbing from where and where it goes. It apparently isn’t going up in LW as you just “proved” so, that leaves molecular bonds, quantum levels, deeper surface warming, or??
I just find it hilarious that the first time through the energy seems to be all passed from incoming SW to outgoing LW with little loss, yet, we are now talking about half of the energy going somewhere on the second pass!!
I am going to be walking lopsided for a week at least!!
HAHAHAHAHAHAHAHAHAHAHAHA
Could you stop chortling long enough to make sense?
Cheers.
“Since you claim the pyrgeometer is accurate, what happens to this 200-300 w/m2??”
It warms the earth.
This is why the temperature of the earth is ~290K rather than ~255K.
“It would be nice if you would show a shortened energy balance showing what you think the surface is absorbing from where and where it goes”
Try here:
“yet, we are now talking about half of the energy going somewhere on the second pass!!”
What half? Where?
Time to lose the troll from RC.
Mark, I’ll respond to your comments at Aug 2, 2.34pm above:
You link to the IPCC and Spencer Weart to rebutt the number of peer reviewed papers I present; that is not adequate.
You say convection uplift is more normally cm/sec or less; that is not true; take a balloon outside and see how fast it travels upwards on a calm day; and incidentally, most storms, like the one I am listening to as I write this are due to warm air trapped under cold air creating an inversion.
Retention rate of CO2 is not in the order of “a thousand years”; I gave a link to a comparison of various studies and the IPCC view; here it is again:
http://c3headlines.typepad.com/.a/6a010536b58035970c0120a5e507c9970c-pi
Here is another study:
http://pubs.acs.org/doi/abs/10.1021/ef800581r
But really the retention rate of CO2 is determined by IPCC’s own data; fig 7.3 on p 515 of AR4 shows that ACO2 is 8Gt of 218.2 Gt of CO2 flux annually; so ACO2 is 8/218.2 x 100 = 3.67% of that flux; DoE figures show 98.5% of the total, ACO2+CO2, flux is reabsorbed leaving ~1.5% to accumulate; the human contribution of that is 1.5/100 x 3.67/100 = 0.000552%; so after 1 year 1 ACO2 molecule has 1 chance in 1811.594203 of remaining in the atmosphere; after 2 years 1 in 120772.9469 and so on.
A recent paper by Michael Beenstock and Yaniv Reingewertz [not peer reviewed] really puts the relationship between CO2 and temperature in perspective; they found as the effects of CO2 increase are short-lived, CO2 must keep increasing exponentially for temperature to increase linearly. If CO2 only increases linearly then temperature is constant.
As to the Frank et al “letter” not being peer reviewed; it is you impugning ‘Nature’ not me but you have misrepresented the “letter’s” findings; the IPCC view on AGW is that temperature rise causes more CO2 to be released which causes further temperature rises, etc; if Franks is right about the low CO2 sensitivity to temperature rise then projected IPCC temperature rises will be reduced by at least 1/3 because of the low sensitivity of extra CO2; Franks find the median value of the CO2 increase per 1C increase in temperature to be around 7.7 ppmv. That’s less than 20% of the recently promoted IPCC value of 40 ppmv per °C. This recently popular, large figure of 40 ppmv per °C (or greater) is excluded by the new paper at the 95 percent confidence level.
“take a balloon outside and see how fast it travels upwards on a calm day”
Good experiment, but I think you’ll find, lacking helium, the answer is, hardly at all. The balloon is more likely to end up in a rose bush.
When the lapse rate is less than 9.8 K/m, as it usually is, the air is convectively stable. It then takes energy to drive convection, which requires some marked inhomogeneity, as with thermals.
Your first CO2 link does the usual obtuse muddling of residence time and response time. Essenhigh at least states the matter correctly:
The differential of these two times is then clearly identified in the relevant supporting documents of the report as being, separately (1) a long-term (100 years) adjustment or response time to accommodate imbalance increases in CO2 emissions from all sources and (2) the actual RT in the atmosphere of 4 years.
And yes, for (1), which is the one that matters, 1000 years is on the high side.
“the IPCC view on AGW is that temperature rise causes more CO2 to be released which causes further temperature rises, etc”
Quote, please? It’s wrong. I don’t believe the IPCC has said anything about the tiny role of CO2 feedback in AGW.
Nick, the abstract says:
“Our results are incompatibly lower (P < 0.05) than recent pre-industrial empirical estimates of ~40 p.p.m.v. CO2 per °C (refs 6, 7), and correspondingly suggest ~80% less potential amplification of ongoing global warming."
I don't have access to the full paper; maybe you can get the references; I think I had this discussion with you at Niche some time ago. Failing that I'm not going to chase through the multifarious IPCC literature for a precise quote to back up my summary; I think the Franks paper is self-evident.
I like your comment:
"And yes, for (1), which is the one that matters, 1000 years is on the high side."
I wish you guys would get your terminology straight; you say residence and response; Arthur Smith talks about " transient and equilibrium response values";
http://arthur.shumwaysmith.com/life/content/heat_transfer_in_the_two_box_model
For me, there is the time ACO2 actually stays in the atmosphere, which is not long; then there appears to be a 2-stage response, the transient and the equilibrium, to the extra CO2. The Essenhigh paper lumps for ~ 100 years; the B&R paper puts a different slant on it; it is contentious and speculative; you might as well talk about resonance and harmonics.
As for my balloon; its full of warm air, not warmer than the surface but warmer than the surrounding air; I guess whether the rose bush will come into it will depend on whether it is wet or dry air.
Nick, 1000 years is about the time for deep overturning of the oceans, equilibriating that sink/source of CO2.
Then there’s the weathering which is the only permanent (well, sort of) sink for CO2 in carbonates and that takes ten thousand years to move the significant fraction.
I’d like to see cohen explain the 40% extra CO2 if CO2 only lasts 8 years in the atmosphere before being absorbed and never being seen again…
“You link to the IPCC and Spencer Weart to rebutt the number of peer reviewed papers I present; that is not adequate.”
To you, maybe.
To refute someone open to the idea that science can be known, plenty.
“the human contribution of that is 1.5/100 x 3.67/100 = 0.000552%; so after 1 year 1 ACO2 molecule has 1 chance in 1811.594203 of remaining in the atmosphere; after 2 years 1 in 120772.9469 and so on.”
And when that molecule is taken up, it takes the place of another CO2 molecule.
Here’s proof your assertion is absolutely and incontrovertibly wrong:
http://en.wikipedia.org/wiki/File:Mauna_Loa_Carbon_Dioxide-en.svg
“A recent paper by Michael Beenstock and Yaniv Reingewertz [not peer reviewed] really puts the relationship between CO2 and temperature in perspective; they found as the effects of CO2 increase are short-lived, CO2 must keep increasing exponentially for temperature to increase linearly”
Indeed. This too is what the IPCC say. Except for the short lived part: see the CO2 record: 40% up after 150 years).
A doubling of CO2 produces the same linear increase of temperature.
“As to the Frank et al “letter” not being peer reviewed; it is you impugning ‘Nature’ not me but you have misrepresented the “letter’s” findings”
Nope, not impugning Nature. Just your attribution of unearned relevance to that LETTER.
You are the one misrepresenting the findings. See the Mauna Loa pix above.
“if Franks is right about the low CO2 sensitivity to temperature rise then projected IPCC temperature rises will be reduced by at least 1/3 because of the low sensitivity of extra CO2”
1 degree of warming per doubling of CO2 is a result of accurate calculation (not simulation, CALCULATION) using physics that would show up obviously wrong in unaligned fields like your DVD player if it were incorrect.
And many threads of evidence lead to a 3C feedback increase of that 1C from CO2. Indeed the current effect of GHG shows just this.
“Franks find the median value of the CO2 increase per 1C increase in temperature to be around 7.7 ppmv.”
Which is contraindicated by the Beenstock and Reingewertz paper you wanted to parade earlier, where it’s a logarithmic not linear response.
Do you not know what you’re proposing here?
Coho, no, Arthur’s talking about something different. The CO2 residence time issue is thus. Say someone gives you $1000 in $20 notes. How long will the money last? Residence time relates to how long you keep the actual banknotes – response time (maybe not the right term, but Essenhigh used it) is how long you stay prosperous, whether or not you put the money in the bank.
Re CO2 feedback, my issue is not its particular value, but its relevance to AGW. It isn’t – WV feedback is far larger. But when the IPCC did feel the need to say something, their numbers were similar to Frank.
Yeah, I get the difference between residence and response [and thanks for using money; the last person to explain it to me, used cookies and I’m on a diet]; the difference I was looking at was Transient and Equilibrium sensitivity or response if you like, but I can’t talk, my mouth is full.
Coho,
I’m on a diet…
I can’t talk, my mouth is full…
Hmmm…
but anyway, equilibrium sensitivity is the eventual response to a sustained change. If you get a salary rise, how much will your steady cost of living grow. Transient response relates to that $1000 question (but not the banknotes).
Again, it’s an overlooked distinction. CO2 sensitivity is an equilibrium figure – double CO2 and the temperature will eventually rise 3C (or whatever). Too often people say, but we’ve had a 30% increase and only 0.7C. But we haven’t reached equilibrium.
Uh, what do they mean?
That 10Gt added into the atmosphere remains 10Gt added into the atmosphere?
You seem to require handholding because your understanding is absent.
Nick; if I understand you correctly, you’re saying that until the CO2 stops any interim, that is Transient effect, is not indicative of the complete process; but doesn’t AGW science rely on the the Transient ‘symptoms’ of AGW to base the predictions of Equilibrium sensitivity on?
Mark, don’t bother responding; if I want to be told what reality is by declaration or be insulted I’ll visit some of my clients in the holding cells.
“but doesn’t AGW science rely on the the Transient ‘symptoms’ of AGW to base the predictions of Equilibrium sensitivity on?”
No.
We have 40% increase in CO2.
This is going to cause heating.
As long as it’s 40% increase, it will be hotter.
“if I want to be told what reality is by declaration or be insulted”
Rather a good example of projection here.
cohenite is declaring what AGW is and what his ballon WILL do by declaration.
And insults others quite freely.
“And insults others quite freely.” That is not true. I have insulted noone, not even you.
Indeed you have insulted, else what was this little tangent about:
“the last person to explain it to me, used cookies and I’m on a diet]; the difference I was looking at was Transient and Equilibrium sensitivity or response if you like, but I can’t talk, my mouth is full.”
Or this one:
“I’ll visit some of my clients in the holding cells.”
?
Maybe they aren’t clients, but compatriots…
“As for my balloon; its full of warm air, not warmer than the surface but warmer than the surrounding air; ”
But surrounded by rubber heavier than air.
And if it’s hot enough to rise anyway, then you’ve added energy to the system. No wonder it can do work (and rise up).
“For me, there is the time ACO2 actually stays in the atmosphere, which is not long; ”
And how does the greenhouse effect know that CO2 molecule is from a car engine or whatever and so it should use it to increase the temperature of the atmosphere?
It doesn’t.
Adding CO2 adds CO2. It doesn’t go down for centuries once biologic sinks have had their way. And we’ve 40% more CO2 in the atmosphere. We’ve put enough to increase the atmospheric concentration by 80%. And the sea has increased its acidity (carbonic acid, a reaction between CO2 and H2O) by enough to account for that 40% difference.
According to your insane hypothesis, there’s ~30% extra CO2 there from alien sources. Can your hypothesis explain that?
The IPCC can, and it doesn’t assume that CO2 lasts only 8 years in the air.
How does this:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happen?
What is the physical mechanism that makes the atmosphere insulate the surface against outgoing radiation?
“The effects of insulation is well understood” isn’t saying how it happens, just that people understand the effect.
So answer the question.
What is the physical mechanism that causes the atmosphere to insulate the surface?
More evidence for a 3C warming per doubling of CO2 after feedbacks:
Time for the astrophysics. You see when a star like our sun burns it converts hydrogen into helium. Helium is twice as dense as hydrogen and so the core of the star has a higher density, this means it burns hydrogen faster. This is the cause of the very well known fact that as stars age they become more energetic. The sun has increased about 30% since it began burning about 4.5 billion years ago. The rate of change over the past 500 million years is given at about 1% per hundred million years (not the exact figure but in the ball park.
As we run back through the Phanerozoic we need huge amounts of CO2 to keep the earth with liquid water. The 17 time more CO2 is not a problem, it is a solution to why the earth did not freeze over.
The earth is about 287K, for simplicity knock of the greenhouse effect of 33K
254K. Now run back about 500 million years and you have 5% less energy from the sun or very simple (and somewhat wrong) calculation you have 241K.
To get up to similar temperatures for today you need a greenhouse effect of 45K. Or 12K more than today. So now try a 3K climate sensitivity to each doubling of CO2. That 4 doublings to get to 17 times current levels of CO2.
What does that give you? The current greenhouse effect from 280ppm plus 16 times modern CO2 comes pretty damn close to explaining why there was liquid water during the Cambrian explosion.
Maybe cohen would like to explain the Cambrian data if it’s less than 3C per doubling…
The “snowball Earth/faint sun” contradiction is often trundled out by the AGW acolytes as evidence of AGW:
Click to access AIGnewsSC.pdf
http://www.nature.com/nature/journal/v464/n7289/full/nature08955.html
http://www.agu.org/pubs/crossref/2010/2009JD012050.shtml
Click to access shaviv-veizer-03.pdf
Then there is the maturation of the Earth’s core; The Dynamic Structure of the Deep Earth: An Interdisciplinary Approach, Shun-ichiro Karato is a good place to start. Why don’t you go off and read it Mark; or, since you appear to know everything and have read everything, go and read it again. Take your time.
Oh dear, cohen. A little investigation of these works in a SCEPTICAL method would have led you not to produce these.
Schneider: he assumed that there would be no rise of the TOA at IR frequencies. Physically incorrect and untenable and fixing for this one thing alone accounts for much of the difference he gets.
Ask Hansen:
[quote] Hansen: No, no. He was a student. He actually got his degree in engineering, but he got introduced to this topic by Ichtiaque Rasool, who was the guy who was later attacking me. The guy who got me my job and was attacking me, referring to our earlier situation. So Ichtiaque got Steve to work as a post-doc under him, I believe it was just after Steve graduated from Columbia. They wrote a paper on the effect of aerosols on climate, and ended up saying that the Earth may be headed towards the next Ice Age. I actually helped Steve with his calculations because he was not really a radiation transfer person, so I gave him equations for the scattering the solar part of the problem. He did the thermal part of the problem, and actually, he did a kind of slightly sticky CO2 calculation. He understated the greenhouse effect by a factor of two or so. We later figured out looking at his computer program that it was his mistake. [/quote]
Your second link is YET AGAIN a letter, not a peer reviewed paper.
RPSr thinks that back radiation really exists:
That AIG News link is to a trade paper, not a science journal.
And the Shaviv paper has many counters to it, one of which is:
http://www.pik-potsdam.de/~stefan/Publications/Journals/rahmstorf_etal_eos_2004.html
“Then there is the maturation of the Earth’s core;”
As proposed by Plimer who believes the sun is mostly solid iron, since iron is dense and the sun just sorts by mass.
Yah. I’ll pass.
Tell me, did you read any of those links yourself, or have you just been told they have the answers you like?
PS there’s no tropical hot spot problem.
The tropical hotspot is not a fingerprint of GHG warming, but of ANY WARMING WHATSOEVER. Since we can measure warming here no the ground, we know it’s happening.
The measures of tropical hotspots requires radiosonde measures which were intended for meteorlogical measures of profiles in height, not for climatology, therefore interaparatus calibration increases the errors manifold. A much simpler problem plagued UAH and Roy Spencer, after fixing for this has found a stronger warming trend that was hidden by the errors in intersystemic comparisons.
The time of measuring and the number of measures do not manage to contain a long enough period to unequivocably prove any trend or even none in the tropical temperatures.
How do we know there were not other greenhouse gases such as methane that were in greater concentrations 500M years ago? Was geologic (earth source) heating significant 500M years ago? Could continent arrangements at the time cause higher temperatures? Just asking. Not being snarky.
“How do we know there were not other greenhouse gases such as methane that were in greater concentrations 500M years ago?”
How do you know that we did? If we have NO OTHER EVIDENCE, then we have to stick with what we know we have now, since that is DEFINITELY possible. Please remember that Methane breaks down after a few years and becomes CO2. On the timescale of 1000 years, methane hardly exists.
Paleoclimate research is what you want to look at to find the answer and Michael Mann has a few papers on it.
SoD may decide to do some work on this, but would’t it be nicer to do your own and, say, show SoD what you got? At the least it reduces the work he needs to do for you.
“Could continent arrangements at the time cause higher temperatures? ”
Yes they have. A considerable one is the Antarctic. When it moved over the south pole, there was no moderation of water beneath ice in the southern winter and so it got colder. And more ice occurred.
These are all explained in the paleo section of the IPCC WG1 reports.
http:/www.ipcc.ch
There’s a lot there if you don’t want the potted history.
“Just asking. Not being snarky.”
No need to be defensive. 🙂
No, they’re good questions. I’ve only briefly followed the paleo reports but they all state that these effects are seen and when accounted for affirm the CO2/temp sensitivity and constrain it to within 2-4.5C per doubling of CO2.
Since they have been peer reviewed (necessary but insufficient as proof of veracity) AND have been cited expanded and stood the test of other peer reviewed papers, I’m willing to accept that as a working hypothesis that they have it right.
The Sun entered main sequence about 4.55B years ago. Since that time, it has increased in luminosity by about 29.7% which has increased in close enough to a straight line over that time period. It has also grown 10% bigger, its surface temperature has increased by 3.4% and it has lost 0.03% of its mass.
The correct formula for determining solar irradiance at Earth 500M years ago would be:
500 Mya TSI = 1366 * (1-(0.297*500/4550)) = 1321 W/m2
[or a reduction of 3.3% and actually just slightly more than this since the Sun is now bigger but the the mass has declined so the Earth would have been ever so slightly farther away from the Sun’s surface but probably close enough].
Earth Equilbrium Temp = (1321 W/m2*(1-0.298) / 4 / 5.67e-8) ^ .25 = 252.9K
[or just 2.1C less than today].
Add in 150 W/m2 greenhouse effect and 4.5 doublings of CO2 at 3.7 W/m2 and we have:
Surface Temp 500 Mya = ((231 + 150 + 18) / 5.67e-8) ^ .25 = 289.7K
[or about 1.7C above today’s temperatures which is not far off the temperature reconstructions which are about +6.0C for the time. Note that the Earth Albedo was probably lower than today since there were no continents at the poles at the time and very little high-Albedo ice at the time.]
Mark,
Why do you assume that the Earth atmosphere always had the same mass of today?
What do you think the change has been in the past 200 years?
Mark,
I thought you were using 0.5 billion years time span and theoretical concept of faint Sun as an evidence for strong influence of CO2 concentration on Earth climate.
Let’s try again: “Why do you assume that the Earth atmosphere always had the same mass as today?”
To Al,
One thing that has varied is the level of Oxygen (currently 20%) which is not likely to have displaced Nitrogen, so the mass of the atmosphere has varied with the level of Oxygen (irrespective of the impact of other gases).
2.7Bya, no Oxygen, no Ozone, but more Methane and probably more CO2.
2.4 Bya, rise of Oxygen and Ozone, Methane reduced significantly. Oxygen at only 5% however. Two Snowball periods.
600 Mya, last of 5 Snowball periods has ended, Oxygen increases to 15%. Earth now slowly warming up.
378 Mya, Devonian period, Oxygen increases from 15% to 30%. Rapid warming occurs that is not really explained by other factors.
300 Mya, Carboniferous, Oxygen increases to 35%, Massive forest fires, giant DragonFlys, Cool overall as Gondwana is transiting the south pole. Warmer than it really should be given the large continental land mass at the south pole – think Antarctica times 10.
100 Mya, Cretaceous, Oxygen spikes again to 30%, Major warming event.
Calculate the atmospheric pressures that would have resulted with these varying levels of Oxygen.
KuhnKat
98% of the “backradiation” or DLR is absorbed.
What happens is:
– a surface absorbs energy
– it heats up
– it keeps heating up until the energy radiated (which is proportional to T^4) is equal to the energy absorbed
So yes, it is “stored”, and as it is stored the temperature rises. Eventually a new energy balance is reached at a higher temperature.
I don’t understand your comment here. Take a look at my answer to your previous comment, maybe it is now answered.
Otherwise the surface fluxes here should be the approximate answer:
I would like to share in the entertainment but don’t quite understand your comment.
It might be explained in Do Trenberth and Kiehl understand the First Law of Thermodynamics?
“So yes, it is “stored”, and as it is stored the temperature rises. Eventually a new energy balance is reached at a higher temperature.”
what is the temperature change of a liquid, gas or solid undergoing a phase transition?
hmm?
“what is the temperature change of a liquid, gas or solid undergoing a phase transition?”
Different molecules undergo state changes at different temperatures, the temperature of things not undergoing state change will not be a problem.
PS how hot does the earth have to be to undergo a state change? How about how cold does CO2 have to go before undergoing state change?
Mark,
I answered your question:
“How does this:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happen?”
I said “You point out that these concepts of insulation/backradiation are not contradictory. I thought that was obvious”. (yet your short attention spans translates this into ‘blah blah blah’) What could I possibly mean other than to say that backradiation is the manner in which the atmosphere insulates against outgoing radiation?
Moreover, there was no point in my original comment where I contradicted SOD’s point that backradiation (from colder body to a warmer body) exists. Pointing out that there is obviously still a net flow of radiation from the (warmer) surface through the (cooler) atmosphere but that the atmosphere insulates against the surface losing all outgoing radiation to space simply makes more intuitive sense to most people than arguing about whether cooler objects increase the temperature of warmer objects. It’s a different way of describing the same thing. Why do you frame these arguments as contradictory?
So like I said to you before. You obviously don’t read other people’s comments before replying. Or you are just a troll who intentionally misrepresents what other people are saying.
Either way I think SOD’s (and other prominent commenters’) more cautious and respectful approach is much more engaging and effective at demonstrating examples of misinformation/errors on the skeptic side of the debate. The approach that every alternate argument (or use of the word convection) is evidence of stupidity or denial has been tried elsewhere (like RC) and more closely resembles preaching of dogma.
I said “You point out that these concepts of insulation/backradiation are not contradictory. I thought that was obvious”
Which isn’t saying how the phenomenon happens.
Just that everyone knows it does.
Not the same thing.
Why can’t you answer that question, if you “know” that backradiation can’t be the answer?
Hopeful wishing?
“You obviously don’t read other people’s comments before replying. ”
No, I read your message and you still haven’t answered the question. Here it is again:
How does this:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happen?
As I said before, and apparently you didn’t read, saying “I know it happens” isn’t saying why it happens.
How does this:
’2) The atmosphere insulates the surface against outgoing radiation thus reducing the ‘net’ amount of radiation leaving the surface.’
happen?
The short answer would be:
Some of the outgoing radiation is absorbed by the atmosphere and radiated back towards the earth, hence prohibiting it from leaving.
“Some of the outgoing radiation is absorbed by the atmosphere and radiated back towards the earth, hence prohibiting it from leaving.”
Indeed, this is the case.
But Blackadder hasn’t agreed this is the mechanism.
The important thing here is that there is radiation COMING THE OTHER WAY that then warms the emitting body.
Or, in other words, back radiation.
Mark,
“Why can’t you answer that question, if you “know” that backradiation can’t be the answer?”
Honestly Mark, this comment is disturbing.
Slow down, read again and you’ll see that I am in full agreement about the existence of backradiation and never suggested otherwise. And, yes I understand the mechanism of backradiation and I’ve discussed this topic on other threads at this site and elsewhere. That’s why I was able to point out that it can be understood as a form of insulation against radiation.
Please, don’t continue to include me in disputes that have arisen out of your imagination. It’s weird and frustrating.
Mark wrote:
“No, it’s at the top end, but if it isn’t responsible for anywhere near 11C of that 33C warming, then the sensitivity of the system to CO2 increases is much higher than 3.”
While it is feasible that current concentration of CO2 contributes about 11C of that 33C warming (where the number 33 comes from grossly unrealistic model of uniform surface heating), what makes you think that the system sensitivity is much higher than zero?
“(where the number 33 comes from grossly unrealistic model of uniform surface heating), ”
If it’s unrealistic, you’ll be able to show us a better hypothesis, yes?
“what makes you think that the system sensitivity is much higher than zero?”
Because CO2 absorbs IR radiation and not Visible.
This was known way back in 1824.
There should be a few postings concerning the 33 K warming figure around here somewhere and Arthur Smith, I think, wrote a pretty good thingy about the general subject somewhere else.
The warming compared to effective Temperature should be actually larger than 33 K (it’s sort of a minimum value) because earth is round and “T” and the radiation emitted are linked by a power of 4.
Aye, though that discordance only exists because we’re converting to temperatures (since we feel temperature, not energy content). If you work in energy instead, then that’s a linear problem.
Same problem I had when in Uni lab investigating Faraday Rotation, which depends no B^2, so we graphed not B (easy to read), but B^2.
We were worried when we got the answer right…
Mark, your answer “Because CO2 absorbs IR radiation and not Visible” is not satisfactory nor correct. Let me re-phrase the question, with some guidance:
What makes you think that increase of CO2 concentration on the top of currently given one leads to decrease of OLR for a given current vertical structure of atmosphere, and causes radiative imbalance that allegedly leads to global warming?
(I assume here that you are familiar with basic physics of Standard AGW Theory, the one that uses such concepts as “effective emission height”, “average lapse rate”, “radiative forcing”, etc.)
Mark said: “If it’s unrealistic [33C of GH effect], you’ll be able to show us a better hypothesis, yes?”
There is not much to hypothesize about. It is about realistic or less realistic assumptions. The 33C number comes from basic calculations under an assumption that Earth temperature is uniform around the entire globe. To be so, the Earth must rotate in random direction every other minute, or Sun should traverse every inch of sky every minute, and the Earth surface has infinite thermal conductivity in horizontal direction. Would you agree that this is not a very realistic assumption, Mark?
Now, on the extreme corner of assumptions is a non-rotating non-conductive planet. In this case the “global average emission temperature” (which is by itself an idiotic concept) would amount to -129C, according to exact mathematical solution presented in the notorious Gerlich and Tscheuschner paper. Therefore, one can start count the excess of surface warming (observed “global temperature”) from this bottom point, which gives the GH effect magnitude of +144C. Relative to this number, the alleged 11C of CO2 effect looks quite more moderate.
I am not saying that G&T model is much more realistic than madly rotating Sun, but the argument about 11C form Co2 alone must be taken in proper perspective.
“Mark, your answer “Because CO2 absorbs IR radiation and not Visible” is not satisfactory nor correct.”
Unfortunately for you, it’s both.
” Let me re-phrase the question, with some guidance:”
Oh dear. Coached?
“What makes you think that increase of CO2 concentration on the top of currently given one leads to decrease of OLR for a given current vertical structure of atmosphere, and causes radiative imbalance that allegedly leads to global warming?”
Because of something called “Optical Depth”:
http://www.daviddarling.info/encyclopedia/O/optical_depth.html
or
http://en.wikipedia.org/wiki/Optical_depth
Therefore the outgoing radiation comes from the atmosphere at one optical depth on average.
As you increase the opacity of the atmosphere (by, for example, adding CO2 for IR radiation), the TOA gets higher.
And, as we all know, the lapse rate means that this higher level is colder. Which means that it radiates less.
Since the incoming radiation hasn’t changed, there is an imbalance.
Mark, you said: “the outgoing radiation comes from the atmosphere at one optical depth on average.”
Are you sure about “one optical depth”? Are you familiar with the fact that opacity in CO2 bands is a highly variable function of frequency? That the 14-16um band (blocking about 9% of essential IR spectrum) is extremely opaque, while other bands are absolutely not (with exception of small transitional area)?
Now, do you know that the lapse rate (“higher is colder”) eventually stops, then becomes (“higher is no colder, flat”) which is called “tropopause”, and then it becomes “higher is warmer”? Then, do you happen to know the position (“height”) of opacity at different frequencies relative to the above layers of atmosphere?
Could you picture a situation when absorption peaks are in the layer where “higher is warmer”, and therefore meant to radiate more, not less?
I notice that Mark is unable to answer the simple physics question about temperature change during a phase transition.
I suspect there is motivation behind the elaborate evasion of it.
And I note the marked decline in quality of this thread compared to all previous. I think it’s probably all downhill from here for this site, now.
I notice that it was a silly question.
If you wish to know the answer, a quick google will solve your problem:
http://www.newton.dep.anl.gov/askasci/chem00/chem00378.htm
was the first link.
Mark:
All of your comments will be held in the moderation queue for approval. I’d like to keep this blog as a pleasant place for everyone to discuss climate science.
If you make worthwhile comments or a low enough number of slightly annoying ones so that you are not hijacking the threads your comments will get approved.
Al Tekhasski:
This is not the case.
In fact the total energy absorbed by the earth’s climate system over a year is compared with the total energy radiated by the surface over a year.
For convenience this is converted to an average flux per unit area – W/m^2.
The earth from space radiates 239W/m^2 – an effective radiating temperature of 255K.
And the earth’s surface radiates 396W/m^2 – an effective radiating temperature of 289K.
The hilarious paper of G&T just missed the point completely.
And the effective radiating temperature is not an idiotic concept, it is a handy conversion of W/m^2 into something easier to see conceptually.
SoD wrote: “And the effective radiating temperature is not an idiotic concept, it is a handy conversion of W/m^2 into something easier to see conceptually.”
The problem with “handy concepts” is that they turn to be misleading when it comes to evaluation of more subtle topics (as sensitivity, or conflating global warming with temperature index) as compared to primitive balance estimations. The superficial simplicity of these “handy concepts” leads to completely unwarranted confidence in crude illustrative estimations that become accepted as gospels and “physics cannot be wrong”. Unfortunately, here is the demonstration:
SoD said: “In fact the total energy absorbed by the earth’s climate system over a year is compared with the total energy radiated by the surface over a year. For convenience this is converted to an average flux per unit area – W/m^2. The earth from space radiates 239W/m^2 – an effective radiating temperature of 255K. And the earth’s surface radiates 396W/m^2 – an effective radiating temperature of 289K.”
To compare something you need to measure or theoretically calculate both ends.
No one has ever measured “total energy” absorbed by the Earth. You do not have 800,000 differential radiometers hanging around turbulent atmosphere and recording fluxes 24/7/ 96 times a day. The “total absorbed energy” is crudely estimated using known solar constant but completely fuzzy concept of “average albedo”, which must be derived from highly variable cloudiness, variable wind-induced sea surface roughness, and variable seasonal albedo from vegetation and snow cover, or boldly assumed as “well-known constant” (as per many RC posts).
No one has ever measured the energy radiated by the Earth surface over a year, for about the same reasons as above. What was “measured” is a goofy temperature index from goofy-located stations, and then this goofy number is converted into more “conceptually easy” IR flux assuming black body with emissivity 1.000.
All the numbers you quoted make only sense when the surface has uniform temperature around the entire globe. That where my simplified concept of “madly rotating Sun” came from. Concepts of global averages are grossly misleading when dealing with fluctuating spatio-temporal fields like non-uniformly heated surface and turbulent atmosphere. If these “tiny” details are ignored and averaged, climatology arrives to such idiotic numbers as “2.2Gt ocean sink” or “1.7W/m2 radiative imbalance”.
SoD wrote: “The hilarious paper of G&T just missed the point completely.”
I tend to disagree. This is maybe the only point that they did not miss.
Al Tekhasski:
What you are talking about is measurement uncertainty. What G&T were talking about is something completely different.
Measurement uncertainty can be bounded and people in all fields of engineering are continually doing it.
To believe that there was no provable difference between the TOA radiation and the surface radiation you need to believe some amazing things.
TOA reflected solar radiation is probably the hardest to measure. However, the estimates from the continual measurements by satellite put the reflected solar radiation around 30%. You might see one calculation which is 29.5% and another which is 31%. The difference in absorbed radiation by the climate of each 1% around 30% amounts to 3.5W/m^2.
With an albedo of 0.0 – no reflected solar radiation – the average energy absorbed by the climate system would be 342 W/m^2.
This still indicates an inappropriately-named “greenhouse” effect, as the temperature of the earth measured in so many locations indicates a surface (upward) radiation of 396 W/m^2.
To get a balance here you can have:
Albedo=0.0, “Average surface temperature”= 278.7K (6.4’C)
Albedo=0.1, “Average surface temperature”= 271.4K (-1.9’C)
Albedo=0.2, “Average surface temperature”= 263.5K (-9.8’C)
And putting “average surface temperauture” as a handy measurement to assist people in seeing that there is no way that this can be the case.
As previously noted in Lunar Madness and Physics Basics average temperature is a problematic concept, however, I can just restate all those values in average W/m^2 if required.
Do you think that the measurement of albedo and surface temperature is that far off?
This is why the G&T paper totally missed the point.
What do I think about importance of albedo? I am not sure what do you want to say with numbers for different albedos, but you inadvertently dismissed the entire AGW theory. You said that small measurement “uncertainties” in albedo (of about 1%) lead to radiative imbalance that equal to the entire alleged effect of CO2 doubling. Which is a good first step. I am sure you are aware that most accurate (integral) measurements of Earth albedo (“Earthshine experiment”) have demonstrated the drop in global albedo by 3% from 1985 to 1998? How many CO2 doubling would you need to invoke comparable radiative imbalance?
And I already expressed my opinion on usefulness of “global surface temperature”. It cannot be “far off” something, because this global something has no physical meaning, so being far or not so far from it still makes no sense at all. You probably also believe that increase in “global surface temperature” must be a proxy for warming, right?
My mom’s turkey has a nearly infinite variety of temperatures at any given point when it is being cooked. That does not mean that it is impossible to tell if it is getting warmer as it is cooked or when it has arrived at a sufficient mean temperature to render it safe to eat. It doesn’t even matter if the thermometer is terribly accurate or not.
Regarding, “…entire alleged effect of CO2 doubling…”: We are changing the composition of the earth’s atmosphere and in so doing, changing the earth’s radiative properties. How much effect do you think that will have?
I am sure you are aware that ice has a higher albedo than seawater or land and that we’ve been loosing a lot of ice. Plus, there has been a lot of deforestation and the sahel has expanded, etc. Albedo, temperature, and CO2 are interrelated, you can’t make changes in one without affecting the others. I see your introduction of a change in albedo as an attempt to create a false dichotomy.
Chris G: “My mom’s turkey”…
Have you tried to cook a turkey on open grill in a windy weather? Did it cook well to be safe to eat? Are you trying to compare the quality of climate science with cooking? I don’t think your analogy works in right direction…
You ask: “We are changing the composition of the earth’s atmosphere and in so doing, changing the earth’s radiative properties. How much effect do you think that will have?”
I believe I asked first: “what makes you think that the system sensitivity is much higher than zero?”, which sort of contains my answer.
You conclude: “I see your introduction of a change in albedo as an attempt to create a false dichotomy.”
Nothing false is in the albedo problem. Are you aware that albedo is mostly defined by cloudiness? The tricky part here is: do you have a foggiest clue how, when, and when the fog (clouds) forms?
So glad we’ve moved off of convection. The topic is backradiation. I believe everyone can agree that convection exists.
Does convection remove energy from the earth system? No.
Convection is driven by differences in energy levels expressed as differences in temperature. Will it be 100% or more efficient in moving the energy difference driving it? I don’t think so.
Would there be more convection if there were not an increase in temperature? No
So, I don’t see how the existence or rate of convection precludes the existence of backradiation.
Mark,
Try to maintain the higher ground. There is the possibility that you are being baited.
I don’t know of any breezes blowing through space to cool the earth. The point you are missing is that it’s quiet possible to detect trends and come up with good working estimates without knowing the energy state of every last molecule.
Belief in climate sensitivity greater than zero? Well, there are the laws of physics, particular in the areas of optics and radiation, and the temperature swings in the paleo record would indicate that the equilibrium of system is not highly stable.
Clouds cover about 70% of the earth on average. Do you have any idea what would cause changes in amount, timing, and location of clouds? Levels of energy and its distribution within the system mostly, which is again affected by radiative energy changes. Svensmark did some interesting work with cosmic rays, but his correlations broke down after a while unless he massaged the satellite data in inappropriate ways.
Chris G wrote: “Clouds cover about 70% of the earth on average. Do you have any idea what would cause changes in amount, timing, and location of clouds?”
Yes, I do have a general idea. First, before looking at changes in something, one must understand when, where, and why these objects form and evolve. Not in reverse, like “we have no idea how they form, so we will assume they don’t ever change”. It is a turkey science.
Second, I do know that clouds form when certain parcels of convecting-advecting air masses meet certain temperature conditions and certain concentration of nucleation centers. This is not a secret. But the convecting/advecting air masses follow laws of mass/energy/momentum conservation in a differential form of generalized Navier-Stokes Equations (coupled with heat-mass transfer). And only the Navier-Stokes Equations with their boundary conditions have their own explicit ideas where and when the clouds would form, and how they will evolve in time and space, and especially on the long run. This is something that general AGW climatology has a very little idea about.
Now, you say it is 70%. Did you think about what might be behind this number? Look outside. Is it a clear sky? Or it might be not completely, clearly clear? What is its percentage of cloudiness? 0.5%? 1%? What about rainy weather? Is it 100% cloudy? Which cloudiness is more percentile-wise, for cumulus or stratus? What about a heavy hurricane condition? is it 150% cloudy, or what? Do you realize that just by quoting 70% you make no scientific sense?
Are you going to say that you would not give a turkey about these pesky details? But please keep in mind that 1.4% change in low cloud cover has bigger effect on Earth energy imbalance than the entire alleged effect from CO2 doubling, at least according to the Chicago University radiative model, see
http://forecast.uchicago.edu/Projects/full_spectrum.html
Al Tekhasski,
Great, and again, what would be driving any changes in cloud behavior that is unrelated to the energy in the system?
The last I heard, climate scientists care a great deal about cloud behavior. Are you implying that there is no interest in them?
“what would be driving any changes in cloud behavior that is unrelated to the energy in the system?”
Related or unrelated, you tell me… I have no idea. But so has the climatology. Do you know why would cloud cover go down from nearly 70% in 1986 to nearly 64% in 2000, according to ISCCP observations?
Click to access Palle_etal_EOS_2006.pdf
(BTW, this is also a reference for SoD)
Do you know what regulates the density of nucleation centers in mid atmosphere? What is the dust and soot dynamics? You know, land gets eroded over time, slowly… turns into dust, desertificaion…
As I already posted here, 3% change in albedo did happen recently, for no apparent reason, which caused so much radiative imbalance that no octopling in CO2 can provide. Yet climatology “care great deal”, but mostly about CO2. Being “interested” in cloud behavior is not enough.
Al,
Pesky details and ‘Related or unrelated, you tell me…’.
I would say that it is pointless to argue about the details when you have not yet come to an agreement on the fundamentals. There are two fundamentals I think are most relevant to the current conversation.
a) It takes energy to move a water molecule from a solid or liquid state to a gaseous state.
b) The amount of water vapor in the air is a primary limiting factor on how much cloud cover there is.
To me, your arguments create a false separation between the two when you say that there can be more cloud cover without there being more energy available.
The last I heard there was still debate over the strength of the effect of cloud cover. High clouds have an opposite effect than low clouds, clouds during the day reduce the energy absorbed from the sun while clouds at night reduce energy loss below them, and water vapor itself is a GHG. Regardless of the absolute strength of an effect of a change in cloud cover, relatively, it will be less than the positive change in energy balance which was required to create the additional cloud cover in the first place. Otherwise, it would be impossible for the earth to get above some mean energy state. The paleo record makes it evident that even if such a ceiling state existed (which I do not believe), it would be at some level higher than it is currently.
I’d be interested to know if the changes in cloud cover you mention are part of a sustained trend or change in mean over time, or whether they are simply snapshots in time.
A simple Google scholar search on ‘climate’ and ‘cloud’ reveals a number published works in the area by various authors. I’d recommend Ramanathan and Stephens for starters. I’d recommend Svensmark and Lindzen too, but not without reading some counters as well. Your implication that there isn’t significant work being done in this area is unfounded.
Chris G said: “To me, your arguments create a false separation between the two when you say that there can be more cloud cover without there being more energy available.”
Your philosophical approach about “more energy/less energy” does not work in complex spatially distributed nonlinear systems far from thermodynamic equilibrium. I am not sure if creating more clouds does require more energy, but creating less clouds definitely does NOT require “less energy” – conditions of air could be such that over-saturation does or does not occur, so it could be no clouds at the same “energy”. Presence or absence of microscopic amounts of aerosols and dusts also do not require any noticeable change in “energy”. The point I am trying to make is that this process (clouds-no-clouds) continuously creates huge radiative imbalances that exceed CO2 influence by orders of magnitude. Yet climatology has only rudimentary treatment and grasp of this fundamental factor.
“In Navier-Stokes We Trust”
I’m having some difficulty I’d like some help with. I’m trying to get a mental picture of the amount of radiation emitted directly to space by altitude. I’m aware that the composition of the atmosphere varies with altitude, at least at the tropopause, where H20 drops off precipitously. Also, the tropopause is higher in low latitudes than high latitudes and also varies with season. Other things also, but the point is that I know that any single graph is going to be a simplification or a sort of mean atmosphere representation. Or, a graph that represents something like 30 degrees N|S at an equinox would do just as well. Night versus day would be great, but an average for a day would be fine.
Anyone know where I can find such a thing?
My guess would be that it would be not much, on a gradient inversely related to the atmospheric pressure, up until the tropopause, an almost step-wise transition, and then a continuation of the curve.
Mmm. I think separate or overlaid graphs for SW and LW would be ideal. My guess is these would have to be generated from physics equations rather than actual measurements.
Well, I see I’m asking for a lot, but if someone knows of some existing material…
I too would be interested in this…. i have heard that the average height that LW escapes to space from the troposphere is around the 6km mark… This is from someone whom “i” would consider i reliable source, but it would be nice to find some actual data on this 😉
The layer or height at which OLR occurs is the charasteric emission layer [CEL] as this paper explains:
Click to access 0809.0581.pdf
The paper notes that the CEL occurs where the optical depth is “near 1”.
The OD is a crucial indicator of the greenhouse effect; extra CO2 should both increas the OD and increase the height of the CEL; neither appear to be happening:
“I’m trying to get a mental picture of the amount of radiation emitted directly to space by altitude. ”
This is a meaningless question. If you are in a IR window band, altitude does not matter, all goes straight from surface. If you are in IR-opaque band, whatever comes up from certain altitude faces another layer with somewhat similar opacity, so some fraction gets absorbed and back radiated. Therefore, nothing goes “directly”. What you can only see is a “path integral” along the entire atmospheric profile. If you want to see “direct emission” as a distant observer, it is called MODTRAN. Every AGW stalwart should know this.
i dunno, i would have thought average height that energy escapes to space, as not a silly Q… I can probably deduce, because of the tropopause and stratospheres temp profiles, that the “majority” of the energy that leaves the troposphere, escapes directly to space with out interacting with the stratosphere by n large. Im sure some is absorbed, but co2 is a net emitter in the stratosphere.
The band widths may be interesting as far as deducing the various gases roles in atmospheric radiation… but i was more interested in where the average energy escapes to space, from the troposphere.
And cohenite, thanks for the link, i will give it a read when i have the time.
The MODTRAN access that I know of gives a snapshot of waveband curve at a specified altitude; I’m looking for an integration under that curve over altitude. I suppose I could code for it given what I have, but I’m thinking someone must have done this already.
Obviously, some amount of energy has a final, direct step out of the earth system, and it doesn’t happen exclusively at TOA.
Mike, “…i would have thought average height that energy escapes to space, as not a silly Q… ”
Your question is different, and is not completely silly. I tried to ask this question several times, asking for mathematical definition of OLR escaping height as function of frequency. I found no such definition.
It seems that the”average effective emission height” is derived from simple “average” (and illustrative) considerations: the escaping flux must be equal to 240W/m2 (assuming stationary state of climate and constant albedo of 0.3). This must correspond to about 255K of effective emission temperature (assuming black-body emissivity, again). This temperature can be found at about 5-6km height, depending on which “average” lapse rate one believes. Conclusion: radiation escapes on average from altitude of 5-6km. That’s what the Standard AGW theory says.
This is for “average” in a stationary state at given concentration of all GH gases. However, the question of sensitivity of this state to changes in GH gases is a tricky question. I asked Mark about his view on the details of CO2 spectrum and layered structure of atmosphere, but received no answer so far. Apparently his Professors are not available for consultations in the summer season…
Al Tekhasski:
You can see what I’ve written about albedo in The Earth’s Energy Budget – Part Four – Albedo
How do you know it’s the most accurate measurement? I will be interested to read the paper if you provide the reference.
You can see what I have written about global surface temperature in Why Global Mean Surface Temperature Should be Relegated, Or Mostly Ignored.
So I’ll rewrite my last comment using W/m^2. If that is too troublesome because it is an average and therefore has no meaning, I can multiply all of the numbers by 5.1 x 10^14 m^2 so we can compare total energy.
But as a service to other readers I am trying to make it easier to follow.
Now in W/m^2:
With an albedo of 0.0 – no reflected solar radiation – the average energy absorbed by the climate system would be 342 W/m^2.
This still indicates an inappropriately-named “greenhouse” effect, as the temperature of the earth measured in so many locations indicates a surface (upward) radiation of 396 W/m^2.
To get a balance here, i.e., no inappropriately-named “greenhouse” effect, you can have:
– Albedo=0.0 – energy absorbed by the climate system = 342 W/m^2
Energy radiated upwards from the earth’s surface would be 342 W/m^2.
– Albedo=0.1 – energy absorbed by the climate system = 308 W/m^2
Energy radiated upwards from the earth’s surface would be 308 W/m^2.
– Albedo=0.2 – energy absorbed by the climate system = 273 W/m^2
Energy radiated upwards from the earth’s surface would be 273 W/m^2.
Given that the calculation is 396 W/m^2 this indicates quite a measurement error (or a “greenhouse” effect).
Now just a note that it’s important to remember the calculation is not a linear one – as energy radiated increases with the 4th power of absolute temperature.
To claim that measurement error is actually the reason behind the inappropriately-named “greenhouse” effect is an amazing stretch.
Satellites have 32 years of measuring reflected solar radiation and while it is quite possible that albedo has varied significantly in the past, it is incredibly unlikely that current annual averages of 30% are actually 20% and no one has noticed it.
Let’s suppose that an amazingly shoddy approach has led to albedo being overstated and in fact the earth’s albedo is 10% (0.1).
In that case, the earth’s climate system absorbs 308 W/m^2.
But the earth’s surface apparently radiates 396 W/m^2.
If all of the temperature measurements that go into the various indices – GISS, HADCRUT, UAH – are out by 2’C what would that lead to?
The value varies depending on the temperature measured:
– For temperatures around 35’C, a 2’C drop has an impact of 13 W/m^2
– For temperatures around 15’C, a 2’C drop has an impact of 12 W/m^2
– For temperatures around 25’C, a 2’C drop has an impact of 11 W/m^2
– For temperatures around 5’C, a 2’C drop has an impact of 10 W/m^2
– For temperatures around -5’C, a 2’C drop has an impact of 8.6 W/m^2
So for the sake of not leaving anything to chance – worst case – we will pretend that the entire surface of the earth days, nights and weekends all year round is at 35’C and therefore our 2’C measurement error has led to a calculation of upwards surface radiation being overestimated by 13W/m^2.
So the surface upwards radiation is in fact (with 2’C temperature error) – 383 W/m^2.
Still a long way from 308 W/m^2 that the climate is absorbing.
What temperature error in our measurement system is needed, combined with an amazing oversight in albedo measurement (really 10%) ?
14’C measurement error.
Chilly anyone?
Perhaps you can see that measurement error is not the issue?
This is why the G&T paper missed the point.
SoD, I am confused. Are you really not familiar with publications from Earthshine Experiment? Try to Googe these keywords.
And why are you trying to convince me that GH effect does exist? There is no need to. My question is not about how overstated albedo is or not. My concern is that the solution to applied problem of climate change involves determination of meteorological fields down 0.3% accurate, which is highly ridiculous to claim. My concern is that climatology has no clue where and when clouds form, when clouds being a factor that modulates ground insolation with 90% depth, and change global albedo by up to 3% for absolutely unknown reasons. Cloud variation imposes radiative imbalances that 30-50X stronger than the entire hypothetical CO2 doubling, yet cloud formation remains the biggest problem in weather-climate models, and will remain as such until the global hydrodynamical circulation of atmosphere will be numerically solved (which likely means “never”).
Al Tekhasski:
There’s a lot of hits from Google. Do you have a reference?
That’s wonderful. Sounded like you thought that G&T had a point, glad I’m wrong about that.
Cohenite,
In the past, you have tried to tell me things like water vapor has a surface and that the characteristics of H2O that make in sensitive to microwaves will cause IR photons to be absorbed by it in preference to CO2.
With all due respect, I’d just as soon hear from someone else.
Chris G; you have verballed me in this fashion before at comment 392 here:
http://joannenova.com.au/2010/07/the-great-leap-forward-professors-et-al-realize-they-need-to-talk-about-evidence-instead-of-insults/
And comment 102 here:
http://joannenova.com.au/2010/07/the-unskeptical-guide-to-the-skeptics-handbook/
I reply to your verballing at comment 126 here:
http://joannenova.com.au/2010/07/the-unskeptical-guide-to-the-skeptics-handbook/
The last link in my comment at 126 is here:
http:joannenova.com.au/2010/07/the-great-leap-forward-professors-et-al-realize-they-need-to-talk-about-evidence-instead-of-insults/#comments [// excluded]
Nothing with Chris G has changed I see.
Indeed.
Your original statements were:
“The problem for AGW is that CO2 is not ’surface’; so even though backradiation from atmospheric CO2 is the backbone of AGW and is supposedly measured at being dominant in the 15um wavelength, that wavelength is dominated by water emission which is most definitely a surface as either vapor or a cloud.”
and
“…water has a permanent dipole moment, CO2 does not; that means water will preferentially absorb IR in the overlapping wavelengths”
The dipole rotation of H2O is induced by radiation in the microwave band, not the IR. Hence the name ‘microwave oven’.
At least Chris G you have quoted me accurately, even though it is out of context, which I’ll get back to. First, that micro-wave oven you are talking about is no doubt this one:
As to context; I would have thought if you were going to have a shot at me about whether CO2 or H2O dominate absorption in the overlapping wavelengths you would have used this spectrum comparison SoD put up:
Pretty convincing, eh? Based on this H2O is definitely the poor relation when it comes to preferential absorption of IR; if IR were customers the business of H2O would be on its last legs. This is where context comes in; and once again I give a hat-tip to Dave in Delaware who did the research for this; Dave’s research showed this and I refer to my previous reply to you in the above links:
“Measurements of the Radiative Surface Forcing of Climate, W.J.F. Evans & E. Puckrin, American Meteorological Society, 18th Conference on Climate Variability and Change (2006). From Evans and Puckrin we see in tables 3a and 3b);
Winter
H20 94 to 125 W m-2
CO2 31 to 35 W m-2
Summer
H20 178 to 256 W m-2
CO2 10.5 W m-2
Not only did the relative CO2 contribution drop in Summer, but the back radiation value decreased from about 30 Winter to about 10 W/m2 Summer.
How do these Evans and Puckrin (2006) values compare with and confirm the energy estimates in K&T?
The back radiation shown in the K&T chart is 323 W/m2.
Data from Evans and Puckrin suggests that CO2 accounts for at most 10% of K&T, and in Summer, CO2 is only about 3% of the K&T back radiation.
To get close to the K&T back radiation values, there apparently needs to be a LOT of water in the atmosphere; CO2 would only be relevant if there were no water.”
This seems to contradict the absorbance dominance of CO2 over H2O in the overlapping wavelengths. But wait there’s more; SoD has also put up this:
This table shows the relative effects between CO2 and H2O in terms of OLR; SoD concludes:
“Therefore, guessing at the overlap effect, or more accurately, assigning the overlap equally between the two, water vapor has about 2.5 times the effect of CO2.”
If H2O has 2 times the, what in effect is the greenhouse impact, of CO2, how can CO2 be said to dominate backradiation?
I don’t see a clear indication of whether or not you are standing by your original statements regarding water vapor having a surface and water vapor’s dipole nature having an effect on IR radiation. A simple yes or no would suffice.
[…] AND much more about the downward radiation from the atmosphere – The Amazing Case of “Back-Radiation”, Part Two, and Part Three […]
[…] In the The Amazing Case of “Back-Radiation” series, which included Part Two and Part Three, someone commented that it would have been good to see more than a few days of DLR (downward […]
[…] The Amazing Case of “Back Radiation” – Part Three […]
[…] and The Amazing Case of “Back Radiation” – Part Three […]
[…] measured DLR and some of the measurements, Part Two reviewed the spectra of this radiation, and Part Three asked whether this radiation changed the temperature of the […]
[…] see The Amazing Case of “Back Radiation” – Part Three which includes the EBEX experiment as well as a brief explanation of fundamental […]
Interesting article, but somewhat confusing. It manages to come up with a conclusion which of cause makes no sense due to the implied logic.
First it postulates that here is no way to distinguish radiation (especially at equal wavelength).
Second it postulates that radiation is either absorbed or reflected by matter giving a bunch of numbers for absorption rates. Cautiously leaving out the fact that matter also emits radiation and thus avoids the trouble to explain how rates for absorption / reflection can be given.
The first postulation would imply that you can not distinguish emitted from reflected radiation which in turn makes any reliable measurement of reflected radiation impossible. Thus these two postulations contradict each other and so their combination constitutes a false statement.
But of cause a viable conclusion can not be drawn from false premise.
[…] Note how it matches the measured value. You can see this in more detail in The Amazing Case of “Back Radiation” – Part Three. […]
[…] – Part Three now […]
[…] The Amazing Case of “Back Radiation” – Part Three […]
I notice that SoD wrote in his essay
“If a surface absorbs radiation it must have an
effect on the temperature…”
Not much of an effectif there is a big effective heat sink on the other side.
nigel raymond:
Define “big effective”.
What is the thermal conductivity of the ground?
You can see the effectiveness or lack of it by seeing how the temperature swings at 1m and 10m deep vs the surface.
Was there a point to your comment? Radiation doesn’t affect the surface because there is a big effective heat sink?
What are you trying to say?
A very late comment, but I have not been reading this site for very long time. I do apreciate the way SoD is exposing the problematics of climatic change. Or just maybe variation. And this article touches upon the main issue of global warming, and the kind of problem that is most interesting to me. But, I am sorry to say, I didn’t become much wiser.
If the intention is only to show that DLR exists its ok, but the impact became a bit diffuse.
SoD says:
“As you can see the upward longwave measurement is around 400-500 W/m2. The paper itself doesn’t record the temperature on that day, but typical August temperatures in that region peak above 35°C, leading to surface radiation values above 500 W/m2 – which is consistent with the measurements.”
As a ground for explananation this is a bit thin. Was the sun shining? I would believe it did. Direct radiation from the sun (with absorption-reradiation) must be the main responsible for high downwards measurement, not the background radiation, the DLR.
Inspired by Roy Spencer I bought myself an IR thermometer and tried it out last autumn on a walk to a nearby small mountain (Trondheim-Norway), 700 meters high. I started measuring against a thin leaf in the shadow to get the air temperature at 250 meters high. It showed 12`C. At the top of the mountain the meter gave -20`C directed straight up against the blue sky. If this had been a black body it would have corresponded to 232 W/m2. Directed straight down against the ground it showed +20`C, which is corresponding to 418 W/m2. This could have been explained the same way as I feel it is done above. This “extra” downwelling radiation is the reason. But it is obviuos that this relatively high temperature of the ground was a result of the direct radiation from the sun on this wonderful day, not the far, far more weaker DLR. It seems to me that the situation is the same situation with these cotton fields masurements. A downward measurement in the shadow for me would have given far less, something like the first of 12`C corresponding to 374W/m2, directly related to the air temperature and its equilibrium with the ground.
I do not doubt some sort of back radiation, but the impact is not as clear as the blue sky over these cotton fields.
Trond A:
This is an important point to understand.
The longwave measurement noted here is upwards from the surface. This is thermal radiation emitted by the surface. Solar radiation reflected from the surface would be shortwave and not measured by this instrument (see The Sun and Max Planck Agree – Part Two).
The reason the surface is hot and radiating is that it is being warmed by the sun.
Does this explanation make sense?
We are discussing – from this specific quote – emission of radiation from the surface.
Thank’s for the reply SoD.
I mean i have the directions of up and down correct, not at least by reading your: “… while upward measurements of both shortwave and longwave were taken at every site.” Which must mean that downwards measurments is meausering radiation from the ground. Pure IR radiation.
But I get a feeling that you explain the higher IR radiation from the ground first of all as a result of the “extra” DLR, the back radiation effect, and not the direct heating from the sun. At least I dont`t see this expressed explicitly in the post the way you do now in your reply.
Then it’s more clear as far as your article is concerned. I am still wondering about the impact of the DLR on surface temperatures. There is more to learn.
In case it’s not clear:
Downward radiation is measured by pointing a measuring device up at the sky – to measure radiation coming down from the sky.
Upwards radiation is measured by pointing a measuring device at the ground – to measure radiation coming up from the ground.
Longwave radiation is greater than 4 μm and therefore not solar radiation. Shortwave radiation is less than 4 μm and therefore is solar radiation. We can easily determine the origin from the wavelength – to 99% accuracy (as explained in the link I gave in the last comment).
So if you point a measuring device down at the ground and have it is measuring greater than 4 μm it is definitely radiation emitted from the ground. If you have a measuring device which filters for less than 4 μm it is definitely reflected solar radiation.
My explanation may not have been clear. But the purpose of that part of the explanation was around elementary physics which many people are confused about and wrong about. This is the point I was trying to convey there: The ground is not reflecting the downward longwave radiation (DLR) from the atmosphere. Therefore the ground must be absorbing the DLR from the atmosphere.
This is not an attempt to explain the actual temperature of the ground by only reference to DLR – it is just another attempt to point out the obvious conclusion that people who say DLR cannot be absorbed by the surface (due to erroneous ideas of the 2nd law of thermodynamics) are wrong.
As always, explanations focused on one groups’ delusions confuse everyone else who are trying to understand the basics.
The reason the ground is hot in this EBEX 2000 experiment is primarily because of the sun.
Trond A,
The SURFRAD network records radiation and meteorological data from seven sites 24/7. You can access the data in graphical form here:
http://www.srrb.noaa.gov/surfrad/pick.html
You can tell if the sun is shining or it’s cloudy by looking at the direct-normal solar plot. Besides that you can get downwelling solar, upwelling (reflected) solar, diffuse solar, downwelling IR, upwelling IR, photosynthetically active radiation, solar net radiation, IR net radiation, total net radiation and UVB radiation. You can also get air temperature, relative humidity, barometric pressure and wind speed and direction and various temperatures related to the infrared pyrgeometers. The data are probably also available in digital form.
You can’t close the surface energy balance, though, because there is no data for convective heat transfer.
DeWitt Payne
Thanks for the link!
Yes, so the surface absorbs heat, more so during sunshine. I knew that all along simply because I have burnt my feet a bit on hot sand which, never-the-less, would have been cool again by, say, 4am the next morning. I also fully understand and accept everything you have said so far in Parts 1, 2 and 3, having majored in Physics and subsequently spent years in private research, including climatology and recent developments in Physics. Chances are I know at least as much about Physics as you do.
Now, let’s consider what happens to that heat generated in the surface and oceans. Actually, the oceans hold nearly 20 times the heat in the land surfaces, and nearly 30 times that in the atmosphere.
Firstly, none of the energy budget diagrams shows any net input of energy into the surface (except the 2008 NASA one which shows 1 unit, probably due to rounding rather than intentional.) It is assumed that, in fact more heat does go in during summer months and less in winter, so that, as everyone knows, the local surf is warmer in summer. So we know it can happen both ways and the surface can cool as we approach winter in any particular location in a particular hemisphere. Yes I know it averages out between hemispheres, but that doesn’t negate my point.
Let’s consider half way between seasons and just the land surfaces at this stage. The extra heat (in a rock surface, say) from back radiation flows into the rock at a certain rate (related to its conductivity) during the morning/early afternoon warming period, and flows out during the afternoon, evening and night and maybe even in cloudy periods during the day. Some will then be conducted and then carried upwards by convection. (NASA 2008 says 5% of total incident radiation energy transfers this way.) The rest will add to the radiated heat coming from the original incident radiation that was stored as heat earlier in the morning. Again some of this will be captured by CO2 et al and photons emitted, some up and some down. But some will not be captured at all and will go direct to space. So somewhat fewer come back the second time to be conducted and radiated up again, and even less the third time, and so on. Of course what is measured is actually some on their first trip down, fewer on their second trip etc. (Strictly speaking I should be talking about energy in the photons, not the numbers. Of course some energy also gets left in the atmosphere if any net warming occurs.)
But, the important point is, that less than half of the total energy that was radiated up on the first trip will be radiated up on the second trip, then less than half what went up on the second trip will be radiated up on the third etc.
Any such geometric progression has a limit which implies that all IR radiation will get to space sooner or later. For example, one half + one quarter + one eighth + … =1
In fact, the satellite measurements comparing inbound and outbound radiation do not show a difference which can be proven to be statistically significant because there are so many other factors – such as variations in potential energy (latent heat) – for which insufficient data is available for proper climate analysis, and probably never will be.
The heat flow from the core, albeit very slow, has over the life of the Earth raised the temperature of the land and oceans from absolute zero (-273.15 deg.C) to a “base” temperature something like that we would observe on a calm winter night an hour or so before sunrise. Solar insolation durng the day warms mostly on a daily basis, but with some storage of heat as summer is approaching, and loss of that heat as winter approaches in that hemisphere.
The only issue is whether there is or is not a build up of heat in the oceans from one year to the next. Data is so inaccurate on this that, for example, comparisons of ocean heat in 1961 are not statistically different from those in 2003 because the error bars overlap. They do appear higher, and that is in keeping with the temperature records. It is not really telling us more than the temperature records do – except for the fact that the temperature records should be weighted by the heat in the oceans (say,> 93%) and land (<7%) rather than surface area of oceans (~ 70%) and land (~ 30%.) This means NASA "sea surface" temperatures are a much better guide than anything else. It is shaping up that the mean for 2011 will be less than the mean for 2003, implying a slight heat loss in that 8 year period. Keep watching yourself on their site.
Now, because there is so much more heat under the floor of the ocean (including the liquid core at around 5,700 deg.K) we cannot be certain as to whether any warming has come from natural variations in the "base" temperature supported by the sub-surface heat in the rest of the Earth, or by variations in heat retained from solar insolation, or a combination of these.
Doug Cotton,
The surface was originally molten due to conversion of the kinetic energy of the accumulating mass, not absolute zero. It wouldn’t have been 0 K even if that weren’t true. It would have been at whatever the cosmic microwave background temperature was at the time (2.7 K now). The core is only hot now because there is sufficient energy input from radioactive decay to keep it warm. Mars, for example, doesn’t appear to have a molten core, nor does the Earth’s moon.
And don’t you think the surface would have cooled (to say nothing of the atmosphere) in billions of years if the outer crust had not stayed warm at liveable temperatures. How much more heat i sthere under the ground (right down to the centre) than in the oceans, land and atmosphere? Don’t even strat to guess! But the heat flow is outwards not inwards – that is all theat matters, not the rate of flow.. That’s what Physics says. (You can have high voltage electricity with low watt output.) There is a lot more detail on my site.
There is a temperature gradient from the core. Boreholes in Germany indicate 270 deg.C at 9,000 metres down to about 10 or 12 deg.C at the surface. Would you like to live there if that 10 to 12 degrees had been 50 degrees, or well below freezing point, perhaps like in Antarctica. It’s hot enough in Singapore where underground and ocean temps are about 25 deg.C – which by no coincidence is also about the minimum temperature every day. The sun there can only warm the place up by 6 or 7 degrees to 31 or 32 deg.C every day of the year. But it also cools every night of the year to 25 or 26 degrees, as is supported by the sub-surface temperature.
How do we get on in Antarctica even in summer when sun shines all day, even though at a bigger angle. It can warm Melbourne at nearly the same angle in winter to over 20 deg.C sometimes, but this is hardly likely at the South Pole, because the underground temp is not high enough.
Sorry, that’s all I have time to write. Visit http://earth-climate.com for more.
PS – DeWitt – OK I’ve altered that para on second page of my site to read …
The heat flow from the core, albeit very slow, has, at least since life started on Earth, maintained the temperature of the land and oceans at a “base” temperature something like that we would observe on a calm winter night an hour or so before sunrise. This is at least more than 250 degrees above what it would now be in the absence of such heat. Solar insolation durng the day warms mostly on a daily basis, but with some storage of heat as summer is approaching, and loss of that heat as winter approaches in that hemisphere.
Doug Cotton on August 15, 2011 at 4:32 am:
Why is that the important point?
Heat transfer calculations don’t follow the life cycle of a packet of energy. They calculate heat flows and consequent temperature changes by applying the equations of conduction, convection and radiation.
Perhaps we can nail all heat transfer problems with the same approach – the energy eventually makes it out of the system so that’s the important point?
Except usually we want to know – as a result of the physical properties of the system and the energy input at various boundaries, values like: temperatures at boundary X, heat flow through material Y, and so on.
If you have a point – an important point – it is so far lost on me.
I’m not how this links to your previous important point. Satellite measurement of OLR and reflected solar radiation do not have the absolute accuracy required to determine an energy imbalance. This is correct.
There’s plenty of papers calculating the heat flow from geothermal sources to be less than 0.1 W/m2.
From Impact of Geothermal Heating on the Global Ocean Circulation:
Check out The Heat Flow from the Continents, by Pollack, which talks about some of the measurements techniques and the first calculation by Kelvin in the 1880s for the UK where he determined it was 68 mW/m2.
And by the same Henry Pollack: Heat flow from the Earth’s interior: Analysis of the global data set, REVIEWS OF GEOPHYSICS, VOL. 31, NO. 3, PP. 267-280, 1993:
Doug Cotton,
Nope. You’re neglecting the effect of the rather large heat capacity of the Earth’s surface and atmosphere. If the Earth were teleported to inter-galactic space, the surface temperature would decline to about 36 K in order to radiate the ~0.1 W/m² energy flux from the core. Solar energy absorbed averaged over the whole surface of the planet on the daylight side over a year after correcting for albedo is ~578 W/m², more than three orders of magnitude higher. That absorbed energy can’t be radiated away in the ~12 hours of darkness at a temperature of 36 K. It is the sun that keeps the surface warm enough to support life.
There are several other points mentioned now at http://earth-climate.com/CaseAgainst.html
Firstly, you may wish to consider the scavenger role of GHG gas molecules. As they collide with oxygen and nitrogen molecules they collect energy and assist thus these molecules to shed their energy, because GH gases can radiate far more easily. If air was only O2 and N2 it would heat continually to extreme temperatures before these molecules start to radiate high energy UV photons. Hence there is also a cooling role for CO2, not just in the stratosphere but also everywhere else.
Secondly, and most importantly, the satellite measurements are in fact very accurate and if you look closely at Tremberth’s chart here http://earth-climate.com/energy.jpg you will see a close tracking of ocean heat with net radiation energy until 2005 when ocean heat broke away from the trend and started to decline, leave what he calls “missing energy.” http://earth-climate.com/energy.jpg
It is no coincidence (I say anyway) that this was merely an expected lag in the downturn in the 60 year cycle which had a maximum in 1999 (not coincidentally correlating with the biggest El Nino on record.) The lag coukl be due to time taken to conduct energy underground as explained below.
The rate of heat flow from the surface is irrelevant, and hasn’t been measured under the oceans anyway. The missing energy is in fact energy which has flowed back into the crust beneath the floors of the oceans. Just like you can have high voltage electricity without having to have high current, likewise a certain temperature in very low conductivity material such as rock and clay can “support” a similar temperature above ground and in the oceans. If this did not happen you could never see such uniform temperatures as, for example, in Singapore. If the “break out temperature were, say, 30 degrees higher or lower humans would not be living on tis planet.
The temperature is “set” by an extrapolation to the surface of the underground temperature gradient which originated at around 5400 deg.C in the liquid core. German boreholes showed a linear trend from 270 deg.C at 9,000 metres to about 10 or 12 deg.C at the surface. In Singapore it is about 25 deg.C or 26 deg.C at the surface, which is also the minimum temperature every day.
If the gradient “breaks out” at a slightly lower temperature (as happens due to natural cycles) then heat from the ocean (and crust) can flow inwards to fill the gap (like a valley in the gradient plot) and build up until it meets the temperature of the outward heat flow. So it just “dams up” some heat there until, perhaps around year 2028 the gradient starts to break out at higher temperatures so heat flows back out and we have 30 years of warming to 2058.
If yoiu don’t accept this, then you explain the missing energy.
The 60 year cycle is statistically confirmed with information on my home page, and it is no coincidence that it correlates with the Saturn/Jupiter resonance every 59.6 years. The communication between planets and Earth could be through magnetic fields, but more probably through the acceleration due to gravity. This varies dramatically between Jupiter and Earth, having a significant impact on the moon’s gravity. Hence the solid Earth tides (in the outer crust) caused primarily by the moon, will have some variation in magnitude, and hence variation in friction. This builds up or declines over many daily rotations of the Earth, and is a plausible explanation for the observed 60 year cycle.
There is also a 934 year cycle correlating with the eccentricity of Jupiter and this plot (based on orbits of the sun and 9 planets) gives a remarkable correlation with recorded temperatures. http://earth-climate.com/planetcycles.jpg
DeWitt You will not affect the liquid core temperature and you cannot alter the conductivity of the rocks, clay etc in the mantle/crust – the temperature gradient being inversely proportional to such. Hence the “break out” temperature (that is the intercept at the surface determined by extrapolating the underground temperature plot) will still be the same. See linear trend line here for this German borehole http://earth-climate.com/crust-temp.jpg
You keep talking about radiated heat from the surface. Where is your calculation of conducted heat which NASA shows as 5% of incoming solar radiation and evaporated heat which they show as 25% here: http://earth-climate.com/calculations.jpg ?
The conduction and evaporation still happen to some extent at night, so where is the energy coming from? Out of the crust and the surface of the water bodies. During the day the inflow of heat from the sun dams up this potential flow from the core. The potential heat flow is thus far more than just what is measured as radiation, or even what is measured between two points underground during the day. One way or another, all the massive heat being generated in the core by fission, friction, magnetic field interactions with charged ions, and decay of radioactive isotopes must come out as fast as it is generated..
Doug Cotton:
The solution is calculated in the radiative transfer equations. This means that the heating rate/cooling rate vs altitude of CO2 and other radiatively-active gases is well-understood.
You can see this explained in Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Eleven – Heating Rates, for example:
I can’t make sense out of your statements. If the rate of heat flow from the surface is irrelevant why are you talking about it. It appears you believe the temperature of the earth’s surface is largely determined by geothermal heat?
You imagine that it hasn’t been measured under the oceans? Or you actually researched the subject and this is your conclusion? Let me know how sure you are before I tell you the answer.
What do you think about Henry Pollack’s paper of 24,000 observations?
Still, I’m having to guess what you are actually claiming because it’s not clear.
In any case, perhaps you haven’t understood this article about DLR. What do you think this article is claiming?
Regarding the heat flow from ocean ridges quoted as “up to 200 mW/m2 on mid-ocean ridges” and other areas of ocean floor, I would want to be seeing measurements in the 1970 to 1999 period (when ocean heat was rising compared with 2006 to 2011 when it has been falling. Do you know of any such comparisons? Even 0.1 to 0.2 W is a significant component of the net radiation in this chart (prior to 2005) which then starts to show the missing energy as ocean heat starts to reduce after 2005: http://earth-climate.com/energy.jpg (There are of course many other weather conditions – cloud cover etc – that could explain the net radiation gain, instead of having to attribute it to CO2.)
The cooling effect considered in the models is totally different from cooling by collision with warmer O2 and N2 molecules (diffusing heat) and I defy you to show me exactly where and how it is taken into account in the models: documentation – page number etc please. If they had taken it into account they would have realised that it is very similar in magnitude to the warming effect. CO2 molecules themselves do not get warm for long when they capture a photon because they rarely transmit heat by collision before the next photon is released.
I am also pointing out the importance of GH gases in preventing extreme heat in a hypothetical O2 / N2 only atmosphere.
Regarding the heat transfer: the heat in the ocean relative to the heat in the rest of the Earth under the surface is absolutely negligible. (The mass of the Earth is far greater, and the temperature of the core 5400 deg.C far higher.)
Anyway, let’s assume it’s like a glass of water at 15 deg.C in an air conditioned room at 23 deg.C representing the rest of the Earth. Which is going to dominate? If you think the temperature in the room (Earth) will not affect that in the glass (oceans) then you are incorrect. Equilibrium will be extremely close to 23 deg.C, not 15 deg.C. So the temperature as “seen” at the outer surface of the Earth (and floor of oceans etc) dominates and controls everything above the surface. Solar insolance each day is an absolute drop in the bucket (or a drop of boiling water in the glass would be pretty near.) That “seen” temperature depends on the intercept at the surface of the extrapolation of the temperature plot fro heat coming from the core.
Heat from the room passes slowly through glass walls by conduction and warms the water eventually to 23 deg.C. But, at equilibrium, not much (if any) heat transfers through the glass – which way would it go anyway?
So no further heat flow is required. No radiation is required. We have equilibrium. (Blackbody radiation theory is irrelevant here.)
Now, give me a Physics argument that refutes the above before we go any further. (Maybe just read all of http://earth-climate.com/CaseAgainst.html and that will save me typing it all out again.).
Of course in the real world, winds and other weather conditions upset equilibrium a bit – usually with more net cooling effect in the atmosphere than net warming, so we do see a low mean value of outward heat flow to bring it all back to equilibrium.
Just think! How on Earth would air near the ground stay at anywhere near the same temperature as the ground itself if this equilibrium was not being achieved by conduction. Radiation alone could never get the balance right. Even Wikipedia on “Heat Transfer” will explain that in the second paragraph..
Doug Cotton:
When people write authoritative comments like “If they had taken it into account they would have realised that..” about basic physics I just assume the writer hasn’t read a textbook on radiative physics, or if the writer has, he or she hasn’t understood it.
In radiation there is absorption and emission. Absorption of radiation is thermalized (i.e. “shared”, in the vernacular) with other molecules in the lower atmosphere. The equations are laid out for consideration in Part Six – The Equations. These are just what you find in textbooks. If you can explain where they are wrong you will revolutionize 60-100 years of physics. These are the equations which determine heating rates in the atmosphere.
Your statement is quite vague, and therefore open to interpretation, but it looks to me as if you don’t understand the basics. Or you imagine that people who study atmospheric physics don’t know what thermalization of absorbed radiation is. Yes they do. Try Atmospheric Radiation: Theoretical Basis by Goody & Yung, 2nd ed 1989.
A physics argument against:
That’s not very challenging is it? The physics argument is that you have to do a thermal conductivity calculation with the correct boundary conditions and material properties. Not one for a glass of water.
Present your calculations and explain why all the papers are totally wrong about the flow of heat from geothermal sources.
Have you read any of these papers?
Any textbooks on the subject back you up? Any papers? All wrong?
What did they get wrong?
-The material property of thermal conductivity of the various rock types?
-The formula for thermal conduction?
-Measuring in all the wrong places (all 24,000 of them)?
-Incorrect measurements (24,000 times)?
In your next comment please explain exactly what equation for heat conduction you have used, what boundary conditions you assumed, what parameter for thermal conduction, what value of heat flow you get, how you averaged it across the surface of the earth and it might be interesting.
Otherwise I will just ignore it.
There are much more interesting subjects to study than why one random person believes that no one else (including Emeritus Professors of Geophysics) can calculate heat conduction from the earth’s interior.
For those wondering if Doug Cotton knows his stuff I thought this might be interesting in the light of his confident claim:
Here is a graph from Pollack et al, 1993 demonstrating the number of measurements under the ocean compared with land:
It’s one thing to say “I don’t know” or to ask a question. But when someone make claims like this then readers are entitled to assume the confident claimers have no idea what they are talking about on any other aspect of their favorite subject.
Emeritus Professors of Geophysics probably know a little about the subject of Geophysics.
If Doug Cotton is ahead of them, his first step should be demonstrating he has even read what they have written in their many decades of research.
Having majored in Physics I am quite aware of what radiation is all about. It is yourself who has missed my point altogether.
Maybe you would like to answer a question I have asked elsewhere (believing I do know the answer, but just wishing to check that we are on the same wavelength) …
How do O2 and N2 molecules lose their heat, which we can assume they initially gained by conduction on contact with warmer surface molecules?
Pollack’s paper is one I quote at http://earth-climate.com/caseagainst.html I acknowledge that many readings have been taken, particularly on ocean ridges where figures of up to 0.2W/m2 have been measured, which is fairly significant when you consider Trenberth and Fasullo (2010) total net energy changes at TOA The ocean would not have to warm very much above the very finely tuned equilibrium to cause the heat flow to change directions. Maybe you have another solution to Trenberth’s “missing energy” dilema in the yellow shading in: http://earth-climate.com/energy.jpg
I should have enlarged my point to clarify that I meant heat flow both into and out of the very floor of the ocean (not ridges) over an extended period of at least several decades on a worldwide basis, even under ice caps, so that trends in any variations could be analysed, as with surface temperature data.
I trust you agree that if two conducting bodies are in contact having different temperatures (say two flat steel plates) and one contains considerably more heat energy than the other, then the equilibrium temperature will be much closer to the initial temperature of the one that had the greater heat. When equilibrium is reached, how much radiation passes between the sheets and, if any, in which direction(s)? How does the energy in that radiation compare with the energy that transferred by conduction during the initial warming period? Do you see any analogy with the surface of the Earth and the immediate atmosphere?
Considering the relative mass of the whole Earth beneath the surface and floor of oceans, and considering the far greater temperature of the core (~5400 deg.C) compared with the oceans (which themselves store about 90% of the stored heat above the surface) then I trust that helps you understand my analogy with a glass of water in an air-conditioned room. Probably a thimble of water in a huge pavilion would be closer.
,
Regarding temperature gradient: At
Click to access dekorp4_ftb-85_ktb-bore-hole.pdf
you will find this plot http://earth-climate.com/crust-temp.jpg where you will note the yellow linear trend line intercepts the surface at, say 10 deg.C. This saves me having to do the calculations myself.
To keep it simple, suppose this were the same all over the globe. What do you suppose the world would be like if that went up to 50 deg.C in the next million years say, because the liquid core temperature rose a bit for some reason?
Would the oceans, perhaps after a billion years, warm to the new “surface intercept” temperature, or would they stay where they are and somehow alter the conductivity of the sub-surface rock such that the temperature gradient became steeper leading to a surface intercept of 10 deg.C again? Obviously the latter is impossible.
A third scenario is that the ground surface on land were 50 deg.C and yet the atmosphere stayed as it is. The NASA 2008 model shows only about 3,4% heat transfer by conduction, so that won’t be very effective in bringing about equilibium while 79.6% of all heat from the ground is going up in radiation – actually more when we apply SB Law. If the model is right, this may be a possible scenario. Get out your rubber boots.
PS In your response to an earlier post you appear to have overlooked “cooling by collision with warmer O2 and N2 molecules” which was the point of the question – not radiation. So I’ll rephrase my question and invite answers from anyone …
(a) How does heat leave an O2 molecule? (ie. by what physical process?)
(b) What type of other molecules, if any, are involved in the process?
(b) Where does that heat eventually go?
I repeat and stand by my comment “The rate of heat flow from the surface is irrelevant” because …
I am speaking about temperatures just under the surface “supporting” a ground level atmospheric temperature which is fairly similar to ambient temp, say, an hour before sunrise on a calm night. (examples: Germany about 10 to 12 deg.C; Singapore about 25 deg.C, The poles well below zero.)
It would not matter if there were no heat flow at all. It can still support a base temperature. (Note that just 3 or 4 metres underground there is little sign of summer to winter variation.)
If the ambient temperature drops below the support level (eg on a windy night) then the sun can have more effect because the heat of the morning cannot enter the ground while it is still hotter than the adjacent atmosphere. But once the atmosphere is warmer, heat can flow in, and back out the next night But, when cooling once the “surplus” heat (usually stored very close to the surface) runs out, the surface stops cooling even if the atmospheric conditions further cool the ground level atmosphere. At least we know that 3 or 4 metres underground it will be stable and non-seasonal.
But that temperature is controlled by the heat from the core as I have shown with the previous reference to the German borehole tests which showed, for example, 270 deg.C just 9,000 metres down.
Surely there is a possibility that variations in the core temperature, or other heat generated in the mantle/crust, could alter this near surface underground temperature.so that this could be the cause of long term tremds over a thousand or more years. Any variations in any heat generated fairly close to the surface (by friction in solid earth tides, or magnetic fields acting on charged ions or something else no one has thought of yet, could affect climate more quickly.
If you are still agreeing, then surely it follows that any climate predictions should look into possible methods for predicting any such changes which could well dominate any others. I suggest we are seeing one such change right now which could explain Tremberths “Missing Energy” http://earth-climate.com/energy.jpg
See the big picture …
All the calculations regarding radiation are incorporated in the IPCC, NASA, Trenberth etc models, and all the models show either zero or up to 0.3% of total incoming radiation as being absorbed. That is, 99.7% or more still gets out to space.
Now just how accurate could they really be? It’s obvious that they have looked at current data (as shown here http://earth-climate.com/energy.jpg courtesy Trenberth) and see a current net input of about 0.3%. So they have to build that known result into the models and adjust the other figures to give this value. See this and read the margin: http://earth-climate.com/calculations.jpg and the difference between warming and cooling is only of the order of the rounding amounts!
Now the first diagram shows “Missing Energy” which they cannot explain, but which is not warming the oceans.
Finally, you have to ask yourself, what does this say about the last 8 years – where is the assumed extra heat in the oceans actually affecting temperatures at sea level? http://earth-climate.com/2003-2011.jpg Maybe it’s all missing energy and their estimates of heat in the ocean are a little out. (The NASA sea-surface temperature data is very accurate to 0.01 degrees I believe, but measuring heat at various depths in the ocean and then weighting measurements by volume has a much bigger margin of error.)
How do we know the missing energy has not just filled temperature “valleys” that have developed naturally (probably in cycles) under the surface? If it’s not really in the oceans, it has to be underground, because the rest of the above surface earth system can only hold 10% of all heat – 90% is in the oceans.
For much more detail please read: http://earth-climate.com/CaseAgainst.html
and the Home page.
See this post on scientific form “The Conversation” under Carbon Tax item in Environment section ….
Gerald Thomas
Dr
insightful +
Talk of “unmitigated climate change” and “glimmer of hope” just adds to the increasingly obvious fact that the whole issue of climate change is shaping up as a worldwide debate between Climatologists (such as Trenberth) who are no doubt good at forecasting local weather, and Physicists (such as Prof. Nahle, Knox and Douglass and Cotton) who are now coming in on the act and pointing out in no uncertain terms (a) that there has been no climate change since 2003 and (b) that the physics of quantum mechanics does not support the “theory” that CO2 can have any significant effect, other than perhaps a slight extension (by a few minutes) of the warming of the day into the early evening. All heat from the day dissipates to space usually by the early hours before the next dawn, and, on a seasonal basis, by the next winter.
Read about Prof Nahle’s experiment pointing out that heat transfer from the surface to the atmosphere is mostly by conduction then convection, and that it is warm air rising by convection that got trapped in the original laboratory experiments in the 19th century – not air that was heated by trapped radiation. http://climaterealists.com/?id=8073
Then read how Knox and Douglass rubished Trenberth’s figures and his “missing energy” which would be even worse if he had used correct data. They showed that ocean heat has not increased at all since 2003. http://www.pas.rochester.edu/~douglass/papers/KD_InPress_final.pdf
There is a good review of the debate at http://www.reportingclimatescience.com/news-stories/article/global-warming-missing-energy-row-erupts-as-new-research-says-oceans-are-cooling.html
Now, ocean heat is 90% of all heat, so NASA “sea surface” data is the best indication of trends. So, see for yourself how 2011 is shaping up as being cooler than 2003: (Select “sea surface” in the listbox and tick 2003 and 2011 in the checkboxes.)http://discover.itsc.uah.edu/amsutemps/
I suggest that NASA data confirms what Knox and Douglass are saying. There has been no net positive radiation since 2003 and consequently no accumulation of heat in the oceans.
Yet CO2 levels have been the highest in modern history.
At the very least, why is the world worrying so much now, when the rises last century have passed by and probably just had something to do with a build up to what was the biggest El Nino on record in 1999.. Such El Ninos can, at least to some extent, act as a safety valve releasing excess heat straight through the atmosphere and out to space. Certainly that seems to have been the effect once it all settled down by 2003.
Climate science reminds me of this quote:
“”Instead of applying observation to the things we wished to know, we have chosen rather to imagine them. Advancing from one ill founded supposition to another, we have at last bewildered ourselves amidst a multitude of errors….
…When matters have been brought this length, when errors have been thus accumulated, there is but one remedy by which order can be restored to the faculty of thinking; this is, to forget all that we have learned, to trace back our ideas to their source, to follow the train in which they rise, and, as my Lord Bacon says, to frame the human understanding anew.”” Lavoisier
Since climate science has been so heavily politicized, it is time to follow the idea back to it’s source:
(1) The basic source is the simple idea, more CO2 will result in more of the longwave radiation emitted by the earth being absorbed by CO2 and then re-emitted back toward the earth, result, more longwave radiation, a hotter earth. This is known as backscatter, in this case, increasing backscatter due to CO2.
(2) There is more CO2.
(3) We should expect to see more longwave radiation at the earth’s surface (backscatter), specifically in areas where the surface upward longwave radiation has not changed much
(a) If upward radiation goes up by a large amount, such as due to an urban heat island effect, increased solar output, decreased clouds, changes in aerosols etc, one would expect that downward radiation would go up as well (you have to have something to backscatter). However, that would not be the result of increased CO2, but simply the result of more longwave to absorb and re-emit.
(4) Methods of detecting longwave radiation exist, and have for some time.
(5) We should therefore be able to see a long time series graph showing that, as CO2 has increased, the amount of backscatter specifically from CO2 has increased.
(a) We would have to isolate backscatter from CO2 from other sources, such as especially H2O. This may mean that we need to know a frequency that CO2 radiates at, that no other longwave source in the atmosphere does, especially including H2O (including both water vapor and clouds) and also including simple rising hot air (oxygen and nitrogen).
(b) Failing that, we would need to know how much of the detected longwave is from sources other than backscatter from CO2. That could include a lot of measurements, temperatures and pressures and humidities and amount and type of all gasses and cloud details and the amount of longwave emitted by the surface, and possibly others.
(c) Failing that, we might be able to tell at least something if the time series is long enough and covers a very wide multitude of sites, where we could at least see a few basics that might effect the measurement, such as weather records of the time the measurement was made (which we know exist).
In short, CO2 has increased, therefore, according to the theory, backscatter from CO2 has increased. We can observe this directly. So, could I see it? There should be a plot somewhere that shows that as CO2 has gone up, backscatter from CO2 has also gone up, this is called direct observation. Theories are all very nice, but the scientific method requires them to be verified or falsified by direct observation. If that is not done, it is not science. In this case, it can be done. So why have I never seen, or even heard tell of, it actually being done? If this theory is correct, CO2 has risen enough that we should be able to detect increasing backscatter right now. I would expect that the pro “climate change” people would have been shouting that news from the housetops by now if it existed, yet they are not. Meanwhile, they claim that secondary effects of climate change are occurring right now (warmer temperatures, rising sea levels, etc), these cannot occur if the increase in backscatter from CO2 does not happen. Therefore if they are detectable, the increase of backscatter must also be detectable. So why have I not seen or even heard a report of observed increase in backscatter?
The information I have been able to gather to date is two reports of anecdotal evidence, from people who detect backscatter, who say that it has not changed in up to 35 years (one reported that it actually went down some). This suggests that the entire theory of climate change due to CO2 induced backscatter has been falsified by direct observation. However, I would like more than anecdotal observation. So where is there, at the very least a long time series of direct observation of longwave backscatter? Better yet would be one, which manages to isolate the backscatter from CO2 from all other sources. And if it exists, why is it not seen on this site?
If more CO2 —–> more backscatter
More CO2
More backscatter observed?
Not theories, direct observation, that is called ‘science”.
Yes – good point. Another related farce is the fact that if there is warming there should be net downward radiation at top of atmosphere (TOA) but even the models only claim that this would be about 0.5% of total inward radiation. Now the problem is that the errors in the models are acknowledged to be at least plus or minus 1% to 2%, so they cannot realy even predict a net positive result – just a range from, say +2.5% to -1.5% – hardly “proof” of warming. In fact Trenberth’s curved trend shows the world starting to cool from about 2006 – see http://climate-change-theory.com and so we could hardly expect increases in downward radiation anyway. The very fact that the world can cool proves CO2 has no effect. There are even ways in which GHG might cool the atmosphere as I explain on my sites.
Another experiment which should be done by someone is this: build two “tanks” say about 5 metres high, 2 metres diameter with removable lids. Place their (open) bottoms on soil and rock, and include a tray of ocean water covering 70% of the base to simulate whole of Earth. Pump in extra CO2 into one, but for the other pump in an 80% nitrogen 20% oxygen mix to flush out the normal air and Co2 etc. Open the lids at midday with the sun shining directly in. (The tanks could be leaning a bit if sun not directly overhead.) Measure temperatures of soil, rock, water and air mix in each for several minutes, then close lids and check the relative concentration of CO2 so that a mean can be calculated for each tank. Compare the temperatures!
Legatis:
It is important to understand that it is not “backscatter” but “re-emission”. Absorption and re-emission of longwave radiation is not known as back-scatter at all.
The definition isn’t material to any of your points but might lead to confusion on the part of other readers.
If you read Part One then you would know that high quality measurements of downward longwave radiation (DLR) are limited.
If we had one location where the surface temperature had not increased yet the DLR had increased we would need to know:
a) the spectrum of DLR (e.g. from an FTIR) to understand the wavelength dependence (to therefore know the source – water vapor, methane, ozone, N2O, CO2..)
b) the temperature of the atmosphere (because a higher temperature atmosphere would radiate more than a lower temperature atmosphere for the same concentration of radiatively-active gases).
Let’s suppose that we had a long well-calibrated time series of FTIR measurements which showed an increase in downward emission in the 15um band with no temperature change in the atmosphere. How much of an increase in the 15um band do you think we would see?
What measurement accuracy would we need over what time period for all of the parameters?
Let me ask you a few more questions. Having read Part One and Part Two do you think that:
a) CO2, water vapor and methane absorb and re-emit terrestrial radiation?
b) The values can be accurately calculated from the theory of radiative transfer given the concentrations of the gases and the temperature of the atmosphere?
Your questions indicate you have not understood the material on this site. And while you would like more than anecdotal observation the fact that you report these “sources” with confidence and without attribution indicates that you believe you already know the answer.
It’s nothing to do with climate change. If more CO2 doesn’t cause more DLR for the same temperature of atmosphere then you will have falsified a significant branch of physics.
Well done in that case.
The field of science that you are questioning is the un-politicized field of physics called “radiative transfer”. This has been well-proven since the 1950’s and probably well-before.
Have a read of Radiative transfer by S. Chandrasekhar or Atmospheric Radiation: Theoretical Basis by Goody (1964).
Chandrasekhar and Goody are physics heavyweights and if you can understand their theory and the experimental evidence that backs up their theory you will make good progress.
Doug Cotton:
A brilliant experiment to prove that with the atmospheric temperature at effectively the same temperature as the surface you will see zero “greenhouse” effect.
You have created a wonderful challenge for people who don’t understand atmospheric physics. I expect that at the end you will say:
“Right, now you have spent all this time building this experiment and finding nothing you wished you have spent a few hours trying to understand the physics behind the inappropriately-named greenhouse effect”
I applaud your thinking. You will have got them to waste their time, but at least they will have learnt a valuable life lesson.
Better to spend a few hours trying to understand well-proven physics than to spend weeks building experiments doomed to failure – or spending weeks writing rubbish in blogs around the internet..
Which part of this equation is hard to understand?
dIλ/dτ = Iλ – Bλ(T)
If the atmosphere is at the same temperature as the surface then the change in intensity of the radiation field is zero.
People who think the experiment demonstrates something – go to the bottom of the class.
If, on the other hand, you have an atmosphere more than 10km tall and decreasing temperature with altitude then the absorption and re-radiation will have an effect. This is clearly demonstrated by measurements of upward and downward radiation.
For example, Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Ten.
There is absolutely no valid logical argument by which you can deduce that warming will occur because back radiation due to CO2 increases. Of course it will increase as the proportion of CO2 increases. So what? So will upward radiation. This does not mean that more heat will remain in the oceans or land surfaces or even the atmosphere. Any excess warming (as happens every hot sunny summer day) will be followed by extra cooling (eg at night and in winter) and heat can be transferred to TOA by other means apart from radiation – what about good old convection?
And how do oxygen and nitrogen molecules cool? Not by radiation but by diffusion (due to molecular collision) to cooler molecules, some of which are GH gas molecules. GH gas molecules can emit far more radiation than O2 and N2 molecules. So there is a good argument that more GH gas could lead to more cooling than would occur if there were less GH gas.
In order to “prove” warming your models have to show on a world wide annual basis that there is marginally more downwelling flux at TOA than there is upwelling flux. Measurements indicate this is unlikely to be more than 0.5% of total incoming radiation at TOA. Yet the models are acknowledged to have errors of the order of 1% to 2%. Hence they are simply not accurate enough to prove that the net difference is definitely positive. Empirical measurements show variations between positive and negative, just as temperature records show periods of warming and cooling. This is hardly surprising. But you should be asking, how could any period of cooling persist over a few years with increasing carbon dioxide levels. There has been no net increase in temperatures from January 2003 to September 2011 for example, despite the highest levels of anthropogenic carbon dioxide ever.
That is the question you have to ponder. It has never been proven and so the whole hypothesis of global warming being due to carbon dioxide has never been proven either in theory or in empirical measurement. So prove me wrong on that! More detail is at http://climate-change-theory.com where you will see other more likely explanations for the observed climate change.
If you have any “feel” for physics you will understand that the vast majority of the heat in the Earth’s system is under the surface of the crust – not in the oceans and atmosphere. This heat creates what we might call “temperature momentum” and it controls climate. We can easily observe how air temperatures just above ocean surfaces are at very similar temperatures to the sea surface on calm nights. The oceans are like a small glass of water in a large air-conditioned room. Their temperature is ultimately controlled by the air conditioner setting because of the far greater “momentum” of the heat in the room compared with that in the water.
What we need to look for is variations in core heat (due to natural causes possibly related to planetary orbits) which ultimately control climate and explain observed climate cycles.
This series of articles about back radiation was motivated by the people who think it doesn’t exist, isn’t caused by CO2, and/or can’t be absorbed by the surface.
The argument is that warming will occur because the emission of radiation into space moves to a higher altitude with more CO2. This happens because the atmosphere is more opaque.
A higher altitude means a colder temperature and therefore a reduction in emission of radiation to space.
Therefore, a warming (less energy leaving the planet).
scienceofdoom
It is important to understand that it is not “backscatter” but “re-emission”. Absorption and re-emission of longwave radiation is not known as back-scatter at all.
Here I was simply trying to find a single word that would allow me to not type out “Absorption and re-emission of longwave radiation” each and every time I need it to describe what CO2 is doing. Since you seem to accept DLR as a convenient abbreviation, I will use that instead. The simple fact is that if I include all information in every statement, including ALL the relevant information, with no shortcuts or abbreviations, any post I (or you) make will be VERY long.
Second, somehow you are able to post in italics, I try to paste IN italics, they don’t come out AS italics, so I am indenting what I am replying to (or trying to, that probably won’t come out either).
..(3) We should expect to see more longwave radiation at the earth’s surface (backscatter), specifically in areas where the surface upward longwave radiation has not changed much
(a) If upward radiation goes up by a large amount, such as due to an urban heat island effect, increased solar output, decreased clouds, changes in aerosols etc, one would expect that downward radiation would go up as well (you have to have something to backscatter). However, that would not be the result of increased CO2, but simply the result of more longwave to absorb and re-emit.
(4) Methods of detecting longwave radiation exist, and have for some time.
(5) We should therefore be able to see a long time series graph showing that, as CO2 has increased, the amount of backscatter specifically from CO2 has increased..
If you read Part One then you would know that high quality measurements of downward longwave radiation (DLR) are limited.
Limited as in none exist, or what? What I am saying is that it is claimed that the effects of “global warming” are happening right now, and that these effects are entirely due to CO2 (and not from some natural cause). The only way CO2 can do this is by increasing the amount of DLR, either directly itself, or by causing more evaporation and more of the “greenhouse gas” H2O. If the EFFECTS are actually SEEN now, and they are caused by DLR, then should we not be able to detect more DLR, if there is so much more than formerly that it is causing actual effects we can see? If it is large enough to see it’s effects, it would seem more likely that it’s primary source, DLR, will also be large enough to detect.
Now, it may very well be possible that the records we have are too few and too inaccurate to detect this, despite the claimed bad effects from it. It may be that the signal is lost in the noise. However, if the effects of this DLR are said to be happening right now, it would suggest that the amount of increase of DLR is enough that we can detect it after all. We should at least TRY, no one seems to have done that. After all, you said “limited”, limited is greater than zero.
If we say that the increase of DLR is great enough that it is causing bad effects now, yet we still cannot detect this increase of DLR, I would have to ask whether an increase so small we cannot detect it after decades of being able to is enough to cause any effect at all. After all, we do know that there was the little ice age, the medieval warm period, the dark ages, the roman warm period etc, all clearly not caused by humans creating massive amounts of CO2. If such warming and cooling, often greater than or equal to today’s, can exist independent of CO2 and it’s DLR increase, then perhaps the so-small-we-can’t-detect-it increase of DLR is not such a big worry after all. That is, to some extant, a different subject.
Also, there may be more sources of DLR records than you know. One of the anecdotal posters was working with observatories. They need to know the DLR for two reasons. The first is they must flex their mirrors (using motors) to adjust for the heat distortion of the atmosphere. To do that, the need to know it’s heat, hence, they need to measure DLR. Second, some of their telescopes are, in fact, infrared telescopes, obviously those can detect DLR on their own, and also need to be flexed. These observatories have been operating for quite some time. They are not climate scientists. Therefore, they can provide a source of measurement of DLR which, being outside of climate science, you may not be aware of. There may also be other non climate science sources of DLR measurements you are not aware of, therefore, the total amount of quality measurements may be greater than you know. And we may be able to do what many climate scientists do, take a very large number of not so great quality measurements over a very long time period (as long as we can get) and average them out.
If we had one location where the surface temperature had not increased yet the DLR had increased we would need to know:
a) the spectrum of DLR (e.g. from an FTIR) to understand the wavelength dependence (to therefore know the source – water vapor, methane, ozone, N2O, CO2..)
b) the temperature of the atmosphere (because a higher temperature atmosphere would radiate more than a lower temperature atmosphere for the same concentration of radiatively- active gases).
You would not necessarily need to know the spectrum to see if global warming was happening or not. It is claimed that more CO2 will increase DLR, which will heat the ground, and result in evaporation, more H2O, thus both “greenhouse gasses” will increase, which physics says must increase DLR. Now according to this site, these two gasses account for 85% of the “greenhouse effect”. It would take a very large change in amount of the remaining gasses for them to skew the results, and I have not heard of such a very large change. Therefore, since the CO2 and water vapor are both said to be involved in “global warming”, and they form the lions share of all DLR causing gasses, we do not necessarily need to know the exact spectrum of DLR. If it goes up, that may be “global warming”, because if DLR goes up, it is most likely due to CO2 AND H2O.
Let’s suppose that we had a long well-calibrated time series of FTIR measurements which showed an increase in downward emission in the 15um band with no temperature change in the atmosphere. How much of an increase in the 15um band do you think we would see?
If the ground temperature were the same over time (or was the same as the time it was measured in the past), then I would expect that the increase from downward 15um band radiation would be right in line with the calculations you have made on this site. This would, of course, have effects, which would likely change things such that everything else, including upward and downward radiation, would also change. These effects could warm, or they could even cool, the surface, and thus change the amounts of longwave in either direction. Example, heat makes evaporation, that makes clouds, clouds act as a sunscreen, that cools the surface (as does any wind and rain), and that reduces the amount of upward longwave, and thus reduces the amount absorbed and re-emitted, and thus reduces the amount of DLR. There is also the fact that hotter ground results in more rising air, it rises, and thus gets nearer to space, and thus is more likely to radiate it’s heat out into space, where it is lost. Thus the hotter the surface gets the more the air conditioners kick in and cool it. This does not change the physics of CO2, it simply counteracts the DLR effect with other effects. This counteracting will lower the surface temperature, so less upward longwave, so less DLR, thus the amount of DLR may be less than predicted from a given amount of CO2 increase, even with all the physics of CO2 being what they are. One might say that these no DLR directly related effects are outside the scope of what you are talking about here, the physics of radiative transfer of CO2, but they effect the amount of actual DLR in the real world, thus the radiative transfer equations stay the same yet the actual amount radiated differs from the prediction.
What measurement accuracy would we need over what time period for all of the parameters?
The same accuracy as the theories predict, or at least enough to see if we can see an actual increase, enough that the “greenhouse” theory some propose may be shown to be true or partially true. However, the theory of “global warming” is not limited to CO2, but includes H2O as well. As such, the amount of total DLR will be far greater (and over a wider frequency range), and far easier to detect. If CO2 goes up, and H2O does not, the total amount of DLR will be far less, so much less that the name of this site, “the science of doom”, would not even be relevant.
Let me ask you a few more questions. Having read Part One and Part Two do you think that:
a) CO2, water vapor and methane absorb and re-emit terrestrial radiation?
They most certainly do.
b) The values can be accurately calculated from the theory of radiative transfer given the concentrations of the gases and the temperature of the atmosphere?
Once again, yes I agree with all of that. However, as I showed above, other physical processes may very well change all that by reducing upwards longwave. Also, the theory of “doom” states that H2O must also get involved, if it does not, what CO2 does alone won’t change much (no doom).
.The information I have been able to gather to date is two reports of anecdotal evidence, from people who detect backscatter, who say that it has not changed in up to 35 years (one reported that it actually went down some). This suggests that the entire theory of climate change due to CO2 induced backscatter has been falsified by direct observation. However, I would like more than anecdotal observation. So where is there, at the very least a long time series of direct observation of longwave backscatter? Better yet would be one, which manages to isolate the backscatter from CO2 from all other sources. And if it exists, why is it not seen on this site?..
Your questions indicate you have not understood the material on this site. And while you would like more than anecdotal observation the fact that you report these “sources” with confidence and without attribution indicates that you believe you already know the answer.
That is just it, they are anecdotal and I can give no attribution, as such I do NOT have confidence in them. However, they are the ONLY, I repeat the *O*N*L*Y* data I have of ANY kind of DLR over a long enough time period to tell anything. Thus, the ONLY data I have says that DLR over a time as long as 35 years has not gone up. That is WHY I am posting here, I obviously need more data. I want to know if such data exists, and I want to see it if it does, I want to know why it does not if it does not, and I especially want to know why no one has even TRIED to see if such data exists and can be used, or at least tried and told us why it cannot. If the bad effects of global warming, “doom”, are seen now, I would expect that we should be able to detect at least some DLR increase, yet I have yet to see any report of anyone even trying, whether they succeed or fail. There must be record of DLR, either of all spectra (the global warming theory does not depend on only CO2 spectra, as explained above), or of selected spectra. If it can be shown that this is not accurate enough to see if global warming is or is not happening, due to other factors (and I can think of many), then this can also be shown.
It’s nothing to do with climate change. If more CO2 doesn’t cause more DLR for the same temperature of atmosphere then you will have falsified a significant branch of physics.
Well done in that case.
Incorrect. Let us say I am Isaac Newton. I have discovered gravity (apples lifted up and dropped through thin air drop to the ground), published the results, others have tested my theory and found it correct, etc. Now, I go to the beach, and I observe a bird hovering over a spot on the beach (as they sometimes do) in thin air, and not dropping. Question, have I just falsified the theory of gravity? The answer is no, other physics are involved which counteract gravity, or at least it’s effect (falling). I now know that there is something I don’t know. “The most exciting phrase to hear in science, the one that heralds new discoveries, is not ‘Eureka!’ (I found it!) but ‘That’s funny…’ – Isaac Asimov ” If I detect DLR over time, and see no significant increase (assuming such is possible), and know that CO2 has increased (as it has), and that physics say that thus must result in such and such increase in DLR (which it does), then I know that here is something I do not know yet. I would then need to find out why that DLR has not gone up as predicted. (This does not include the “doom” necessary matching increase of H2O and it’s DLR, both together they are larger and much easier to detect).
The field of science that you are questioning is the un-politicized field of physics called “radiative transfer”. This has been well-proven since the 1950′s and probably well-before.
Have a read of Radiative transfer by S. Chandrasekhar or Atmospheric Radiation: Theoretical Basis by Goody (1964).
Chandrasekhar and Goody are physics heavyweights and if you can understand their theory and the experimental evidence that backs up their theory you will make good progress.
I am not questioning radiative transfer at all, even in the slightest. I AM questioning the theory of “global warming”, or “doom”, which says that if CO2 goes up, H2O will go up, that CO2 has gone up, and that this has increased DLR enough that we are seeing the bad effects of it right now. I am questioning that if these effects are so great we can see them now, then the DLR increase must be large enough to detect. I am further questioning why no one seems to have even done so, ever, anywhere that I can find, or even stated why they cannot do so, and what we would need to do to be able to do so. I am looking for an alternative to the only data I have on this, two posters of anecdotal information that say that DLR has not gone up at all. Surely there must be something somewhere, even if it comes with many provisions that show that it is inaccurate and why.
I am questioning how much relevance the theory of radiative transfer as it exists, in models, in laboratories, has relevance to the real world of the atmosphere, which is wild and does not obey the rules that exist in the models or labs (or may not). I am saying that the map is not the territory http://en.wikipedia.org/wiki/The_map_is_not_the_territory . I am saying “The difference between theory and practice is greater in practice than in theory”. I am saying “I never guess. It is a capital mistake to theorize before one has data. Insensibly one begins to twist facts to suit theories, instead of theories to suit facts”, and that therefore we should at least TRY to see if we can actually observe the central, beginning, basic assumption of global warming theory.
We have made great big computer models of the atmosphere.
They say that, given the theory of radiative transfer, and other things, if CO2 increases, such and such is predicted to happen.
CO2 has increased.
Such and such has not actually happened.
I then look at this quote:
“”Instead of applying observation to the things we wished to know, we have chosen rather to imagine them. Advancing from one ill founded supposition to another, we have at last bewildered ourselves amidst a multitude of errors….
…When matters have been brought this length, when errors have been thus accumulated, there is but one remedy by which order can be restored to the faculty of thinking; this is, to forget all that we have learned, to trace back our ideas to their source, to follow the train in which they rise, and, as my Lord Bacon says, to frame the human understanding anew.”” Lavoisier
Observation shows that the predictions of the models are not happening. Therefore we must go back to the beginning and trace the idea of global warming back to it’s source. The source is obviously CO2 and radiative transfer. This is physics, and does not change. However, in the wild, other factors may interfere with it, or it’s effects, possibly reducing DLR below what global warming theory says it should be, or counteracting it’s warming effects. The “simple” way to check that is to directly observe DLR over a long period of time, to see if it has increased as much as the models say it will (see above about CO2 AND H2O). It is, after all, the central, beginning idea of global warming. If we cannot do so, at least we tried, that is science. If we cannot do so now, perhaps we are going about it wrong, or we need to take some of the money we pour into models that fail to predict accurately and put it into direct measurements (some real data to plug into those models).
To mark up text you need to use tags, as explained in Comments and Moderation under the heading Nice Formatting, or Making it Stand Out.
You say “it is claimed that the effects of “global warming” are happening right now, and that these effects are entirely due to CO2 (and not from some natural cause).”
So let’s discuss what is really happening. First, I suggest that, because about 90% of heat (excluding sub-surface heat) is in the oceans and sea ice (4% in atmosphere and 6% in land and other ice) then similar weighting should be given to temperature data. So we get a pretty good idea from NASA sea surface data which is measured to 0.01 degree C accuracy. It also exhibits very regular patterns at the same time each year which suggest that climate is indeed very sensitive to natural (cyclical) fluctuations.
So I suggest Trenberth’s trend (curved with a maximum in about 2006 and now starting to decline) is a very realistic picture of what has been happening for the last 18 years or so. There has been a very definite flattening of the curve as others like Knox and Douglass have observed. The NASA data shows 2011 as being a cooler year than 2003 for example. Hence we can deduce that there has been no net accumulation of heat since January 2003.
So it follows that, whatever your “logic” about carbon dioxide, it simply has been having no effect since 2003, even though there has been a continually increasing percentage of it up there. And after all it is you who rules out natural causes – even though such natural causes obviously did cause variations in climate for the last billion years and more. You say everything is “entirely due to CO2” and it is you who refuses to accept the evidence.
You never got back on my hypothesis that GH gases can also have a cooling effect by absorbing heat by collisions with oxygen and nitrogen molecules and then radiating that heat.
All your argument about radiating at higher altitudes etc ignores many other processes involved. You forget that a significant percentage of radiation misses the CO2 and gets through to space. And about half of all re-emitted photons head upwards towards space. You also forget about heat moving upwards by convection. You overlook energy considerations too in that, if a molecule is at a certain temperature then it has to emit a certain amount of energy in cooling to (near) absolute zero.
Not even the models can prove that heat is being trapped because they are not accurate enough to predict a net “trapping” of only 0.5% when the error in the models is 1% to 2% as is admitted – see links on http://climate-change-theory.com
As I keep saying, the models can’t “prove” the hypothesis (and so nor can your type of verbal argument) and there is no empirical evidence of CO2 in the atmosphere causing warming. Indeed, the opposite is the case and the world is not warming these last few years. In fact, it is heading for further cooling until about 2027 or 2028. Then about half a degree of warming for the next 30 years, followed by over 400 years of cooling towards another Little Ice Age. It’s all in the stars – well the planets actually. http://earth-climate.com/planetcycles.jpg
I accidentally replied to Legatis instead of yourself – please read that reply.
PS And here is more about how the IPCC models fail …
http://www.c3headlines.com/2011/10/ipcc-models-spectacularly-fail-reality-test-key-predicted-components-exaggerated-overestimated.html
and more on the current cooling and sea level falls ….
http://www.climatedepot.com/a/13151/Warmist-faithful-being-tested-as-AGWs-scientific-collapse-continues-HadCrut-acknowledges-temps-have-declined-this-millennium-NASA-acknowledges-sea-level-has-declined-for-past-2-years?utm_source=feedburner&utm_medium=email&utm_campaign=Feed%3A+ClimateDepot+%28Climate+Depot%29
Doug Cotton on October 5, 2011 at 1:03 pm:
Who are you addressing this to?
Because you said on October 5, 2011 at 1:12 pm:
If “yourself” means me then you have invented statements on my behalf.
Please confirm who you have attributed these statements to.
I thought you made the quoted statement “entirely due to CO2” but correct me if not. Meanwhile I add the following ….
Like others, you talk only of the increased back radiation which we all acknowledge does happen due to carbon dioxide. What you fail to even think about is the fact that, when the ground warms (eg hot sand on the beach) it then transfers more heat back upwards by both radiation and diffusion followed by convection. Unless you can prove that (upward) heat energy is less than the extra energy from the extra downwelling radiation then nothing whatsoever is proven about the net effect. If there can never be times when local upwelling exceeds downwelling radiation, then we would never see winter cooling.
Think of GH gases as being like a mirror with holes in it facing Earth Another mirror on Earth has no holes. So light bounces back and forth, but every time a ray goes up it has a reasonable percentage chance of escaping to space. Clearly in the limit virtually all light would get to space after a very brief time.
This is close to what happens in practice, but some heat takes longer because it goes back up via convection rather than radiation. Hence GH gases can and do delay the cooling off process each afternoon and evening. The Earth will tend to cool down on a calm winter night to the base temperature supported by the internal heat. That is why and how the gradient of the temperature plot from the core to the surface is critical to climate and thus why natural small variations in heat generated under the surface will control climate, not carbon dioxide. But for a full explanation you will have to read and study all three pages at http://climate-change-theory.com
You see (I hope) that you can’t just generalise that warming must occur if DLR increases. You would have to consider the effects at every point on Earth and in every season. Cooling can and does occur, both locally (at night and in winter) and globally – as it has been happening for at least nine years now.
Carbon dioxide emission is extremely insignificant when compared with water vapour emission – less than 0.5% in fact. Note what Prof. Nahle said (below) then show me the fault in his argument if any …
“A brief and simple calculation of the emissive power of the carbon dioxide at its current mass fraction, taking into account the results of many experiments done by reputable scientists and engineers like Hottel, Leckner, Sarofim and many others, the total emissivity and absorptiviy of the carbon dioxide is quite insignificant.
The following formula is for calculating the total emissivity of the carbon dioxide:
ΔE = [[ζ / (10.7 + 101 ζ)] – 0.0089 ζ ^10.4] (log10 [(pH2O + pCO2) L] / (pabsL) 0) ^2.76
Considering the data obtained by many researchers on this matter, the total emissivity of the carbon dioxide is low. It is 0.0017.
This value is very important for calculating the amount of energy that the carbon dioxide absorbs and emits each second. Given the specific heat capacity of the carbon dioxide at its current density and temperature, which is of the order of ~871 J/Kg K, the carbon dioxide is not the cause of any change of the Earth’s climate.
The formula for obtaining the amount of energy transferred by radiation between two thermodynamic systems is as follows:
Φq/s = e σ (A) [(Ts^4 – Tg^4)]
For example, at an atmosphere temperature of 310.4K (27 °C), the usual temperature in Summer at my location, and a surface temperature of 340.65 K (67.5 °C) the energy emitted by the carbon dioxide is 0.403 W*s.
On the contrary, the water vapor emits 102 W*s.
It is clear what is the main protagonist in the warming of the Earth.”
Doug Cotton:
Most people would be concerned to have discovered they have just made up statements, attributed them to people, and then asked those people to justify said statements.
But not you.
While I applaud your avant garde approach to discussion, I have decided to invest time with people who follow the old-fashioned approach and stop and reconsider when they have blundered.
Now, if Nahle is correct, water vapour has an effect which is more than 200 times that of carbon dioxide. Hence, as happens in El Niño for example, if the water vapour (humidity) doubles the effect is comparable with what we would expect from multiplying the carbon dioxide level by about 200.
And what do we see during El Niño? Perhaps less than half a degree of temporary warming much of which came from an initial warming of the oceans which caused extra evaporation and which had nothing to do with carbon dioxide anyway..
He isn’t. I’d direct you to the appropriate comment thread if I thought it might actually change your mind, but I agree with SoD, you’re hopeless, so I’m not going to bother.
Terribly sorry – but hardly a reason not to address the new topic therein – just because the truth is, you probably have no answer.
I take it that no one has any valid contradictory evidence that refutes any material in my last three posts. This does not surprise me because the truth of the matter is that the whole AGW hypothesis is fallacious and is now being supported primarily by the “establishment” who want to save face, and who are no doubt actually hoping it warms up a bit. This is pretty unlikely given NASA’s sunspot predictions and the fact that climate seems to lag lomg-term sunspot cycles (not the 11 year ones) by about 20 years.
There’s no need to worry guys – there won’t be any “doom” – there is no valid “science” that predicts “doom.”
Your choice is either to continue being bluffed and fooled by pseudo “warmist” claims, or go and enjoy a good sunbake before it gets any cooler over the next 18 years. Think for yourself and ponder everything I have written in the three pages at http://climate-change-theory.com If you have any “feel” for physics you will see I must be on the right track.
You are welcome to direct me to the comment thread which supposedly shows the above calculations by Nahle are incorrect. It’s only a small piece of the puzzle though and does not negate that fact that no one has proved that carbon dioxide caused the warming seen late last century. The Chinese don’t look like cutting their carbon dioxide much http://www.smh.com.au/world/chinese-sceptics-see-global-warming-as-us-conspiracy-20111007-1ldl1.html so we should see lots of warming pretty soon to get back on the IPCC trend line, shouldn’t we? I’ll be laughing in the background for a few years yet.
It’s all rather ironic that Australia’s Carbon Tax bill should be passed in the Lower House in a month when NASA announced falls in sea levels – which are now already back to the levels of about two years ago – see http://www.jpl.nasa.gov/news/news.cfm?release=2011-262
… that it should happen in a month when we have seen that 2011 is very clearly shaping up to be cooler than 2003 – see NASA data: http://earth-climate.com/2003-2011.jpg which took a plunge these last three weeks especially.
… that it should happen in a month when we have just heard of “consensus” opinion about the universe being turned on its head by Nobel Prize winning studies – and yet we continue to believe that other AGW “consensus” that bases its thinking on century-old experiments that were debunked this year anyway – see http://climateclash.com/category/authors/nasif-nahle/
And yet, without any valid proof whatsoever that carbon dioxide caused the observed warming late last century (which has now stopped) we blunder into an extremely expensive carbon tax venture which will probably be undone by 2013 anyway – all because our Lower House voted 74 to 72.
Let’s hope one or two senators start thinking about the lack of evidence for AGW before they vote next month – like those Chinese “sceptics” perhaps who stand a good chance of influencing Chinese officials. It simply is not happening and the models are not accurate enough to establish whether warming or cooling should be the outcome.
The potential cooling effect of carbon dioxide is ignored ( see http://climate-change-theory.com ) and it is quite wrongly assumed that nearly all heat transfer from the Earth’s surface is by radiation, rather than diffusion followed by convection.
The huge heat “momentum” resulting from all the heat under the surface (right through to the Earth’s core) far exceeds the heat in the oceans and stabilises climate, though such heat can vary slightly by natural means. These natural variations have caused the cycles of about 900 to 1000 years that resulted in warm periods in Roman times, Medieval times and the present, and cool periods in the Dark Ages and the Little Ice Age.
Anthropogenic carbon dioxide neither has had nor will have any effect at all on climate. No one has proved that it can, it does or it will.
Try Google. I’m tired of doing your work for you.
You are really out of your depth. Prof Nahle answer his critics more than adequately here: http://www.biocab.org/Induced_Emission.html
Sound a bit like what I’ve been saying?
“There are many scientists who have found errors in the calculations of the proponents of the idea that carbon dioxide is causing planetary warming. The most serious of these errors resides in believing in a downwelling photon stream which, as AGW proponents say, overwhelms the surface emission of radiation and warms the surface during nighttime. However, when we analyze the issue of downwelling radiation emitted by the atmosphere, we find that such warming of the surface by greenhouse gases does not exist.
“The problem with the AGW idea is that its proponents think that the Earth is isolated and that the heat engine only works on the surface of the ground. They fail to take into account that incoming heat from the Sun is transferred by conduction from surface to subsurface materials, which store heat until the incidence of direct solar radiation declines, explicitly during nighttime.
“At nighttime, the heat stored by the subsurface materials is transferred by conduction towards the surface, which is colder than the unexposed materials below the surface. The heat transferred from the subsurface layers to the surface is then transported by the air by means of convection and warms up. The upwelling photon stream affects the directionality of the radiation emitted by the atmosphere driving it upwards, i.e. towards the upper atmospheric layers and, from there, towards deep space. This process is well described by the next formula:
FSH = -ρ (Cp) (CH) (v (z)) [T (z) – T (0)]
…… “
Nahle is the one out of his depth. I really feel sorry for his students. Induced emission/absorption plays no significant part in atmospheric molecular emission/absorption. It’s actually quite easy to calculate the emission intensity for CO2 using the Einstein A_21 coefficient. You might try reading my post at The Air Vent ( http://noconsensus.wordpress.com/2010/08/17/molecular-radiation-and-collisional-lifetime/ ).
Did you even bother to look at the comment thread here? (Hint: start here: https://scienceofdoom.com/about/#comment-10901 ) Besides ignoring path length and having problems with simple algebra, Nahle couldn’t even correctly calculate the amount of CO2 in a vertical column of air extending to the ‘top of the atmosphere’. The amount, ~6 kg/m² for a volumetric mixing ratio of 390 ppm or 3.1 cubic meters/m² at STP, was absurd according to him. During one exchange I had with him some years ago, he was surprised to find out that the mass of the atmosphere in a vertical column was 10,379 kg/m². The calculation is trivial, divide the surface pressure in Pascals, 101325, by the acceleration of gravity, 9.81 m²/s². But apparently he had never done it or thought about it.
He also completely ignores the fact that atmospheric emission has been measured and found to be in accordance with theory. You can measure it approximately yourself with an inexpensive IR thermometer. Just point it at the sky. It’s best to find one that goes to -60C. If the sky weren’t radiating in the IR, you wouldn’t get a reading.
I took a closer look at the induced emission section of Nahle’s paper you linked above. All I can say is wow! So many errors, so little time. Let’s just look at the critical one:
Compare that equation to equation 10.6 in Modest:
Ibv = 1/(4π)*[(Aul/Bul)/((g1*Blu/gu*Bul)*e^(hν/kT)-1)
Parentheses added for clarification of evaluation.
Actual screen shot of Equation 10.6 ( http://i165.photobucket.com/albums/u43/gplracerx/ModestEquation106.png )
Notice anything different? That’s right Planck’s constant only appears in the exponential. The real howler, though is this: “k is the absorption coefficient variable”. Wrong, wrong, wrong. k is the Boltzmann constant. hν has units of energy so the denominator of the exponential must also have units of energy. That will be true if k is the Boltzmann constant, but not true if k is an absorption coefficient. Also, because k is the Boltzmann constant there is no concentration term in the equation. That’s fine for a black body, for which equation 10.6 applies. Not so much for the atmosphere, though.
Out of my depth indeed! Still think Nahle is a reliable source?
out of my depth
Oops. There should be another bracket at the end of the equation. Another pair of parentheses wouldn’t hurt either
Ibv = 1/(4π)*[(Aul/Bul)/(((g1*Blu/gu*Bul)*e^(hν/kT))-1)]
OK – I’ll pass on your comments to Nahle and see what he says. It’s not an important issue for me anyway. The main point I make is that the models are not accurate enough to predict whether net radiative flux at TOA should be positive or negative. The last 8 years have demonstrated that all incident thermal energy can escape to space leaving sea surface temperatures in 2011 actually lower than those in 2003. What have you to say about that?
Maybe you’d like to specifically fault what I say on the second and third pages at http://climate-change-theory.com
On another point, I quote John Nicol: “There is also no doubt that back radiation does occur. It has an intensity at ground level of about 1/3 of the intensity of the radiation upwards from the ground. The main difference between what actually happens and what the warming industry claims happens is that increases in carbon dioxide do not cause an increase in back radiation, because as the back radiation increases from each sample or layer of air, the absorption coefficient below increases by an exactly compensating amount. What is measured at ground level is actually just the radiation from the emitting molecules which are just above the measuring instrument with a field which itself is in thermal equilibrium with the ground. If you pointed the instrument downwards, you would measure exactly the same field which would carry some signature of the green house gass pectrum but will not show any increase if the gas density increase over time.”
Maybe you’d like to fault him too.
Please don’t bother to pass that on. He’s hopeless. I did have a faint hope that you might see that he’s hopeless, but apparently not. Bye.
Well, Nahle also considers you to be in error. He will correct the reference to k and apparently the difference in the formula was just a problem with his site-builder. Neither point affected his calculations.
He says the expresion hv depends on frequency, which is variable.
“Obviously, it is not a formula strictly functional only to blackbodies, although we could apply it to blackbodies. Induced emission, as well as spontaneous emission, happens every day, everywhere, whenever and wherever radiation happens.
“A second fallacious argument is that the formula only applies to lasers. However, Einstein discovery happened many years before lasers were invented. Actually, lasers are the corroboration of Einstein’s theory on induced emision.
“There is a great danger to the anthropogenic greenhouse effect hypothesis on this issue because Einstein’s theory says that the possibilities of induced emission from any particle capable of absorbing energy are the same as the possibilities of spontaneous emission, including at those frequencies related to the greenhouse effect. In addition, spontaneous emission does not depend on the density of energy of the radiation field, while induced emission depends on the density of energy of the source of thermal radiation (emisor) and the number of excited particles of the absorber.”
Please point me and your readers to some (peer-reviewed) information supporting your viewpoint or contradicting Nahle’s.
And I take it you have no valid argument against all the other points I raise which you ignored in your two line answer.
And still, not once in all your replies, have you ever quoted any scientific paper which actually proves (with good old common logic) that carbon dioxide causes warming. Correlations do not prove cause. Models with error bars of 2% do not prove TOA net radiative flux differences of 0.5%. Missing frequencies for carbon dioxide do not prove the thermal energy did not escape to space by another route.
Furthermore, current sea surface temperature trends are curving around, having passed a maximum, and now declining. (Only ridiculously long moving averages and incorrectly assumed linear trends show significant warming, but these are not a best fit for the data.) No valid trend which is based on, say, a 13 month to 5 year moving average, now provides a plausible extrapolation in keeping with the IPCC projections to 2100. I rest my case.
Sure I do. But what’s the point when no matter what I say, you won’t change your mind or admit that you’ve made an error. In that respect you are just like Nasif. I need to restock my supply of patience which was exhausted by an exchange with other clueless people at The Blackboard so I’m not going to waste any more of my time on you.
In regard to the topic of back radiation supposedly causing warming I would comment that there are obviously many other factors involved. For a start, it does not cause net warming at all times in a 24 hour daily cycle, nor in a seasonal annual cycle at any particular location at, say, mid-range latitudes. Clearly variations in solar insolation dominate there.
Now I know “climate” measurements eliminate such variables, but the fact that they occur means it is not “easy” for the Earth system to “remember” what temperature it has to return to at the same time next year. The very fact that it does remember so well (usually to a small fraction of a degree) suggests to me at least that the “momentum” (as I shall call it) of all the thermal energy in the rest of the Earth (below the surface) acts as a controlling mechanism.
Whether you accept this or not, there is a very fine balance between warming or cooling over the course of any full year. And, indeed, we see situations (now and in historic records) where no apparent net warming has happened for several years. For example, NASA sea surface data for 2011 is pretty sure now to end up with a mean for the year less than the mean for 2003. (There really is no proof of Trenberth’s missing energy which Knox and Douglass rubbished anyway.)
So you can argue all you like that the higher levels of carbon dioxide should now be causing even more warming than we saw, say, in the 1980’s and 1990’s, but it simply isn’t happening and the temperature gradient has taken a plunge this century no matter how you look at it, whether or not you consider it now in negative territory.
So you will say there are other factors – maybe aerosols – maybe natural. But, if there are, then you cannot blame all the previous rises on carbon dioxide. Hence any predictions based on such assumptions are wrong.
Looked at another way, if you are honest in fitting the most appropriate trend line you will almost certainly find a curved trend (convex from below) fits better than any linear trend. But as soon as you acknowledge such a curve is a better fit, you immediately create doubt as to the extrapolation of such, simply because you cannot with any certainty say whether we are passing through a flex or a maximum. In the 20 to 25 years or so after about 1880 and 1940 there was slight cooling, so such could be happening again now. And also, even on a longer term basis, you cannot say that a long term maximum has not been reached, such as in Roman and Medieval times, or will not be reached in the next 100 years or so.
There simply is no valid empirical proof that carbon dioxide levels are controlling climate or even having any effect over and above natural variations. The hypothesis stands unconfirmed.
Well, well – just the sort of unsubstantiated reply I expected.
There is not one iota of conventional proof in anything you have written on this site which establishes that carbon dioxide causes noticeable warming of any part of the Earth system.
Measurements of back radiation do not proof it. Missing spectral lines for carbon dioxide do not prove it. Correlations (if they even are anywhere near convincing) do not prove cause. All you have is a lot of head nodders who thought it sounded a plausible hypothesis.
It is you who is wasting you time propagating stuff than anyone can read elsewhere on the web anyway if they feel comfortable agreeing with the majority and doing good for the planet as they put a bit of ethanol in their car or get solar power or pay carbon tax or whatever.
Looking forward to your red face one of these years.
PS Do you ever wonder why people like Nahle, myself and 1,000 others http://www.climatedepot.com/a/9035/SPECIAL-REPORT-More-Than-1000-International-Scientists-Dissent-Over-ManMade-Global-Warming-Claims–Challenge-UN-IPCC–Gore don’t change their minds about the AGW hypothesis? Simply because neither you nor anyone else has as yet published a proper proof that carbon dioxide must cause any significant warming.
Yes, if it’s a “fault” in your eyes for us all not to be misled by incorrect logic and the pseudo science of AGW then so be it. But I suggest that 1,000 “thinkers” might be onto something which you too should start to think about, rather than just repeating consensis thinking and half-baked arguments.
Future comments not on topic about the subject of this article will be snipped, please read the Etiquette.
This article is about “back radiation”, also known as DLR.
As you or some readers may not choose to visit the above linked site, I will just quote one example:
[moderator’s note – commented snipped because not on topic – please read the Etiquette
A note to lurkers. Saying a scientific theory or hypothesis is unproven is making a nonsensical statement. There is no proof in science, there is only disproof or falsification. There are no measurements that falsify atmospheric radiative transfer theory. There are a lot of measurements that are in very good agreement with the theory. Downwelling IR can be measured. The measured values are in agreement with theory. The spectrum of downwelling IR can be measured. The observed spectra are in good agreement with calculated spectra. There are several articles (including this one) at this site that show this in detail. But that doesn’t mean the theory is proven. No theory is ever proven and most will likely be found to be flawed in some way like Newton’s Laws of Motion, which do not include relativistic effects.
One can be skeptical that the potential damage from possible future increases in temperature are enough to justify drastic measures now. For example, Professor Judith Curry has said that the IPCC Working Group 2 and WG3 reports, which are supposed to document the effects of increased temperature and evaluate mitigation strategies, “belong in the dustbin”. One can also, like Professor Roger Pielke, Sr., think that the WG1 report on the science “suffers from ‘sins of omission'”.
But anyone who claims that atmospheric radiative transfer theory is wrong has no measurements to support their claim. Anyone who claims that a theory which is agreement with measurements can be ignored because it’s unproven has failed to grasp the fundamentals of science and should be ignored.
I’m a “lurker” DeWitt! What can I say? Perhaps Prof Nahle is ‘old school’ like myself and being such I’d prefer to keep the units of ‘measurement’ as equivalent as possible (‘Pascals/gravity constant’ is ‘obtuse’ to much of a student’s ‘learned’ logic). You only ‘really’ comment upon his teaching curriculum.
“Saying a scientific theory or hypothesis is unproven is making a nonsensical statement.”
No they’re not! They’re reaffirming the ‘status quo’!
“There is no proof in science, there is only disproof or falsification.”
I concur, but on occasion this basis needs reaffirmation. Especially when the ‘premise’ is ignored in such cases as yourself. Lab data is not a ‘real simulation’, it’s a ‘specific scenario’ simulation and has to be accepted as such!
In this instance ‘lab data’ is ‘falsified’ by it’s inability to ‘reproduce’ the true physics of Earth’s systems.
“There are no measurements that falsify atmospheric radiative transfer theory”
Wrong! Why is ‘cloud-base’ altitude warmer than ‘near surface’ where ‘cloudy conditions’ prevail (adiabatic principle taken into account)? I really do wish you were more open to ‘other interpretations’. 🙂
I’m out of here now, I’ve not the time to deal with the many-fold issues that ensue from your statements.
Best regards, Ray Dart.
Atmospheric Radiative Theory is not the same as the AGW hypothesis.
[moderator’s note: rest snipped – This article is about DLR or “back radiation”. This theory is also not the same as the theory of relativity. I haven’t claimed that DLR proves the theory of relativity. I haven’t claimed that DLR proves the theory of AGW.
If you have a comment to make about the content of this article it might be interesting. If you don’t have a comment then you already have enough (too many) essays published on this site.
Please read the Etiquette. Post comments that follow those guidelines, which include being on topic and not essays explaining your world view.
Otherwise your future comments will just be deleted. If you want a platform, start your own blog.]
Scienceofdoom
Pointing to EBEX 2000’s close agreement between observed and theoretically calculated values for DLR is a flimsy basis for promoting confidence in the predictive skill of climate models. The close agreement is surprising given a turbulent real atmosphere and a stable model one. The close agreement reflects the fact that, in both the models and the field measurements, DLR is calculated using the Stefan-Boltzmann equation. The sensing plate in the KippZonen pyrgeometer used by EBEX loses heat by radiation to the atmosphere and to space through the atmospheric window and it cools. The plate also absorbs DLR from the sky, which counters the heat loss, and it reaches temperature equilibrium, cooler than initially. The temperature reduction, being a measure of the excess of OLR over DLR, together with known OLR allows calculation of DLR.
No it isn’t. DLR is calculated in models by integrating the atmospheric emission spectrum, which doesn’t have constant emissivity, so the S-B equation doesn’t apply. The S-B equation is the integral of the Planck function for a body with constant emissivity and temperature over the full wavelength (or frequency) range. A pyrgeometer is effectively an integrator as it is sensitive to radiation over most of the thermal IR band. The S-B equation is used to calibrate pyrgeometers using blackbody sources at different temperatures. That gives the parameters to convert the measured temperature of the plate in the pyrgeometer into total radiant power. The pyrgeometer doesn’t care if the radiation it sees has a complex spectrum or not. It simply measures the total power.
Thanks for that. It was this formula from the manufacturer’s manual under the section called “Operation” that led me to believe DLR was calculated using S-B::
Ld = Uemf/S + 5.67*10^-8*Tb
The S-B equation does apply to the detector of the pyrgeometer because it is a gray body with constant emissivity. Teff for the sky can also be calculated using the S-B equation, but it isn’t a real temperature because the sky isn’t a blackbody. That’s why it’s called the effective temperature because it’s the temperature of a blackbody that would emit the same power. So the detector emits according to the surrounding air temperature. But if the effective temperature of the sky is cooler (or warmer) than the near surface air temperature, the detector will be emitting more radiation than it receives and will cool slightly. If it were perfectly insulated, it would cool to the effective temperature of the sky. But it isn’t. It’s coupled to the temperature of the instrument by the thermopile.
Stoffel, et. al., 2000, goes into more detail on the calibration procedure against a blackbody source. The advantage of a blackbody source for calibration is that the temperature of the detector and the temperature of the source can be controlled independently. The Kipp & Zonen pyrgeometer differs from the EPLAB pyrgeometer in that K & Z claim they don’t need to correct for dome temperature. They also calibrate against a standard reference pyrgeometer rather than a blackbody.
Or emitting less if Teff is greater than the near surface air temperature.
[This comment may appear first as I had links in the original which put it into the moderation queue.]
John Millett
Who made that connection? Do you think that is what I am claiming? If so you haven’t understood the article at all.
True, this is not the message of this essay. But unless my memory fails me badly, it is incorporated in another of your essays. Paraphrasing: it would be prohibitively expensive to deploy pyrgeometers as we do thermometers; but where measurements have been made the close agreement with theoretical calculations allows us to be confident about the theory. The question in my mind is this: Is a measurement which relies on a theoretical calculation a true test of the theory informing the calculation?
Your memory fails you badly.
Find this message in any of my essays.
When you don’t find it, ask yourself why you came to believe it.
[moderator’s note: comment deleted for being off topic of this article, again, and also attributing random statements from someone else to me, again]
The former post of course referred to John Millet’s comment.
Regarding Atmospheric Radiative Transfer Theory, the “close agreement” still exhibits error bars of 1% to 2% (uncertainties if you wish) and the only link between such theory and the AGW hypothesis would be established if ART theory actually predicts net warming which would be of the order of, say, 0.5% of total incoming radiation at TOA. But it is not that accurate, now is it?
So any comparison between ART theory calculations of net radiative flux at TOA and measured values for such neither proves nor disproves anything, and could just as easily be purely coincidental.
Referring to the end of the above article, you say “DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.” I totally agree. After all, sand on the beach and rocks get hot on a sunny day.
So how do they cool off at night?
..
[Moderator’s note: rest of comment deleted for being one of the many repetitions of the commenter’s opus, see comment at August 15, 2011 at 4:32 am and following.]
Scienceofdoom, Part 1 said::
“However, if you want to look at the surface, the values are much “thinner on the ground” because satellites can’t measure these values (see note 1). There are lots of thermometers around the world taking hourly and daily measurements of temperature but instruments to measure radiation accurately are much more expensive. So this parameter has the least number of measurements.”
Extracts from a number of papers followed comparing calculated and measured values showing close agreement.
Although not stated explicitly, implicit in the presentation of these extracts is a vote of confidence in the validity of the models to calculate DLR.
My question stands: Is a measurement which relies on a theoretical calculation a true test of the theory informing the calculation?
There is a world of difference between:
– and being able to calculate the radiative transfer through a known climate state.
Here is what I said in Theory and Experiment – Atmospheric Radiation:
I recommend you read the whole article and try and understand the substance without trying to jump into a “For” or “Against” mode of what it might possibly be implying in the “climate wars”.
In essence, if we know today at a specific time and location what the temperature profile and atmospheric composition (of trace gases) is then we can accurately calculate the total flux and the spectral composition of DLR and TOA radiation.
If we know this today what does this tell us about our ability to predict the climate future?
Nothing.
Read the article already linked and try to understand this essential point.
I think what John Millet may be saying is that the measurement of DLR requires a theoretical calculation, i.e. the use of the S-B equation to calculate the radiative flux from the surface of the detector which is then corrected for heat flow out of or into the block to which the detector is attached. Or possibly the use of the S-B equation to calculate the flux from the blackbody sources used to calibrate the pyrgeometers or the use of the Planck equation to calculate the flux density from a blackbody source to calibrate the FT-IR spectrophotometers used to measure atmospheric emission spectra. He would still be wrong that this somehow invalidates the measurement, but not wrong in the way you think he’s wrong.
John wrote: “So any comparison between ART [Atmospheric Radiation Transfer] theory calculations of net radiative flux at TOA and measured values for such neither proves nor disproves anything, and could just as easily be purely coincidental.”
If you are interested in radiation transfer, the atmosphere isn’t the right place to “prove or disprove” anything or – expressing your concern in the normal scientific terminology – to test the theory to see if it can be invalidated. In the process of testing theories, we learn how closely a theory and the most precise observation agree; within 0.05%?, 1%?, or 20%?. Even if a theory is later proven wrong, we still can use calculations based on that theory under conditions where theory and observation are known to agree. We can waste time debating about whether a theory is “proven”, but there is no reason to argue when a theory is being applied to a situation where theory and observation have been shown to agree.
Most, if not all, of the parameters used in radiation transfer calculations were established (long before global warming became an issue) by LABORATORY experiments which measured the absorption spectra of gas under a wide range of temperatures and pressures (and admixture with other gases). To get data at very low pressure, one paper I read used a path length of nearly 1 kilometer (multiple reflections through a chamber)! Since emission of radiation is just the reverse process of absorption, the parameters obtained from absorption experiments tell us how gases emit. IMO, it makes little sense to argue with such laboratory experiments.
To demonstrate that radiation transfer theory is useful in the real atmosphere, we first need to know the precise composition (especially humidity) and temperature of the atmosphere (at all altitudes) that is emitting DLR towards our detector. Therefore, a radiosonde is sent through the atmosphere above the detector. The results are in reasonable agreement: best below Antarctica skies, where there is little humidity; within a few percent below cloudless skies over tropical oceans, where the humidity is high; and less well below cloudy skies, where the emission can come mostly from heterogenous droplets – not homogeneous, well-mixed gases.
Unfortunately, radiosonde data isn’t available for climate change experiments done with GCMs – we rely on a GCM to tell us the temperature and humidity of each atmospheric grid cell. A GCM makes massively bigger errors predicting the temperature and humidity of a grid cell a week in the future than it does in predicting how much DLR will be emitted from that grid cell. Here is the real problem with the “scientific consensus” about global warming.
IMO, alarmists want us to believe that climate change is all about the interaction between radiation and greenhouse gases, because that really is “settled science” – at least settled within a few percent. You are falling into their trap. SOD has many posts on radiation because it is the one thing we really do understand. He has less to say about clouds, aerosols and convection – things we don’t understand. For example: 1) Stainforth has shown that the temperature rise predicted for 2XCO2 changes about 8-fold when the parameters used by a GCM to describe the behavior of clouds was varied at random within a range compatible with laboratory measurements. 2) The grid cells in climate models are too big to accurately represent convection, a process takes more energy away from the surface of the earth (ca 100 W/m2) than NET radiation (390-330 = 60 W/m2) and dwarfs radiative forcing.
Science of Doom and Dewitt Payne.
Thankyou both.
(And thankyou Frank, though I suspect you’re replying to Doug).
“In essence, if we know today at a specific time and location what the temperature profile and atmospheric composition (of trace gases) is then we can accurately calculate the total flux and the spectral composition of DLR and TOA radiation.
If we know this today what does this tell us about our ability to predict the climate future? Nothing”
This statement encapsulates the reality that, in my opinion, puts the close agreement between theory and measurement in the “too good to be true” category – the state of the atmosphere under field measurement is unlikely to be the same as in the theoretical model.
Flux in both theory and measurement is calculated from temperature which is what the pyrgeometer measures (via laboratory calibration to an electrical signal) – those of the instrument case and of the detector. Calibration goes further to link radiation balance at the detector surface to detector temperature, the radiation balance assuming unit emissivity for both sources. In field measurement, emissivities of the opposing sources are different, neither being unity. The net radiation reported by the instrument will therefore differ from the true balance. Adding surface upward flux, calculated with an untrue emissivity, to a faulty net radiation balance gives a doubly faulty DLR.
Like flux, emissivity is calculated, not measured. Wilber 1999 measured reflectivity from which he derived absorptivity (zero transmissivity) and thence, via Kirchoff, emissivity. Does the condition under which emissivity equals absorptivity, local thermal equilibrium, apply always and everywhere in the real world?
Wilber also qualified his data for water and for barren ground which together account for about 80% of the planet’s surface. He noted that reflectivity of the oceans depends on the state of the surface and the sun’s angle in the sky. His data are for near-zenith sun and ignore surface conditions. They are likely to overstate the true average emissivity. His data for barren ground are for just one type of the many actually existing. Thus the blackbody assumption underlying radiative transfer theory and estimates of global energy budgets could be further from reality than reliable results demand.
John Millet,
The emissivity of the detector has nothing at all to do with the emissivity of the source of the radiation that is being measured. It only affects the calibration of the detector. The emissivity spectrum of clouds is, for example, very different than the emissivity spectrum of the clear sky.
And as long as we’re getting picky, we don’t measure temperature directly either. We measure a physical property that has been shown to be proportional to the the thermodynamic property we call temperature, the volume of a fixed mass of fluid like mercury, the resistance of a thermistor or a coil of very pure platinum wire or the voltage output of a circuit consisting of one or more thermocouples in series. For a thermopile, that’s on the order of hundreds of junctions. In the case of non-contact thermometers, radiant intensity is measured. The source emissivity is important if we’re converting radiant intensity to temperature. But in the case of atmospheric radiation, we’re not. An effective temperature can be calculated by assuming unit emissivity, but that’s more of a mathematical construct than an actual physical property since for the clear sky anyway, the emissivity isn’t constant with wavelength and isn’t equal to 1 on average.
If people are starting to wonder what’s really going on, this summary may help …
The physics referred to in Section 2.2 at http://greenhouse.geologist-1011.net shows why the original “physics” on which greenhouse theory was based was not even in line with new theories that were already put forward at the time..
[Moderator’s Note – the rest of Doug Cotton’s essay snipped for irrelevance to this article. Even the bit I kept is irrelevant to this article. This article is about whether the surface absorbs “back radiation”. Part Two was about the source of back-radiation, revealed via spectroscopic analysis, and Part One was about the existence and magnitude of back-radiation.
If people want to comment on these specific claims with reference to theory and measurement their comments will appear.
If people want to write essays about their pet theories their comments are most likely to be deleted.]
Actually the comment was all about radiation, including what happens after the surface absorbs thermal energy from such radiation. People can read a very similar comment and subsequent discussion on http://theconversation.edu.au/climate-change-threatens-seaweed-4079
I’m sure it would be within your means to either transfer a comment to what you consider an appropriate thread, or allow a bit more flexibility within a thread. To simply delete a post (as they do at Skeptical Science) when you really have no valid counter argument is just weakening the credibility of your site, or at least any honest attempt to seek truth.
First, I draw your attention to the paragraphs on radiation in this screen capture of a post I made on another thread this morning. http://climate-change-theory.com/ScienceOfDoom.jpg
You refer to the surface being warmed but you appear to leave it there. Obviously the surface also cools at night and even more so on winter nights. The issue is whether there is any net accumulation of thermal energy from one year to the next, particularly in the oceans. As the above linked post points out, there has been no such accumulation since 2003.
Now you appear to imply that about 99% of all net thermal energy leaving the surface does so in the form of radiation from what you consider to be a near perfect blackbody – the land and ocean surfaces – even though the atmosphere comes between these surfaces and space.
The big problem is that your assumptions do not leave room for any significant amount of temperature equalisation leading to equilibrium between the surface and the first millimetre or so of the atmosphere. Yet such temperature equilibrium obviously does exist.
Why is it so? You can have no answer, because your 99% radiation uses up all but 1% of the energy available, and radiation itself will not bring about such temperature equilibrium. The linked post gives a clue as to where and why your thinking on this is simply incorrect.
Doug Cotton continues his theme of inventing ideas and putting them in the mouths of others (usually me on this blog).
Here he says:
Given the response of Doug Cotton to earlier invented statements attributed to me it is pointless to try and “engage”.
I won’t bother to respond except to say:
I had to moderate out my own next comment.
[snip, breach of etiquette] .. and all future comments by you, or believed by me to be by you will just be deleted. You can start a blog and claim that your comments are moderated because this blog can’t handle the truth. Whatever will make your heart sing.
Just don’t do it here.
This blog is for people able to engage in a discussion which means they have to be able to read and understand and also remember what others write.
[…] […]
Four simple questions for you all – true or false?
(1) When the refective (mirror-like) internal surface of a vacuum flask reflects radiation back into the coffee the coffee does not get any hotter – true / false ?
(2) If you hold a mirror over a batch of earth (which is radiating) at night so that the mirror reflects that radiation back to the patch it does not get any hotter, just like the coffee – true or false?
(3) When carbon dioxide captures radiation from the surface and then re-emits it back again it is acting rather like a mirror because the radiation going back has no more energy than that which it captured – true or false?
(4) Hence, when such back radiation meets the surface it does not warm the surface – true or false?
Full marks if you answered true to all questions – you are now a well-informed —-er. [Moderator’s note – please read the Etiquette]
Take a two identical heating coils and place one in an uninsulated glass of water. Take the other and but it in a dewar flask with the same volume of water. Put the coils in series and pass a constant current through the circuit. Which will be hotter, the uninsulated cup or the dewar flask?
Your example has no relevance. Of course I am familiar with the process of boiling water in a (reasonably) insulated electric jug. Insulation in the vacuum flask will of course slow the rate of cooling, just like the rate at which warm air rises by convection slows the rate of cooling of the Earth, so it doesn’t get as cold as the Moon’s surface in its (much longer) night.
Now answer the four questions I posed above.
Hint: Radiation with frequencies below a cut-off frequency (determined by Wien’s Displacement Law) is captured and immediately re-emitted with the same frequency (thus same energy) without leaving any energy behind to warm the surface. Only direct incident solar radiation has frequencies above the cut-off and cannot thus by re-radiated (because it does not resonate) and so its energy is converted to thermal energy. Hence backradiation cannot under any circumstances (when it returns to a warmer area of the surface) actually cause that area of the surface to get warmer still. Your coffee will never get warmer in the vacuum flask, even if you put your radiating hand over the top of the flask instead of the cork stopper.
Jack. What total ——! [Moderator’s note – please read the Etiquette]
“Radiation with frequencies below a cut-off frequency (determined by Wien’s Displacement Law) is captured and immediately re-emitted with the same frequency”
What constitutes immediately? If some IR radiation is absorbed by a GH gas. How long will it be before that GH molecule reradiates that energy? Answer. It depends on the frequency involved. For visible light we are talking about timescales of nanoseconds. Hoever in the IR regions which we are dealing with in the GH Effect, this timescale is 1-10’s of milliseconds. This calculation goes back to Einstein.
In contrast any molecule in the lower atmosphere will undergo billions of collisions per second. So while waiting for those milliseconds to ever so slowly pass by, each molecule, including our GH molecules will undergo millions of collisions. spreading their energy around. Molecular collisions are the main process by which GH absorbed energy is spread around.
Your questions are irrelevant, not my example which is, in fact, completely relevant. The fact that you think it’s irrelevant is determinative of your lack of knowledge.
You again demonstrate that your understanding of emission and absorption of radiation is severely flawed. Wein’s Displacement Law gives the wavelength or frequency of maximum radiative emission from a blackbody. It has nothing whatsoever to do with absorption of radiation. Ignoring the angle of incidence, which can be important for solids and liquids, the absorption of radiation is determined by the absorptivity of the material at that frequency. Temperature plays no part at all. Emission is determined by the emissivity and the temperature. For materials where local thermal equilibrium applies, emissivity equals absorptivity. If it didn’t, it would violate the Second Law. In a gas, the molecule that absorbs a photon is almost never the molecule that emits again because the excess energy is transferred to other gas molecules by collision. That’s called local thermal equilibrium. Emission is mainly from molecules that have been excited by collisions. The fraction of molecules in an excited state can be calculated from the Maxwell-Boltzmann distribution. The rate of emission is then the number of molecules in the excited state multiplied by the Einstein A21 coefficient for that transition. The HITRAN database contains the information, including the Einstein A21 coefficient, to calculate complete spectra of a gas mixture given concentration, pressure and temperature.
DeWitt @ Jan 9, 5:17pm
A perfect blackbody has no conductive interface with its surrounds. The Earth’s surface does have such an interface with the atmosphere and a considerable fraction (probably over half) of all thermal energy transfer from the surface (including oceans) is by means other than radiation.
The process you describe is indeed what happens when the surface absorbs incident solar radiation. It cannot re-emit at such frequencies because those frequencies are above the cut-off frequency as per Wien’s Displacement Law. (That’s where temperature comes in, my friend.)
For the very reason that such radiation cannot be immediately re-emitted it has to be converted to thermal energy and that’s where your molecular collisions do indeed come into play. The surface acts as a transformer, converting high energy SW radiation to thermal energy, some of which is then radiated as low energy LW radiation and some transferred by conduction (diffusion) and evaporation plus some other processes to a smaller extent. The timing may be hours or even years later.
But the process is totally different for incoming radiation with frequency below cut off which is thus either (a) within the band(s) which can be re-emitted with the same frequencies. – this radiation going into resonance or near resonance, causing excited states which immediately relax by emitting, or (b) it does nothing to the molecules it encounters, probably experiencing multiple deflections until it escapes again (unaltered) into the atmosphere. [The excited states in (a) do not represent sufficient energy to convert to thermal energy, as this involves a physical process converting from a coherent state to an incoherent state, possibly involving ionization such as happens when x-rays penetrate matter.]
You cannot simply apply the S-B equation (even with correction for the temperature of the surrounds) to the surface/atmosphere interface because not all energy transfer at that interface is by way of radiation. When looking at the Earth from space, a large portion of the radiation emanates from the atmosphere rather than the surface, and all those calculations about how the surface would be -18 deg.C are totally fallacious. Even applying the -18C to a point 5km up is also erroneous, because you could “calculate” almost any temperature and find it somewhere in the atmosphere, sometimes at two different levels. If you (more correctly) take an average over all times in a 24 hour cycle you get a vastly different (much colder) mean radiative flux anyway.
Sorry, my last sentence was incorrectly worded. If you treat the Earth as a sphere (rather than a flat disk) and use as input the radiative flux at different times (let’s say every 5 minutes) in the 24 hour day/night cycle (it being zero at night of course) and you calculate (using SBL) the temperatures for each time and then the mean of those temperatures, then you get a vastly different mean temperature which is in fact about 90 degrees colder than that -18 deg.C figure. This serves to show just how spurious is the calculation of supposed sensitivity of temperatures to carbon dioxide levels. It is not carbon dioxide which determines the lapse rate – it is the process of convection and the effect of pressure on the rate at which warm air rises, as well as weather conditions of course.
DeWitt / Jack Frost… help me out here.
I’ve seen some of this stuff Claes Johnson pop up here and there and have looked at https://scienceofdoom.com/2010/07/31/the-amazing-case-of-back-radiation-part-three/#comment-15206 some of his material.
It seems to me that what he does is this:
– He sees that Wein’s Displacement Law gives a maximum wave length / frequency that can be expected at a given T.
– He then goes “maximum”? That must mean it’s a cut off. (see comment to Figure 4 and text on P. 18 in the above document “Observe that the cut-o shifts to higher
frequency with higher temperature according to Wien’s Displacement Law”)
– But he sees the standard BB radiation graph that decays away from the maximum and so he says “Oh, well, it’s ‘strongly attenuated'”.
– Then he reasons, well, if it’s a cut off of radiation, it must be a cut off of absorption…
– And and world of confused pain emerges (see, e.g. P. 24 absorption .v. internal heat storage!)
Seems to me, at the root of this, is no more than the confusion between “maximum” and “peak”. But from there on, the theory makes little sense.
Do I miss understand?
Oops, I’ve put the wrong link above…
Should be http://www.csc.kth.se/~cgjoh/ambsblack.pdf
Jack Frost, by failing to understand the relevance of DeWitt Payne’s analogy you also fail to comprehend why your four questions were not appropriate (Hint: the Sun is the heating coil, our atmosphere is the cup, and gradually becoming more and more like a dewar flask).
The answers to your first three questions are true, the fourth is of course dishonest as the back radiation makes the surface warmer than it otherwise would have been with no back radiation; however it cannot (of course) make the surface warmer than it was before the surface emitted radiation.
Back radiation cannot make a surface hotter than its initial state, but keeps it warmer than it would be with no back radiation present.
Frosts subsequent ramblings are entertainingly inaccurate.
By the way, skywatcher, you say “Back radiation cannot make a surface hotter than its initial state ..” which is true.
I think you will find SoD is not saying this. He says temperature is irrelevant and that back radiation will make the surface warmer regardless of whether or not it has cooled since emitting the original radiation, or the radiation itself has “cooled” by way of being at lower frequencies. (Wien’s cut-off frequency is proportional to absolute temperature.)
For example, on a sunny morning when the surface is still warming I think you will find SoD would say the backradiation will warm it even more. But you, quite correctly, say it won’t.
Perhaps you can explain to him how the radiation “remembers” whether or not the surface was originally warmer. It does of course do so by way of its energy which is proportional to its frequency. It warms if that frequency is above the cut-off frequency above which (as per Wien) the surface cannot radiate for its current temperature.
So you have your little argument with SoD.
Jack Frost, I think you’ll find that my argument is not with SoD, who has the physics of greenhouse gases and back radiation down very well (one of the best series of explanations on the Net). The argument is with you, who fails to understand the greenhouse effect.
Your third paragraph is incorrect, and you falsely put words into my mouth. On a sunny morning, back radiation will indeed help the surface become warmer than it would be without the back radiation, as a greater total amount of radiation is being absorbed by the surface. You seem to think that longwave radiation is incapable of adding energy to a surface (thus slowing net energy loss), which makes me wonder if you know why a clear dry subtropical desert night is much colder than a clear humid equatorial tropical night.
DeWitt: I notice it was yourself above who suggested just pointing an infra-red thermometer at the sky to confirm there is some IR radiation coming down. Well, yes, there could hardly be none. But all your thermometer is doing is measuring the frequency of whatever it detects, with no indication of the amount of radiation. Now any attempt to convert temperature to radiative flux using SBL would yield a negative value because you would be considering the radiation from some layer in the troposphere which would usually have a warmer layer just below it. You would need to know the temperature of the warmer layer also, then deduct the two SB values to get net radiation.
The only way to actually measure backradiation would be to measure any warming effect. You would think it would be quite simple to compare different temperatures between identical metal plates one of which was shielded from backradiation. But no. No one ever seems to be able to do so – correct me if I’m wrong! Why? Because the backradiation does not warm the plate. So, instead they do things like point an infra-red thermometer upwards and determine the temperature somewhere up there, the thermometer doing so from the frequency of the radiation which is proportional to temperature. Then they bung this temperature into the SB equation and, bingo, we have a “measure” of backradiation. Or do we?
I’m never quite sure why it’s called backradiation anyway. Quite a bit of incoming radiation from the Sun is in the infra-red spectrum and is thus absorbed by trace gases such as carbon dioxide. That which is sent back to space helps prevent the warming it would have caused. That which gets re-radiated in the direction of Earth (where it was going anyway) gets lumped in with the “backradiation.” So why is it that plots like this only seem to show radiation from the Sun without a lot of extra radiation in there in the infra-red spectrum? http://earth-climate.com/spectral-content.gif We can see the “pockets” where water vapour and carbon dioxide have absorbed some of the incoming infra-red radiation, thus having a cooling effect, but why aren’t these pockets filled up again and indeed overflowing because of all the backradiation?
Now, as I have pointed out, the radiation from the atmosphere which comes from a point which is cooler than the surface it “hits” will not be converted to thermal energy in the surface, but will instead be “bounced off” because it is immediately re-emitted with the same frequency and thus the same energy. This process takes no significant time and so does not slow the cooling process one iota. Only incident solar radiation has enough energy to surpass the threshold frequency and thus produce warming. Such thermal energy can sink deep into the oceans, or not so deep into the land surfaces, and then come back out again hours or even months later. All of which is why the surface does not act like a perfect blackbody and why the so-called atmospheric “greenhouse effect” is a physical impossibility.
Jack
“Quite a bit of incoming radiation from the Sun is in the infra-red spectrum and is thus absorbed by trace gases such as carbon dioxide”
Doh!!
Have you never heard of the idea of NEAR IR and FAR IR? Very little of the incoming Solar is in the same frequency range as the absorption bands of CO2.
If you disagree, please post appropriate data….
For interest, here is the spectrum of solar radiation at the top of atmosphere and at the surface:
From F.W. Taylor, Elementary Climate Physics
You can see that a small amount of absorption is from CO2.
Here is the absorption spectra of CO2 from 0.7 – 4.0 μm first as a linear graph, then as a log graph:
There is also a stronger band at 4.2-4.3 μm, but it’s not very significant for solar or for terrestrial radiation.
For interest for newcomers, there is little overlap between solar radiation (“shortwave” = less than 4 μm) and terrestrial radiation (“longwave” = greater than 4 μm). Radiation of greater than 0.7 μm is known as “infrared” but this description is not at all relevant to the conventions of “longwave” and “shortwave”.
That’s exactly backwards. An IR thermometer consists of a metal block with a thermistor inserted in the block to measure its temperature. The exposed surface of the block has a thin layer of insulating material with known thermal conductivity inside a thermopile. The exposed surface has one set of thermocouple junctions and is treated to have high emissivity in the IR. The back surface has the other set of thermocouple junctions so the temperature drop across the insulator can be measured accurately and is thermally coupled to the metal block. If the thermopile voltage is zero, then the effective temperature of the IR radiation observed is the same as the metal block and there is no flux into or out of the detector. If the effective temperature is higher, then the exposed surface has a higher temperature and the energy flux can be determined by the temperature drop across the insulator and conversely for radiation from a colder source. The effective temperature can then be determined from the equation:
F = εσ*(Teff^4-Tblock^4)
where F is the net energy flux, ε is the emissivity of the detector and σ is the Stefan-Boltzmann constant. A handheld IR detector has a Fresnel lens to focus the incoming IR radiation on the detector. The total flux is measured, not the frequency.
My understanding is that the cheap infra-red thermometers you suggested we all go out and buy do not measure flux in the way a somewhat more expensive “IR detector” does. They work more like an infra-red camera I believe an dthe camera artificially colors areas of the image in accord with the frequencies detected.
There has been a limited supply of the expensive equipment needed to measure flux directly, so historic records at least are somewhat spurious.
In any event, I understand that actual measurements of downwelling IR radiation made by pyrometers at night are only of the order of 60 W/m^2. Have you made any measurements yourself, as Prof Nahle has, or do you just blindly accept what they say?
Jack Frost:
You don’t understand absorption and emission of radiation. Or spectra.
First, take a look at Absorption of Radiation from Different Temperature Sources.
From that article here is an example pair of waveforms from highly emissive surfaces at -10’C and +10’C:
Note that they have very similar spectra. If we considered the atmosphere at -10’C and +10’C the spectra would have a different shape from the above spectra (see Part Two of this series) but importantly the two different temperature spectra would be very similar to each other.
The material property called absorptivity is what determines what radiation gets absorbed and what gets reflected. Absorptivity is a function of wavelength (and direction with non-diffuse surfaces).
So if a surface has an emissivity of 0.8, for example, for a wavelength of 10 μm then it will absorb 80% of photons of this wavelength, regardless of whether the 10 μm photons came from a -10’C or a +10’C source.
This is just basic textbook radiative heat transfer theory. Please have a read of the article.
Secondly, emission of radiation takes place from a surface according to the temperature of that surface and its emissivity (which again is a function of wavelength, and direction). It doesn’t take place according to the temperature of the source radiation that was absorbed.
Yet you write “..but will instead be “bounced off” because it is immediately re-emitted with the same frequency and thus the same energy..” – which is very confused and not anything that you can find in a textbook on the subject of radiative heat transfer.
Either the radiation is reflected or it is absorbed and re-emitted in which case it must have a different spectrum if it is at a different temperature from the source radiation.
You could even try reading the extract from Fundamentals of Heat and Mass Transfer, by Incropera and DeWitt in this very article and trying to explain it.
SoD: You said: “Either the radiation is reflected or it is absorbed and re-emitted in which case it must have a different spectrum if it is at a different temperature from the source radiation. ”
This is incorrect. The LW component of the (IR) radiation is re-emitted exactly as “absorbed” with exactly the same frequencies and intensities, so there is absolutely no energy left behind for any warming. The net effect is the same as reflection. On the other hand, the SW radiation is not re-emitted as such and its energy is fully converted to thermal energy which may well be stored for hours, months or even years before being transferred back to the atmosphere, not necessarily by radiation anyway – eg perhaps by evaporation or conduction followed by convection.
SoD – stop trying to teach your grandmother to suck eggs. I am fully aware of what you have said, and am aware of Wien’s Displacement Law and the fact that the peak frequency is proportional to the temperature. But you are totally wrong in saying the radiation has to be either reflected or absorbed if by “absorbed” you mean converted to thermal energy. If you hold your radiating hand (say ~40 deg.C) over the lid of a “perfect” vacuum flask with coffee at, say, 95 deg.C the radiation from your hand will not warm the coffee. Answer the four questions I have posed. Reflected backradiation will not warm the surface either. If you wish to argue, then argue about the computations to which I have linked you. So far no one in the world has been successful in disproving them in over six months. And, yes, this sort of proof is probably not in a textbook because it would get censored for disproving the greenhouse effect.
Just one final note: The Earth surface and oceans do not act as a perfect blackbody for numerous reasons. The surrounding air is warmed to a very similar temperature by molecular collision. Evaporation also removes some energy from the oceans. Overall, probably more than half the net thermal energy that transfers from the oceans and land surfaces to the atmosphere does so by means other than radiation. If the two temperatures are very close then correct S-B calculations give very low net radiation – nothing like a blackbody in space. Furthermore, incoming solar SW radiation is not re-emitted at all without first being converted to thermal energy. Such thermal energy can be stored for hours and even months, sometimes years. So the timing is by no means instantaneous regarding radiation in and radiation out.
Glen – Yes the carbon dioxide bands (peaks) at 2.58 and 2.76 micrometres are the main ones which absorb some of the incoming solar infra-red radiation and re-emit about half of it back to space, thus having a slight cooling effect. There is another totally different cooling effect resulting from carbon dioxide molecules absorbing thermal energy through collisions with oxygen and nitrogen molecules and then radiating that energy out of the atmosphere. There is no warming effect because backradiation does not warm a surface which has a cut-off frequency (determined by Wien’s Displacement Law) greater than the frequency of that backradiation. Such radiation is immediately re-emitted without loss of energy and thus without any warming effect.
We are talking about surface molecules. The low energy back radiation is never converted to thermal energy when it strikes the surface. There is no issue regarding timing before collisions take place with other molecules. The radiation has to be re-emitted and this results in a blackbody effect (at least for IR radiation) such that IR radiation in = IR radiation out. The radiation has not gone through the process of being converted to thermal energy such as by ionization and so thermal energy cannot be transferred by collisions.
I am having trouble posting messages which include links – so just google “computational blackbody radiation” to see the calculations I have referred to in the replies to SoD.
Emissivity is not emission. The emission of radiation at those lines is quite small compared to the energy absorbed. There is very little emission from the atmosphere at <3μm because the temperature is too low. The net effect is warming, not cooling. Although not much because most of the radiation that is absorbed in the atmosphere by an increase in CO2 would have been absorbed by the surface.
DeWitt and SoD: I am not really interested in arguing about the cooling effect of carbon dioxide. As there is absolutely no warming effect, indeed I hope the cooling effect is not too great because another “Little Ice Age” is inevitable within 450 to 600 years. I have seen calculations that the cooling effect is seven times the assumed warming effect, but you would probably argue about such calculations, so I can’t be bothered discussing same.
My main point is to draw attention to the ground-breaking work of Professor Claes Johnson whose “Computational Blackbody Radiation” proves quite cogently (if you can follow the mathematics) that radiation with frequency below the cut-off (as determined by Wien’s Displacement Law) is immediately re-emitted with an identical spectrum and energy (thus not warming the surface) and only radiation with higher frequencies (normally incident solar radiation) is absorbed and all its energy converted from coherent radiation to incoherent thermal energy.
The net radiation from the surface in calm conditions represents a relatively small proportion of the thermal energy leaving the surface (maybe less than 30%) because the majority exits the surface by evaporation and molecular collision (call it conduction or diffusion, whatever you like) which is followed by convection. The rate at which warm air rises by convection (and weather conditions) is the primary determinant of the temperature gradient. Surface temperatures are determined by the associated lapse rate and the rate of conduction under the surface, because a significant amount of thermal energy from incident solar radiation flows into and out of the surface (including the oceans of course) on a daily basis, and also seasonally in each hemisphere.
Prof. Johnson’s calculations were supported by empirical results obtained by Prof Nahle in his September 2011 experiment, and I understand more experiments are following this year. You owe it to yourselves (and other readers) to investigate the computations carefully. Maybe you will be the first in the world to prove him wrong, but if you think you can, then obviously you should take up the matter directly with him.
I should not need to remind you that there is now clearly no empirical evidence of a causal (forcing) relationship between carbon dioxide levels and temperatures. This was supposed to be most obvious in the Arctic, but in fact Arctic temperatures rose 4 degrees between 1919 and 1939 and were then warmer than they are now – the trend up there bearing no resemblance whatever to that of carbon dioxide.
Well that explains a lot. If you think Claes Johnson’s work is ground-breaking, you not only don’t have a clue, you’re incapable of getting one. In which case, further discussion is pointless. I will point out, however, that if Johnson were indeed correct, molecular spectra of gases would look a lot different than they actually do.
You also haven’t looked closely at Nahle’s experiment. He actually fails to replicate Wood as he claims, as have I (work in progress) and Vaughan Pratt. Look at picture 06 on page 6 of Nahle’s experiment. One box has extra insulation in the form of glass wool. Guess what, it’s the box with the IR transparent polyethylene film window. Now look at the results of Experiment number 1 on page 8. The polyethylene film covered insulated box has the same temperature as the sealed glass and acrylic windowed uninsulated boxes ~70 C. Now go to page 22 and look at the results comparing boxes with polyethylene windows, one with glass wool insulation and one without. The insulated box is 9-10 C hotter than the uninsulated box (averaging readings from 35-60 minutes, 72.2-62.8 = 9.4 C). That means that if all boxes had been uninsulated in his experiment 1, the polyethylene box would have had a significantly lower temperature than the glass and acrylic windowed boxes. Nahle’s experiment, then, has failed to replicate Wood’s results and is in agreement with theory. Note that Wood was rebutted at the time by Charles Greely Abbot and the whole thing was quickly forgotten until someone resurrected it nearly a century later and apparently failed to look for the rebuttal.
PS You should also read this article: http://wattsupwiththat.com/2012/01/09/strange-new-attractors-strong-evidence-against-both-positive-feedback-and-catastrophe/#more-54515
Jack Frost
Of the many supporters of Claes Johnson I have yet to read anyone who understands more than the absolute basics about radiation and yet to read anyone who has actually realized what the Professor actually claims to have proven.
He claims to have overturned not just a small part of climate science but major theories of physics which have stood firm for 100 years.
Have you realized this point?
You might not have because his marketing work stresses the bit about climate science.
Do you believe his writings because:
a) you can’t understand them but like the outcome, or
b) because you have studied the subject, understand what the prevailing view is, why it is accepted, what the experimental evidence is, and what the implications are for the field of physics (not climate science) if the good Professor turns out to be correct
This blog accepts as proven standard theories of physics, which includes gravity, quantum mechanics and statistical mechanics. They have considerable theoretical and experimental evidence to back them up. (They were accepted long before climate became a big topic in science).
As and when the physics world recognizes that these theories are flawed and the undergraduate textbooks start getting rewritten we will evaluate the implications on this blog. Until that time..
DeWitt: It is you who have not looked at the relevant Nahle experiment …
Observations on “Backradiation” during Nighttime and Daytime
By Nasif S. Nahle
Abstract
“Through a series of real time measurements of thermal radiation from the atmosphere and surface materials during nighttime and daytime, I demonstrate that warming backradiation emitted from Earth’s atmosphere back toward the earth’s surface and the idea that a cooler system can warm a warmer system are unphysical concepts.”
Click to access New_Concise_Experiment_on_Backradiation.pdf
And in regard to Professor Claes Johnson’s “Computational Blackbody Radiation” it holds firm while ever no one in the world has as yet been able to disprove it. And it is backup by Nahle’s experiment and common sense.
Remember, the IPCC has never quoted any experiment that proved backradiation can warm the surface. Show me one that does! I won’t publish my book if you can.
Is your radiating hand going to warm that coffee in the vacuum flask? I’ll won’t publish my book if it does and you can prove why computationally, showing me exactly where Johnson’s calculations are wrong and Nahle’s Sept 2011 experiment wrong. While you’re about it, show me two identical electric radiators turned on simultaneously (in open air) helping each other to get hot faster.
It seems that to “back” the assumed warming of warmer surfaces by radiation from cooler atmospheric layers you have to be pretty good at abandoning common sense.
If you had actually read my post rather than dismissing it out of hand, you could verify that I have quoted the relevant data from Nahle correctly. What someone claims to have proved and what they actually proved can be completely different and definitely is in this case.
Johnson actually proves the main theories of radiation computationally and shows that it can be done using only wave properties of radiation. Such wave motion is “two-way” and avoids the need to assume a mass-less “particle” nature for radiation. Einstein was still not happy about the concept of such even in 1954 when he made that well known statement “All these 50 years of conscious brooding …” The assumed particle nature of radiation
There is nothing you can show me in this* which you can demonstrate to be contrary to the physical world. It has never been proved empirically that radiation with frequency below the cut-off can warm a “warmer” surface. So he is not turning any physics on its head. The greenhouse effect hypothesis was never physics. And you, however brilliant you may think yourself, cannot prove him wrong on this, because you would also have to replicate Nahle’s September experiment and get a contrary result. Nature doesn’t work that way my friend.
That is why the Arctic temperatures are all over the place – rising 4 degrees from 1919-1939, then being warmer than current temperatures, and thus experiencing a net decline since the second World War – just when carbon dioxide was taking off. That is why both North American land temperatures since 1880 and over 200 years of Northern Ireland temperatures since the previous century shown a linear trend with absolutely no sign of a hockey stick. That is why world temperatures have not been warming any more for about a decade now. That is why it is natural cycles are controlling climate and IPCC predictions don’t stack up.
Jack Frost aka ?:
You are entitled to your opinions about Claes Johnson’s theories. And you have aired them.
I have explained mine.
This blog’s Etiquette states:
So please respect the blog rules and tell the world somewhere else about your opinions on quantum mechanics.
Johnson does NOT overturn one iota of “standard physics” because your concept of radiation from a cooler body adding thermal energy to a warmer one CANNOT BE PROVED from standard physics.
Please yourself what you believe – that’s your personal choice – but it’s wrong and it is not backed up by physics experiments which you could repeat if you had a genuine desire to promulgate the truth on your website.
But I am fast losing confidence that you are.
And you are misleading people seriously.
Try keeping up with Watts up.
Whatever form you consider radiation can take, if it can pass right through some materials, it can also pass by some individual molecules perhaps being deflected. So, if it doesn’t resonate with such a molecule, and doesn’t have enough energy (ie a frequency above the cut-off) to knock electrons out of orbit and be converted to thermal energy, then it may simply experience multiple deflections in which it has no effect on the energy of the molecules it grazes. Such deflections could be such that probabilities tend towards exiting the surface back into the atmosphere. So the process is like reflection, but the angles are not the same.
There is nothing new in this “physics” but it may help you to understand why there are more than just the two possibilities “if it is not reflected it must be absorbed (and warm the surface?)
The English may not be perfect, but it’s pretty clear what Nahle has proved:
“I demonstrate that warming backradiation emitted from Earth’s atmosphere back toward the earth’s surface and the idea that a cooler system can warm a warmer system are unphysical concepts.”
No, that’s what he claims to have proved. In fact, his data proves exactly the opposite.
For a start, in his experiment Nahle measures downwelling radiation at night as starting at 61.93 W/m^2 and reducing to 46.45 W/m^2 after 7 hours, whereas upwelling radiation from the surface started at 308.33 W/m^2 and reduced to 279.99 W/m^2 after 7 hours. What does that do for your SBL calculations?
So how do these figures compare with typical energy diagrams? The downwelling radiation in sunlit hours would have to be pretty high (~ half that of daytime solar insolation) to balance out the low night time value. Why would it vary so much between day and night? Why wouldn’t it warm your skin about half as much as the sun when you stand in the shade of direct insolation but exposed to a large section of the sky? Why can’t any small amount of warming of any object ever be detected if there is so much backradiation and if it really does warm anything? The proof of the pudding is in the warming, not the pyrometer.
The atmosphere cools faster than the surface at night and is of course somewhat colder anyway. The rate at which the surface cools depends on conductivity (and convection and evaporation in the oceans) because thermal energy penetrates to some depth during the day and is flowing back out again at night mostly by conduction. (A similar process prevents the Moon from cooling to near absolute zero as soon as night sets in.)
The measurements of downwelling radiation come from clumps of rarefied warmer air mostly in the stratosphere (perhaps some in the upper troposphere) where it is possible to emit radiation downwards towards cooler regions. The pyrometer measures all such radiation in an upward facing cone about 30,000 metres in height.
After all, the S-B Law dictates that radiation is from a warmer body to a cooler one – otherwise it gives a negative result, which means the same anyway.
DeWitt – I’ll call your bluff – show me why. You won’t pull any wool over my eyes my friend.
DeWitt has already told you the answer in his comment of January 10, 2012 at 1:42 am.
No he hasn’t. DeWitt mistakenly talked about a totally different experiment – not the September 2011 one I have been referring to and which I pointed out to him. I was not talking about Nahle’s attempt to replicate Wood’s experiment which he reported on earlier last year.
Perhaps you had both better read it first and find out how Nahle measured backradiation and surface temperatures at night, and what the pyrometer was actually measuring coming from a disc about 5km in diameter. http://principia-scientific.org/publications/New_Concise_Experiment_on_Backradiation.pdf
No one has answered my four questions above (January 8th, 9:38pm)
“The Greenhouse Effect is an imaginary process that would be the result of backradiation absorbed by the source of primary thermal radiation, in opposition to the universal trajectory of events, i.e. against natural spontaneous progression of entropy, from low level to high level.” Nahle
Jack Frost:
Please provide your entropy calculation.
Or take a look at The Three Body Problem and explain what is wrong with it.
Extremely simply stuff. But it’s your choice – parrott, or explain.
Jack Frost:
How does he do that?
You have just explained that the theories of Claes Johnson are proven and yet he says:
The error in your three body problem is that the radiation from the crazy body has a frequency lower than the cut-off frequency for the Earth’s surface and therefore is not absorbed and does not warm the surface. You keep repeating the error you make regarding any backradiation.
If you undersatnd and accept what Johnson has proved then his quoted statement should not seem bewildering. Out of context it may well do so.
Once you fully understand Nahle’s September 2011 experiment (which is NOT the one about Wood’s experiment) then you may realise that what is measured as backradiation is most likely radiation from warm, rarefied pockets of air in the stratosphere radiating to cooler regions below, this being possible because of temperature inversion.
I don’t know why you think Nahle would have trouble buying a $500 pyrometer and measuring downwelling radiation at night. Try it yourself with some of the capital gains profits you have made with this site. (Take a tip and sell out before the whole AGW guessothesis collapses publicly.)
Jack Frost,
If you had given me a link to the article in question originally rather than just a publication date it would have made things simpler.
The article in question is pathetic. The first section on calculating the average insolation over 24 hours just demonstrates Professor Nahle’s ignorance of simple geometry. I’m not going to spend any time on it because if the problems aren’t obvious to you, my explaining them won’t help.
As for the IR thermometer readings: An IR thermometer has quite a small angle of acceptance so it only sees a small fraction of the sky. The ground, however, sees radiation from horizon to horizon, i.e. 2π steradians. My IR thermometer, for example has a spot size to distance of 1:10. That is, at a distance of 1 m from the device, the diameter of the observed area is 0.1 m. That’s approximately equivalent to a solid angle of 0.1 steradians or 1.6% of the total sky. The effective temperature of the sky increases as the observation angle decreases from 90 degrees.
To properly measure the downwelling IR, you need a pyrgeometer that has an acceptance angle of 2π steradians. Kipp & Zonen and Eppley Labs manufacture those instruments. The SURFRAD network measures incoming and outgoing radiation at seven sites in the US. The data can be viewed here: http://www.srrb.noaa.gov/surfrad/pick.html
Let’s look at full sky upwelling and downwelling IR and downwelling solar radiation for Desert Rock, NV on January 8, 2012: Graph. Note that the downwelling IR doesn’t change much from day to night and averages about 250 W/m2. From the shape of the graph, it’s obvious that the sky was clear. But I can download the actual data and average over 24 hours. The results:
Downwelling solar: 130.8 W/m2 (It’s January)
Downwelling IR: 237.6 W/m2
Upwelling IR: 346.0 W/m2 (280.9 K or 7.7 C &epsilon=0.98)
Average air temperature at 10m: 6.6 C
So incoming radiation averaged to 368.4 W/m2 and outgoing radiation was 346 W/m2. the discrepancy is convective heat transfer caused by an average wind speed of 7 m/sec with an air temperature close to the surface temperature.
On June 8, 2011
Graph
Downwelling solar: 369.6 W/m2
Downwelling IR: 307.1 W/m2
Upwelling IR: 464.3 W/m2 (302.3 K, 29.1 C)
average wind speed: 4.1 m/sec
Average air temp at 10m: 22.0 C
So taking a simple average of the two days, average downwelling solar is 250 W/m2 and average downwelling IR is 272.4 W/m2 for clear sky conditions. Downwelling IR is higher for a cloud covered sky and downwelling solar is less. Considering the latitude of Desert Rock, NV is 36.6 degrees North, that’s about right.
MODTRAN mid-latitude summer downwelling IR from 100-1500 cm-1 = 307.1 W/m2 and 430.2 upwelling for a surface temperature of 29.1 C and a surface relative humidity of 21%. Mid-latitude winter, 7.7 C, RH 31%: upwelling 323.1 W/m2, downwelling 223.4 W/m2. MODTRAN IR will normally be 20-30 W/m2 less than SURFRAD because SURFRAD has a wider spectral range than MODTRAN.
Nahle’s instrumentation is not fit for purpose so his results are not correct.
Then there’s the view factor. A disk on the surface sees radiation coming from a hemisphere and vice versa. The area of the hemisphere with the same diameter as the disk has twice the area of the disk. If the radius of the hemisphere is twice the radius of the disk, the area increases by a factor of four, but the flux intensity decreases by a factor of 4. So the effective temperature of the sky in equilibrium with the surface at 300 K with unit emissivity is (300)^4)/2)^0.25 = 300/2^0.25 = 252.27 K or -20.9 C or 229.6 W/m2 for an IR thermometer pointed straight up at a small acceptance angle.
At least I’m pretty sure that’s correct. When you convert W/cm2/sr to W/m2 in MODTRAN you multiply by π, not 2π.
DeWitt, SoD and Skywatcher:
DeWitt completely ignores the main point of Nahle’s experiment which shows the atmosphere at night is cooler than the surface and cooling at a faster rate than the surface during the whole 7 hour period he measured at night. I suggest this supports Johnson’s computations that prove that radiation with frequency below (Wien’s) cut off frequency will not be converted to thermal energy: that is, in general, backradiation from a cooler atmosphere will not warm the surface, nor slow its rate of cooling.
I give credit to skywatcher for acknowledging that the first three of my four questions are true, and half agreeing to the fourth. Maybe you might like to consider why, if a mirror placed over the Earth at night causes no warming when it reflects all the radiation back again, then how can carbon dioxide do any better if it sends half the radiation back.
Now, on the question of backradiation, of course you are going to “measure” more the wider the angle of incidence. But if you can’t understand that you should be dividing by the area of the disc you are effectively measuring then we are not getting off square one. Otherwise, standing at various different points on Earth, you could be measuring radiation coming from the same molecule over and over again, simply because you would be viewing something up to 30,000m above from different angles. The ideal instrument would measure just a cross section of 1 m^2 in a vertical column all the way up. If you don’t know the actual altitude from which the radiation is coming (which you don’t) then you don’t know the area of the disc through which the radiation you are measuring is passing. So you can’t calculate a value per m^2, now can you? Thus all the measurements of so-called back radiation are grossly overstated due to the very fact that their expensive pyrgeometers do in fact have a wide angle of view.
Now, as to the use of S-B calculations by “climatologists,” there is an intrinsic assumption that you can work out average temperatures by using average radiative flux. Obviously you can’t because of the fourth power relationship. So the 255 deg.K figure is garbage as Nahle rightly points out. I don’t care if his other calculations are exactly right or not – no one could really get it right because there is a huge flow of energy into and out of the surface every day and night as the Sun warms the ground in the morning and it cools off by the end of the night. There is also a far greater percentage of thermal energy transferring by evaporation and molecular collision which should be deducted before doing radiation calculations, so it is not counted twice. Then you should deduct the calculation for the temperature of the surrounding air in the first 1mm or so of the atmosphere, such temperature being very close to that of the surface. And lastly, any calculation using S-B for backradiation from the atmosphere should deduct the calculation for the temperature of the layer of air immediately below the radiating layer. If it’s warmer (as it usually is in the troposphere) then you get a negative result. Nahle is thus quite right in explaining that the only downwelling IR radiation (other than solar) which we are measuring is in fact coming from warmer air which can radiate to cooler air below it mostly in the stratosphere because of temperature inversion up there.
In summary, all you need to consider is net radiation out of the surface, and there’s no point in even considering such anyway. The surface is nothing like a perfect blackbody because it is not insulated from its surrounds (both above and below) and so evaporative and conductive processes transfer much of the energy both upwards into the atmosphere and downwards into the crust (or by currents into the deep ocean) – all of which energy is no longer available to radiate.
Now I’m off on a 10 day trip this time tomorrow which I have to prepare for, so you have plenty of time before I get back to really study what Johnson and Nahle have proved as to why “cooler” radiation cannot warm a warmer surface – exactly the same reason that your radiating hand placed over the lip of that vacuum flask will not warm the warmer coffee below. If you agree with skywatcher on this, then think about the implications for the imagined atmospheric greenhouse effect which is a physical impossibility.
What is this Wien’s cut-off frequency of which you speak? The only place I have ever heard of this is from you – you seem to think that below a certain frequency, somehow radiation is incapable of adding energy to the molecules with which it interacts.
And though I cautiously agreed with your first three questions, which were badly worded and open to a variety of misinterpretation, I certainly disagreed with your fourth. Back radiation keeps a surface warmer than it would be without the back radiation. How often does this need to be stated? It does not heat the surface beyond the temperature of the surface as it was when the radiation that becomes ‘back’ radiation was emitted. Thus there is no disagreement with the physics of the greenhouse effect, and thus you can technically agree with your poorly-worded first three statements. If you mean in your first three statements that back radiation cannot keep a surface warmer than it would have been had the back radiation merely escaped to Space, then I utterly disagree with your first three statements.
Look up “Wien’s Displacement Law” in Wikipedia and Google ‘Claes Johnson “Computational Blackbody Radiation”‘
I see you failed to answer my questions. As Johnson misinterpreted Stefan’s words and then used a fatal assumption with regard to absorption in his rambling body of work, I didn’t see fit to bother with him anymore. As Johnson’s work is no good, and the Wien’s Displacement Law wiki page does not mention a cut-off frequency, your information is unhelpful.
Skywatcher:
Let’s consider your two sentences quoted below …
(1) “Back radiation keeps a surface warmer than it would be without the back radiation. ….
(2) “It does not heat the surface beyond the temperature of the surface as it was when the radiation that becomes ‘back’ radiation was emitted.”
The second is OK regarding “not .. beyond” but in practice it does not do any warming whatsoever. The backradiation comes from a point in the atmosphere which, in at least 99% of cases, is cooler than the surface. Some of the original energy from the radiation emitted at the surface gets lost in warming some air molecules after the initial capture, before re-emission. In practice, there can be multiple captures and emissions before (a part of) the original energy packet gets back to the surface, so it is inevitably depleted of some of its energy and thus cooler.
Now, when it returns to the surface with its frequency (hence energy) now lower than the original surface emission, it will do ABSOLUTELY NOTHING which would warm the surface one iota, because it is re-emitted with exactly the same frequency and energy that it just arrived with. There is no left over energy to warm the surface.
SoD thinks it will warm the surface regardless, even above the original temperature which he thinks forms no barrier to additional warming. But he is wrong as Prof Johnson has proved.
Only direct incident solar radiation (which nearly always has a frequency higher than the cut off frequency) will not be re-emitted (because its frequency is above the cut off determined by Wien) and so its energy must be converted to thermal energy which does warm the ocean or land surface as we all observe when out in the warm sunshine.
So your first sentence is wrong, and your second sentence misleading at best.
I appreciate that you might argue with me about the measurements and interpretation of downwelling radiation, perhaps saying that an individual photon has only one direction vector and so can’t be detected at two points on the surface. I may agree. But the main point is that whatever radiation is measured must simply be coming from rarefied warmer parcels of air which do radiate downwards because the immediate vicinity below them is cooler.
And, in any event, whatever the source, any radiation with frequency below cut off has no warming effect on the surface and, one way or another, is re-emitted or turned around by deflection and thus re-enters the atmosphere with the same frequency (and hence energy) and intensity. There is no new spectrum created in the IR band by this process. There will of course be additional IR radiation from the surface resulting from some of the incident solar radiation which was previously converted to thermal energy, but not all such energy from the Sun needs to exit by radiation, in fact probably less than half, maybe less than 30% does so. Because of the complex composition of the surface, we get the observed full IR spectrum, not just the spectral lines observed in the downwelling radiation.
DeWitt: I apologise for not including the link to Nahle’s experiment. In the first version of my post it was included, but that post got rejected automatically, probably because it also included a link to a site which SoD’s system rejects and sends to his junk mail.
Regarding measurements of upwelling radiation from the surface, let’s assume that, at night in calm conditions, your instrument is 1 m above the ground and that the temperature at that height is 1 degree cooler than that of the surface.
Now consider 1,000 individual layers of air 1mm in height between the surface and your instrument. Let’s also assume there is a linear temperature gradient, due mostly to conduction and convection, so that the first 1mm of air is 0.001 degrees cooler than the surface, the next 0.002 degrees cooler than the surface etc.
Correct application of SBL for any layer must deduct the calculation for the layer above. Hence, using T^4 = ~(T-1)^4 we deduce that only ~1/1000 (one thousandth) of the radiation being measured is coming from the actual surface, the rest from the layers of air between the surface and the instrument.
Extend this to the TOA and measurements up there are clearly almost 100% coming from the atmosphere, not the surface, and so they tell you nothing about the surface temperature.
It’s not SB that applies here, it’s Beer-Lambert. The absorption of surface radiation by the atmosphere for a path length of 1 m is quite small on average. You will get some absorption at the peak of the CO2 absorption, but since the temperature difference is only 1 degree, the emission of CO2 will almost completely make up for the absorption. It would require an extremely precise measurement to tell the difference.
If you have an IR absorbing gas in a cell and put a black body emitter at the same temperature as the gas at one end of the cell, you won’t see absorption or emission lines or bands regardless of path length. You only get absorption if the emitter has a temperature higher than the gas, which is how IR spectrophotometers work. You only get emission above the Planck curve of the emitter if the emitter is at a lower temperature than the gas.
DeWitt:
You can’t deny that when an atmosphere which is nearly as warm as (and sometimes warmer than) the surface butts up against the surface it must reduce the net radiation from the surface very significantly. For a start it takes thermal energy by evaporation and diffusion, so this reduces the energy left for radiating. Also some energy seeps out the back trap door and into the depths of the ocean and the crust. Even the IPCC energy diagrams show significant radiation from the atmosphere which can occur at any level, and is more likely to occur in warmer regions. There is nothing to stop water vapour and carbon dioxide capturing and re-emitting some radiation in the space between the surface and the measuring instrument.
Now you write: “You only get absorption if the emitter has a temperature higher than the gas”
And that’s exactly what Claes Johnson proved in his “Computational Blackbody Radiation.”
Nice of you to provide a practical illustration. QED
I note that you avoid discussing this issue, so perhaps you are starting to see the light. Whatever backradiation there may be (and some could originate from absorbed incoming insolation as well as from energy transferred from oxygen and nitrogen – thus cooling them) then, since that backradiation comes from a cooler atmosphere in over 99% of cases, I suggest, then it cannot warm a warmer surface. The cut off frequency determines whether absorption and subsequent warming can occur, as obviously does for SW radiation. The cut-off falls approximately between the spectra of SW absorption and LW emission and, as you know, the spectra barely overlap for this very reason.
There is no proof of the contrary in any standard physics documentation. There are just wishy-washy “explanations” of the assumed greenhouse effect, none of these backed up by any proof whatsoever that radiation from a cooler source can warm a warmer surface.
It simply doesn’t happen in nature, which is why the GHE is a physical impossibility.
Are you able to be the first in the world to pinpoint some error in Johnson’s line of argument and mathematical deduction? That’s all I need to hear from you.
SoD You say this site only accepts standard physics. Above you have DeWitt describing a real life situation in which, as he says, ““You only get absorption if the emitter has a temperature higher than the gas”
This is a very clear cut contradiction of your assumption that a cooler emitter can warm a warmer receptor.
Is he wrong or are you yourself wrong?
Are you yourself able to be the first in the world to prove Prof Johnson’s computations wrong.
My point is, something has to go here. Either you explain to DeWitt that he is wrong, and to Johnson that he is wrong (and thus myself too) and you delete all this non-physics which you consider it to be, leaving your own blackbody articles as is, or you wake up to the fact that you have been wrong and should thus delete such articles.
DeWitt and SoD:
Have you ever really thought about why radiated heat transfer is always from warmer to cooler bodies?
What is the actual mechanism that determines this? How does one body “know” that the other is cooler or warmer? We can easily construct examples in which the intensity of radiation from the warmer body is less than that from the cooler body, eg a polished silver warm body and a black cool one. So why doesn’t the cooler one warm the warmer one if the radiation intensity from it is the greater?
The ONLY answer lies in what Prof Johnson has proved.
Jack,
If you really think downward emitted photons in the atmosphere cannot travel from the colder atmosphere toward the warmer surface then how do the photons from the Sun pass through the colder upper atmosphere and reach surface?
Have you ever felt the Sun’s rays on your skin when you walked outside? Does getting a tan violate the 2nd Law?
RW:
I don’t think “emitted photons in the atmosphere cannot travel from the colder atmosphere toward the warmer surface” and I never said I did. If you wish to quote me, please use my actual words.
I do however believe the computational physics which Professor Johnson documents regarding the fact that such photons, having frequencies below cut off, do not warm the surface when they get there.
Physics says radiated heat transfer is always from a warmer body to a cooler one, even if the intensity of radiation is greater from the cooler one than from the warmer one. How then does the cooler one “know” the warmer one is in fact warmer than itself? You can only answer this using what Prof Johnson has proven because the temperature information is in the cut-off frequency, not the intensity. So how about reading what Johnson actually says, ’cause I’m off on an 11 day holiday tomorrow morning and don’t have time to explain again what is already on my website – see link in above post.
Skywatcher:
Your response says nothing, documents nothing and does nothing but make hand waving dismissals of Professor Johnson’s computations which are obviously way beyond your comprehension. How much tertiary Physics do you have to your name? I have been giving tuition in such since the 1960’s. Go and comfort yourself on a pro-AGW forum as you clearly are not interested in learning anything new, such as the truth.
Frost … you’re funny, because you cannot answer straightforward questions, blindly believe incorrect physics, and rant when the physics you put so much faith in is challenged. Good luck with that approach to the world. Johnson is wrong, but I’ll leave it up to you to work out where, as you don’t listen to anyone’s answers anyway!
I can assure you I would be all ears to any vaild debunking of Johnson. I have no desire to publish anything that is incorrect and no vested interest in sitting on either side of the fence. I just seek the truth.
You, I suspect, have no understanding of Johnson’s computations and can’t explain any “error” when I call your bluff. How about telling everyone just how much tertiary physics you have studied, as I asked above?
Funny how fake ‘skeptics’ appeal to authority when it suits them. I see your single eccentric professor and your tertiary physics education and raise you every national science academy and major scietific organisation around the world, including physics ones. Do you really believe they’re all wrong? Good luck with that conspiracy…
Those who say there is an atmospheric greenhouse effect due to radiation from a cooler source being assumed to be warming a warmer surface, in breach of everything that standard physics says to the contrary are indeed wrong, because they have absolutely no proof that such warming is possible, even though it would be very easy to prove empirically if it did happen. Just put two metal plates out in the backradiation at night, but shield one from the radiation and see if the other gets warmer. What’s “funny” is that you can’t show me documentation of any such experiment ever having been carried out with positive results anywhere in the world at any time in recorded history.
Yes, “funny” but sinister.
You. like the typical member of the public who has no real understanding of atmospheric physics, and probably no tertiary education in physics, have been bluffed by them all, and you can’t prove that you haven’t been, because the real world doesn’t work that way.
That’s why Arctic temperatures, for example, show no sign whatever of any response to carbon dioxide levels, having been warmer in the 1930’s than in any recent years. That’s why a temperature rise of about 0.5 degrees late last century was nothing unusual – it happened before with much lower carbon dioxide levels. That’s why temperatures have levelled this century – because they are not influenced at all by carbon dioxide levels.
Notice how others on this forum seem to be thinking before replying – unlike yourself.
That’s quite a row of insults when you have even less a clue about who I am as you do about Arctic climate, global temperature rise both present and past, or how to test for a back radiation effect! What’s even funnier is your absolute confidence in your ability, despite your unwillingness to take on new information. Rather sad, really.
PS Here’s some interesting facts all should read …
Click to access History-of-Radiation.pdf
For the tiny minority of readers fascinated by Claes Johnson’s theories I will probably write an article.
The article will attempt to make clear the very simple point that one who accepts Claes Johnson’s theories accepts that photons don’t exist.
This is not because it is some awkward and difficult to fathom consequence of Johnson’s theories, but because actually he states it nice and clearly.
Furthermore, I will make clear that writers of textbooks on physics and on radiation believe that photons exist.
By use of amazing leaps of logic I will demonstrate that IF someone accepts Johnson’s theories then they have BY DEFINITION accepted that photons don’t exist and BY DEFINITION have claimed that all current textbooks on this realm of physics are wrong.
And thus, those who use Johnson’s theories to back up their “ideas” are requiring as a premise that current physics as taught in basic textbooks is wrong.
Challenging to make all of these wild connections, I know. But I will attempt to do this in my most challenging article yet – a roller coaster ride of writing down what the author says he is proving and comparing it with writing down what textbooks say.
For some like the illustrious Jack Frost, aka a previously moderated out commenter, this will be a step too far, and he will respond:
Perhaps the article will be in vain. Perhaps it will be too taxing for me to write down what Claes Johnson says.
And perhaps Jack “Doug” Frost will continue to use the unproven outlier Claes Johnson as proof of stuff he believes anyway.
The notion of idea A being contingent on idea B being disproven. And idea B being something that the physics world has long accepted as true.
What’s this A, B, proven, disproven stuff? Claes has written down some equations and talked about climate science. That’s enough, right?
Perhaps if just one other commenter says “please can you write this article” I might do it.. no judging, I realize that many people find the subject hard to understand..
What is unproven is the “particle” nature of radiation. Prof Johnson shows that everything can be explained by wave-motion. Before you get too far off the track, you might note his reference to eigen-frequencies and being excited, just as in quantum mechanics. He has already explained (and disputed your claim) that he does not disagree with standard quantum mechanics.
If you propose to “prove” him wrong, then the only way is to prove his computations wrong and not showing results which can be confirmed empirically. No amount of wishy-washy statements about how his theory differs from so-called standard physics will necessarily prove him wrong. History abounds with false concepts originally accepted as standard physics at the time. Einstein was never satisified with the concept of light quanta. You might do well to confront Johnson privately first, as well as reading the article I just linked above.
But keeping to the topic of backradiation and whether or not it warms the surface, Johnson also shows the following which CAN be confirmed empirically. Hence, if you wanted to prove him wrong on that, you would have to construct (or find documented) an experiment which demonstrated backradiation having a warming effect. I have suggested some, and it would be easy to devise plenty. Surely you must wonder why the IPCC does not mention any such empirical evidence for the key underlying assumption of AGW. Bear in mind that Nahle is coming up with more experiments this year which won’t surprise me, but may surprise you in that they further confirm Johnson’s statements below …
“A blackbody acts like a transformer of radiation which absorbs highfrequency radiation and emits low-frequency radiation. The temperature of the blackbody determines a cut-off frequency for the emission, which increases linearly with the temperature: The warmer the blackbody is, the higher frequencies it can and will emit. Thus only frequencies below cut-off are emitted, while all frequencies are being absorbed.
“A blackbody thus can be seen as a system of resonators with different eigen-frequencies which are excited by incoming radiation and then emit radiation. An ideal blackbody absorbs all incoming radiation and re-emits all absorbed radiation below cut-off.
“Conservation of energy requires absorbed frequencies above cut-off to be stored in some form, more precisely as heat energy thus increasing the temperature of the blackbody.”
While already this view of a blackbody is theoretically interesting and new, Johnson’s conclusions from his model are even more interesting:
“Radiative heat can be transmitted by electromagnetic waves from a warm blackbody to a colder blackbody, but not from a cold to a warmer, thus with a one-way direction of heat energy, while the electromagnetic waves propagate in both directions. We thus distinguish between two-way propagation of waves and one-way propagation of heat energy by waves.
“A cold body can heat up by eating/absorbing high-frequency, high temperature, coherent waves in a catabolic process of destruction of coherent waves into incoherent heat energy. A warm body cannot heat up by eating/absorbing low-frequency low-temperature waves, because catabolism
involves destruction of structure. Anabolism builds structure, but a black- body is only capable of destructive catabolism (the metabolism of a living cell consists of destructive catabolism and constructive anabolism).”
Now I really must get ready for my trip driving from Sydney to Adelaide via Victoria where it’s been snowing this week right in the middle of summer.
PS This quote summarises quite well ….
AGW choose to argue that “downwelling longwave radiation (DLR)” is real because it can be measured and there are many government funded programs to measure DLR. However, with respect to AGW the question is not if one can measure the temperature of the atmosphere by means of radiation – which is basically what is done when DLR is measured from earth´s surface. The one and only important question is whether DLR transports heat from the colder atmosphere to the warmer ground.
Particle based radiation models, like Planck´s, inevitably must suggest (to someone ready to believe such a suggestion) that heat bound to the “particles” (quantitized photons) is transported at least statistically from cold to warm, thereby violating the 2nd law of thermodynamics, when these quanta “statistically” also move from cold to warm.
Johnson avoids this violation of the 2nd law.
With Johnson´s proposed mechanism it is OF COURSE possible to measure the temperature of the colder – atmosphere standing on the earth´s surface with appropriate devices like e. g. a pyrgeometer (two-way propagation of emitted waves) but any “downwelling” radiation cannot transport ANY HEAT from the colder atmosphere to the warmer earth surface and thus can neither warm the surface nor reduce its cooling rate by means of downwelling radiation.
Note: Of course the presence of an absorbing/emitting atmosphere can change a planet’s temperature and the cooling rate must be the same as the insolation in stationary state, but the temperature gradient and thus surface temperature can change with changing atmospheric properties. The above statement therefore only refers to a change of cooling rate by “back” or “downwelling” radiation.
Is Johnson’s mathematical and theoretical approach less (or more) credible than Planck’s and Einstein’s in the first place? Unlikely! Planck’s and Einstein’s proofs were as purely mathematical and theoretical as is Claes Johnson’s”
Dr Matthias Kleespies.
Previously I told a commenter known as “Doug Cotton” that his comments would be moderated because he had not respected blog rules.
I wrote an article about said commenter, about the reason for moderating his comments and pointed people to his blog.
This same commenter has posted another 40 or so comments under his new name of “Jack Frost” in less than 3 days. These cover no new ground from the original Doug Cotton.
No doubt he will claim on his blog that his comments are censored. Whatever.
As a totally separate and unrelated issue someone unable to follow an argument or follow blog rules is not welcome at Science of Doom.
So, please don’t reply to Jack/Doug’s comments. After all, he won’t be able to reply and even if he could it would be ..
If a newcomer thinks any are possibly valid by all means pick up a theme or question and ask it.
Roger, SoD, and thanks for your informative series of articles anyway. The D-K is strong in that one. I’d say you should do the article debunk Johnson, but to be honest if it is only Jack/Doug who thinks Johnson was gold-standard physics in the first place, then the debunking isn’t worth your time!
If you want to know just how much of a waste of time it might be (in terms of showing people who agree with this why it’s wrong) a good place to start is at the source
http://claesjohnson.blogspot.com/2011/01/questions-to-lord-monckton-and-roy.html
Of particular note: Judy Curry says “To rebut/refute them would take more time than I am prepared to spend on this”.
Oh, just one more.
I keep asking this question of people like Bryan and Doug/Jack and I keep failing to get an answer:
If an object cannot absorb radiation with a wavelength below some non-zero cutoff frequency that is proportional to temperature, how can you cut steel with a 10 μm CO2 laser? At some point, the steel should heat up enough that it no longer can absorb radiation with a wavelength that long.
DeWitt Payne
Just saw my name here.
Don’t you think that using machines like lasers and microwaves to draw conclusions about equilibrium thermodynamics is misplaced?
A refrigerator is even better.
If you cannot find an explanation involving spontaneously interacting thermal emitters- then ask yourself……why?
And one more on the same vein as DeWitt – I was thinking of the rays emitted by the humble microwave oven. These are much longer wavelength / lower frequency than the supposed “cut-off” frequency of my lunch. Rays below the “cut-off” frequency are allegedly incapable of transferring heat to a surface. Yet somehow my lunch is heated. Impossible!!! Maybe Jack Frost/Doug Cotton would care to test his “cut-off” frequency notions by placing a selected part of his body in a microwave oven and seeing whether they experience heating…
Conclusion
“The world we live in does absorb DLR and adding 300W/m2 to the surface energy budget is the reason why the surface temperatures are like they are”
Your conclusion is wrong. Lets make the following though exercise: we block the surface from emitting any radiation (as done to a thermos bottle). It is night so no sun radiation is coming through. How much will the surface temperature raise with the DLR of 300 W/m2?
Nothing. The DLR has a temperature signal with it and no thermos will explode because of overheating. even if we do not protect the bottles from incoming DLR.
So DLR exists, but is not the same as sun radiation which has a temperature signal of 5000°C+. DLR can only compensate or back radiate the lower energy waves and not add energy in the higher ranges.
1W/m2 from sun is not equal to 1W/m2 from DLR. 1W/m2 from sun would warm the thermos bottle if we manage to make it so efficient as not lose any energy whereas 300 W/m2 from DLR not.
Earth energy budget is also wrong as it does not treat the energy budget of the oceans which makes a great deal of the warming, so you calculate a theoretical 33°C warming that is attributed wrongly to the atmosphere.
Lars P.,
You can’t stop the surface from emitting radiation. It will emit radiation regardless of what you put around it.
Perhaps you mean that you put some shield over the surface to stop it radiating into the atmosphere?
Please be specific about this shield. What are its characteristics? What is its absorptivity (at the wavelengths in question)? Do you mean that it reflects all longwave radiation back to the surface?
How will the DLR reach the surface with the shield in place?
The DLR has a characteristic spectrum with it. Here are two examples provided in the article:
Is this what you mean by a “temperature signal”?
If so, how will a 0’C surface know how to differentiate between the radiation from a -10’C surface and a +10’C surface?
Or do you have some other claim as to what a “temperature signal” is?
What specifically are you claiming?
1. That DLR cannot be absorbed by the surface?
2. That DLR when absorbed cannot change the internal energy of the surface?
What do you mean “DLR can only compensate or back radiate the lower energy waves“?
Can you find this explanation in a textbook on radiative physics?
Can you find an equation (from a textbook – or even invent one for us) which clarifies the idea of "compensating lower energy waves".
Textbooks on the subject talk about the wavelength (or frequency) of the photons that are emitted and absorbed.
Does a 10 μm photon have a temperature signal with it? No.
What is fascinating is that both a -10'C atmosphere and a +10'C atmosphere both emit 10 μm photons. And the surface will absorb 10 μm photons according to its absorptivity at this wavelength. There is no interrogation of these photons to find out their last temperature of emission. There is no way for the surface to know.
Of course you would know that if you had read this article.. Or read a textbook on radiative heat transfer..
Do you think Kramm and Dlugi were wrong with their energy balance equation shown in Kramm & Dlugi On Illuminating the Confusion of the Unclear:
SoD, I made the clear points that you did not answer:
– back-radiation cannot warm the surface to any temperature above the one of its source
– for isolation I gave you the example of the flask isolated to keep warm tea in it. There exists thermal isolation foil one-sided which leave IR pass through from one side but reflect the other one.
http://en.wikipedia.org/wiki/Space_blanket
It is only an example to make it clear that back-radiation cannot warm an object above the temperature of its source. This is unphysical and you know it. This is what I mean by temperature signal. The temperature signal is the frequency and this also you know.
– back-radiation makes sense only within Prevost heat exchange but cannot be taken away and averaged and computed on itself. This is the fallacy of the averaged energy budged.
– back-radiation from -75°C would heat the ground to the dangerous -75°C
– back-radiation from -10°C would heat the ground to -10°C
you cannot compare back-radiation to sun’s radiation which comes as net heat transfer, back-radiation is influencing the net heat transfer from the ground to the atmosphere.
Earth energy budget has several subsystems which interact, the oceans are the most important of them which you miss to address. It is there where 99% of heat accumulation is happening not in the atmosphere but this is a different story.
Lars P.,
You claimed it. You didn’t prove it.
I have proven my claim. You can see it spelt out in The Three Body Problem – a simple example with three bodies to demonstrate how a “with atmosphere” earth vs a “without atmosphere earth” will generate different equilibrium temperatures. This uses the first law of thermodynamics and demonstrates that the second law of thermodynamics has not been violated.
Because the sun and the atmosphere contribute to the temperature of the surface.
Feel free to explain what is wrong with this example, via actual equations of first law and second law. So far lots of unhappy people, but no one with any maths.
Alternatively, when you realize you can’t do that, why not cite a textbook or paper which supports your claim. Remember that the sun warms the earth and the atmosphere keeps it warmer than it would be without the atmosphere.
It’s a three body problem.
Textbook?
Paper?
Lars P.
Of course, no one disagrees with you if there are ONLY two bodies.
One hot, one cold. Like an earth and an atmosphere with no sun. They move towards a temperature between the two. This is the consequence of the second law of thermodynamics.
– What about THREE bodies?
So, find me a textbook, or a paper, or the consequence of a proven heat transfer equation which demonstrates that with three bodies that the energy from the coldest body cannot be absorbed, or cannot increase the internal energy of, the middle body.
You cannot not find such a textbook or paper.
If you read a textbook on radiative heat transfer then you will find that your claims are unsupported.
Prove me wrong. Produce your equation. Produce your text.
I know you can’t do this, so I expect you to continue claiming away. Join the happy crowd of people who make claims but can’t read a simple equation.
If you want to learn, have a read of Amazing Things we Find in Textbooks – The Real Second Law of Thermodynamics – pages from six heat transfer textbooks to confirm the ideas that you are disputing.
SoD – the very paper linked says it in conclusions:
http://www.scirp.org/journal/PaperInformation.aspx?paperID=9233
“1) the so-
called atmospheric greenhouse effect cannot be proved
by the statistical description of fortuitous weather events
that took place in past climate periods, 2) the description
by AMS and WMO has to be discarded because of
physical reasons, 3) energy-flux budgets for the Earth-
atmosphere system do not provide tangible evidence that
the atmospheric greenhouse effect does exist. Because of
this lack of tangible evidence it is time to acknowledge
that the atmospheric greenhouse effect and especially its
climatic impact are based on meritless conjectures.”
I find the paper a step forward.
I missed in my first review the ocean mentioned in Figure 12 in the paper – shame on me – nevertheless the paper is missing the analysis based on it and sea-ice part that I mentioned below which limits the radiation loss of the oceans – as they (the authors) focus is the atmosphere and thus no energy budget for the oceans.
The paper says something in conclusion that you like.
That same paper has an equation that you don’t like. An equation that proves you wrong.
You can’t provide an equation or a textbook or a paper which backs up your idea. And even on your idea – I have asked you for an equation but you are not able to provide one.
I know you believe your idea.
But your idea is not physics. You are confused.
If you would like to write down an equation for your idea we can make progress. I will wait for you to produce one.
How long do you think it will take?
SoD to your question: “Do you think Kramm and Dlugi were wrong with their energy balance equation shown in Kramm & Dlugi On Illuminating the Confusion of the Unclear”
Kramm and Dlugi had an interesting comment: “If it is possible to publish such a physically inadequate comment, we have to acknowledge that the discipline of climatology has lost its rational basis.” referring the Halpern et al paper that tries to falsify Gerlich and Tscheuschner.
Now on their own energy balance I find it odd that climatologists still live in a flat world model. Earth is not a flat world at 300.000.000 km from the sun, so yes, I think that results based on such model are wrong. The flat world model is not a rational basis to discuss Earth energy budget.
For simplification purposes one cannot ignore 71% of the surface (the oceans) and base its calculation only on the remaining.
Lars P.,
I think scientific understanding can only be clarified via specifics.
I asked a specific question about an equation.
Not “are the results of a flat world wrong?“, or any other question.
Please be specific and clear so myself and other readers have no doubt.
If you want to reply “yes it is wrong”, then I ask:
SoD I do not need to prove the model of the flat world is wrong, the supporters of the model should show it describes correctly the 3 dimensional world. It is a much too simplified model ignoring the real heat transfer in the actual world.
To take one member of Prevost heat exchange and base your calculations on it is also wrong.
The Earth energy balance is 3 bodies model by day and 2 bodies model by night. Where do you show that the 3 bodies model does correctly replace the 2 bodies model?
As said the oceans do not behave like solid rock. Rocks heat to 70-80°C when in the sun and radiate much more heat, cooling in the same night to below 0°C.
Oceans are warmed in depth by the solar radiation which penetrates to 200 m. This is why oceans do not warm at the surface to 70-80°C, the heat is distributed to the deep column of water.
Oceans radiate only at the surface. There is no IR inside the oceans as water is opaque to IR. Oceans act as a heat trap at the equator where the radiated heat is less then incoming heat.
Backradiation from the atmosphere does not penetrate the oceans it ends at the very surface.
The heat trapped by the oceans is slowly distributed to the north and south.
At the very north and south areas the oceans are covered with ice and thus do not radiate heat. Ice is a very bad heat conductor so almost no heat from the oceans escapes.
This is enough to demonstrate that ignoring the oceans is wrong, that calculating an averaged outgoing radiation for the oceans is wrong. This is where 99% of the energy stored from sun is on the earth. How can you say you make an energy budget without this? You only look at the fleas on the tail of the dog.
[…] the last few days, as at many times over the past two years, people have arrived on this blog to explain how […]
a**2=b**2+c**2 is a valid equation. Is it? Or not? Well it depends.
Equations are valid within a certain frame. The frame and the logic how we use these equations are relevant to their validity and to their results.
The equation that you posted did not contradict any of my arguments.
I also did not say I do not like the equation.
I pointed only to flaws in the logic.
At a certain time you say: “I know you believe your idea.”
I am not feeling particularly strong about these ideas. Ideas come and go. The way how we see and understand things evolve as far as we learn, am happy to learn.
Fundamentally I want to understand the logic what stays behind.
Now you may say: more blah blah against my equations. Well, if you see it as more blah blah, sorry for having posted, please disregard.
Any radiation from a cooler atmosphere heading for the surface (at some angle in practice) has absolutely no effect on the surface. It does not get converted to thermal energy and so cannot affect the rate of thermal energy leaving the surface. It is merely immediately radiated out again with the same frequency and intensity, never having been converted to thermal energy. How could it possibly affect radiation coming out at different angles from other molecules? When you shine two torches towards each other, but not directly – just so the beams cross – they have no effect on each other’s beams. This I suggest would be a close analogy if backradiation even exists – there are solid reasons why “measuring” techniques may not be measuring backradiation at all, but just making deductions about its intensity from temperatures calculated from frequencies. They don’t measure a warming effect.
The surface does not need to radiate at all to lose heat – it can do so by diffusion, conduction, convection, evaporation and chemical processes. The surface does not act like a blackbody because it is not surrounded by a vacuum or insulated from losses by these other means.
I suspect that most radiation actually starts in the atmosphere, not the surface. But then I also suspect that any backradiation is extremely small compared with upward radiation, because I do not believe radiation has an equal probability of going towards warmer areas than towards cooler areas due to the higher energy of molecules “blocking” it in the warmer direction. If there are numerous captures and re-emissions, then even a slightly higher probability than 50% will, in the limit, ensure the vast majority heads for cooler regions. There are no experiments to my knowledge which demonstrate backradiation warming something, or slowing its rate of cooling.
But whatever happens, the end result (if any gets to the surface) as far as energy and rates of cooling are concerned is just the same as if it had been reflected by a mirror. A mirror neither warms nor cools more slowly when it reflects IR radiation.- it, like the surface, is not affected at all because the radiating energy is never converted to thermal energy. You can only add and subtract like things such as thermal energy. Radiation does not cancel out other radiation as there are different angles involved for a start. The transfer of all thermal energy is in one direction, and the reason it only takes place in one direction is because only the cooler body “receives” it and converts it back to thermal energy.
Hopefully this will help all to understand why an atmospheric greenhouse effect resulting from radiation is a physical impossibility.
Valid Physics,
I look forward to you writing down the equations governing these processes in the new post:
“Blah blah blah” vs Equations
This was inspired by so many commenters writing such amazing words that might sound like physics to readers unfamiliar with textbooks on thermal radiation.
Valid Physics,
And yet this is not what is written down in radiative heat transfer textbooks.
Textbooks say unanimously that the emission of radiation from a surface is given by:
R = εσT4
Where ε = emissivity (a wavelength dependent value), &sigma = 5.67×10-8, T = temperature of surface in K
This means that the radiation emitted by a surface is a precisely defined function of temperature.
You are saying that emission of thermal radiation is also (or instead – who would know?) a function of the amount of incoming radiation from a colder body.
Perhaps:
R = εσT4 + Ea.f(T,Ta)
where Ea = atmospheric radiation and f is a function of the two temperatures such that f=0 if Ta>Ts, and f=1 if Ta<Ts
Amazing that no one thinks to put these equations in textbooks, or even write down the words that you have written.
I look forward to you writing down your confused ideas in an equation or two. This will be of assistance to other readers.
nitpick:
I would use the Planck equation, B(λ,T) rather than the SB equation. So
R=ε(λ)B(λ,T)
So the emissivity is a function of the wavelength and the Planck equation is a function of wavelength and temperature. Emissivity may be a function of direction of emission as well, but I’m less sure about that.
Valid Physics: To people familiar with modern physics, most of your statements sound something like: “If you lean against a wall, you exert a force on the wall, but the wall doesn’t push back.” Hopefully, you will recognize that the commonsense idea that walls can’t “push” anything, violates F = ma under these circumstance. (If your force isn’t balanced by some force from the wall, the wall must accelerate.) Thermodynamics plus commonsense is a recipe for total confusion when talking about molecules and photons. Quantum mechanics is essential.
“Valid Physics” wrote:
“Any radiation from a cooler atmosphere heading for the surface (at some angle in practice) has absolutely no effect on the surface. It does not get converted to thermal energy and so cannot affect the rate of thermal energy leaving the surface. It is merely immediately radiated out again with the same frequency and intensity, never having been converted to thermal energy.”
How does the surface know whether a particular arriving photon comes from the atmosphere or the sun? Does it wear a sign that tells the earth’s surface the temperature of the atmosphere or star from which it was emitted? (On the earth, there is enough separation in wavelength between DLR and SWR that we can make an educated guess where a photon came from. We can calculate something of this nature: 10 um, 98?% probability it came from the sun; 1 um 99.7?% probability from the sun. However, with a hotter planet or a cooler sun, no one would be able to say with any confidence where a particular photon came from, the emission spectrum from cooler and warmer sources overlap.
Your problem is even more ABSURD than described above, because temperature is the average kinetic energy of a group of molecules. The molecule that emits a photon must “talk” to all of its neighbors to learn their average kinetic energy before it can “label” its photon with the correct temperature. (Or the surface must do so before deciding to absorb the photon.) Remember the Boltzmann distribution: a molecule in a gas at a particular temperature can have a range of kinetic energies. Therefore, a single molecule by itself can have a kinetic energy, but we can’t assign a unique temperature to that molecule. All of this is heresy in the macroscopic world of thermodynamics, but real physicists abandoned that world more than a century ago, when they realized that molecules and photons didn’t obey the familiar laws of the macroscopic world. Whether or not you are comfortable with the no-deterministic nature of quantum mechanics, there is no doubt that it accurately predicts the behavior molecules and photons.
Therefore, it is impossible for a surface intercepting a photon to have any idea whether or not an approaching photon was emitted from a warmer or cooler source. There is some probability that a photon will be absorbed by the surface and some probability that it will be reflected/scattered. Those probabilities depend on: the molecules at the surface, the wavelength of the photon, and angle of approach; but NOT the temperature of the source that emitted the photon. Once absorbed, the energy of a photon is quickly distributed from the excited state of the absorbing molecule to its neighbors by collisions taking place quicker than a photon can be re-emitted. The absorbed energy loses its “identity” in general thermal motion (thermalized). I have argued elsewhere that the heat from enhanced DLR MAY be quickly returned to the atmosphere by convection without raising surface temperature appreciably, but the photons will NOT be reflected simply because they came from the a cooler location! The laws of thermodynamics and concepts such as temperature, warmer, cooler, and heat apply to large groups of molecules, NOT single molecules and photons – they obey the laws of quantum mechanics. Don’t confuse the macroscopic world of thermodynamics with the microscopic world of quantum mechanics (even when photons are traveling through kilometers of air).
“Valid Physics” also wrote:
“The surface does not act like a blackbody because it is not surrounded by a vacuum or insulated from losses by these other means.”
The surface doesn’t know what surrounds it: vacuum, air, liquid or another solid. It emits photons at an appropriate rate with a mixture of wavelengths obeying Planck’s Law AND the emissivity of that substance at each wavelength. Many solids and liquids emit nearly blackbody radiation, but gases do not.
“Valid Physics” also wrote:
“I also suspect that any backradiation is extremely small compared with upward radiation, because I do not believe radiation has an equal probability of going towards warmer areas than towards cooler areas due to the higher energy of molecules “blocking” it in the warmer direction.”
Molecules in the atmosphere don’t “know” where it is colder or hotter, they emit equally in all directions. Photons going opposite directions don’t usually interfere with each other: Pulses of light from two lasers can even travel through an optical fiber in different directions at the same time (and probably do every time you use the Internet).
Statistical mechanics has shown that large numbers of molecules obeying the laws of quantum mechanics will follow the laws of thermodynamics (which were devised before we understood molecules or quantum mechanics). The “Laws” of Thermodynamics can be derived for large groups of molecules from quantum mechanics, in much the same way that Kepler’s “Laws” of Planetary motion can be derived from Newton’s Laws of Gravity and Motion. The orbit of a comet moving around the sun will violate Keppler’s “Laws” if it gets too close to a planet, because Newton’s Laws are more fundamental. In the same way, quantum mechanics is more fundamental than thermodynamics. You can’t sensibly discuss the behavior of photons and individual molecules without it. It’s time to abandon the religion of 19th century physics and enter at least the first half of the 20th century. (Belief in string theory, dark matter, dark energy and the multiverse is not required.)
Frank said, truly:
“Molecules in the atmosphere don’t “know” where it is colder or hotter, they emit equally in all directions”.
Presumably the energy density of the emission varies inversely with the square of the distance from source. The radius of a molecule is calculated approximately at 10^-10 m. At a distance of one metre, energy density would be a quite small fraction of that at source. Only emissions from molecules very close to the surface would be candidates for absorption there.
John Millett, the radius of a molecule is irrelevant in this case, as the molecule is emitting a single packet of energy (a photon) in one random direction. The ‘energy density’ as you call it, will depend if you are in the way of that photon, or not. If you are in the way of that photon, the energy density is essentially identical to that of emission, if you are not in the way, you won’t notice it.
How did you think astronomers see extraordinarily distant galaxies, quasars or supernovae (heck, even nearby stars)? Not many photons arrive from the sources, but each photon was emitted by a single atom, sometimes billions of light-years away. The photon may be redshifted if it’s come a really long way, but otherwise arrives safely and be ‘absorbed’ at the astronomer’s CCD detector, despite coming from a source with a radius “approximately 10^-10 m”. Why do you think the molecules in the atmosphere behave any differently to those from a diffuse nebula or dust (colder) or from stars (hotter), that somehow succeed in making the journey?
Thank you for that. Not up with astronomy. Scienceofdoom drilled into me a little while back that solar flux density diminishes with distance. At the sun’s photosphere it is 6.4*10^7 Wm-2. Here on earth it is 1367 Wm-2. The reducing factor is the square of the ratio {sun’s radius/earth’s distance from the centre of the sun}. My question is: Why wouldn’t the same thing happen to radiation from an atmospheric molecule?
John Millet,
If you look at a single photon emission from a molecule, there is no decrease of the photon energy with distance, presuming the observer is at rest with respect to the emitting molecule. It’s only when you look at a flux from lots of molecules in a given isolated volume, say a sphere that the observed intensity diminishes as 1/r².
[moderator’s note – retrieved from the spam queue]
DeWitt Payne,
Are you saying: The inverse square law of radiation intensity reduction, as it applies to molecular radiation, varies directly with molecular density/concentration? Therefore, because radiatively-active molecules exist in the atmosphere in such insignificant proportions, the law doesn’t apply?
I see things differently: radiation intensity, with dimensions Wm-2, may be expressed {number of photons*energy per photon} per square metre. While the inverse square law doesn’t apply to energy per photon, it does to the number of photons and, hence, to intensity.
Incidentally, several attempts yesterday to post a comment to you on Blah blah failed. I’ll try again today.
Not at all. When you’re inside the atmosphere, there is no effective point source from which to measure distance. You don’t have a single lamp at a variable distance. The sources are all around you. If you move horizontally, there is no change in radiation as long as there is no temperature gradient. In absence of an atmosphere, even vertical movement close to the surface does not follow the inverse square law exactly because as you go up, you see more of the surface. Gravity above the surface follows an inverse square law because you can model the field as if all the mass were concentrated at a point. But if you go below the surface of the Earth, that no longer holds true. You have mass above as well as below. Something similar is true for molecular radiation inside the atmosphere.
DeWitt Payne,
Since you introduced gravity into the analysis, I would have thought that the forces on an element of rock below the surface would be of the same type as those on a parcel of air above it – gravity downwards and pressure differential upward, ignoring vertical shear.
“You don’t have a single lamp at a variable distance. The sources are all around you”.
True, an observation point within the atmosphere would be surrounded by a 3-D universe of radiating molecules, each separated from its nearest neighbour by about a thousand molecular diameters. Why would the observer, of molecular scale, sense the same intensity of radiation from the nearest molecule as from the farthest one? I suppose an answer might go along the lines that the observer wouldn’t be able to sense radiation from a discrete molecule at a particular distance; only universal radiation (akin to the CMB?). But the observer’s inability to distinguish different intensities from near or far radiators doesn’t rule such differences out.
My understanding of the relevant physical principles is: molecular radiation results from periodic acceleration of electrons in the atoms of an oscillating molecule; it is non-directional leaving the molecule; and it propagates on an expanding spherical surface. If this be true, the intensity must reduce as the surface area increases. For intensity to remain constant, wouldn’t radiation leaving the molecule have to be directional?
John Millett, you keep treating a single molecule as if it were the only source of thousands of photons of radiation, whose intensity declines with the inverse square of distance. Then you could consider and expanding sphere of influence centred on the molecule. Except your single molecule is not the only source, and your single molecule is the source of a single photon. There is no sphere of interactions, just interaction in one direction, that which the photon is emitted. You have a whole atmosphere worth of sources, and the intensity declines as you move significantly far from Planet Earth, not from the individual molecules.
Each molecule, at any one time, is responsible for a emission of no more than a single photon, in a single direction. If that photon is directed towards you, its intensity is essentially equivalent to that at the time of emission. The photons do not get “”tired” on their jorney from source to destination; by the same principle we can see stars and galaxies that are very, very far away. Not many photons in that galaxy get emitted in exactly the right direction to intersect with Earth, but those that do are not notably weaker than when they departed. The image of the galaxy is faint, not because the photons are weaker, but because few got sent in the right direction. However, if that photon from the single molecule was emitted in a direction away from you, its intensity is zero, zip, squat, whether you are 1mm from the molecule, or whether you are 1 million light-years from the molecule.
Hence you cannot use the radius of a molecule when considering the inverse square law, but you must use the whole atmosphere.
DeWitt Payne,
Nope, failed again today, both on Blah Blah and here.
Skywatcher,
We seem to be in agreement:
You wrote: The image of the galaxy is faint, not because the photons are weaker, but because few got sent in the right direction.
I wrote: radiation intensity, with dimensions Wm-2, may be expressed {number of photons*energy per photon} per square metre. While the inverse square law doesn’t apply to energy per photon, it does to the number of photons and, hence, to intensity.
John Millet, nope, we’re not in agreement. You said:
“The radius of a molecule is calculated approximately at 10^-10 m. At a distance of one metre, energy density would be a quite small fraction of that at source. Only emissions from molecules very close to the surface would be candidates for absorption there.”
This is incorrect. You cannot use a single molecule for that calculation, or else no radiation from anything would get anywhere in the Universe, according to your mangling of the inverse square law. One photon comes from the single molecule, either you see it or you don’t, you don’t apply the inverse square law to it!
John Millett,
Probably pointless to try.. but here goes:
Take a look at this picture from Wikipedia on the inverse square law
Have a read of the wikipedia article.
As other commenters have already pointed out, if you were correct then no radiation could ever be measured.
Once someone was 1m away from an emitting surface the reduction in intensity would be [10-10]2 = 10-20.
But this is not the case. This is why textbooks on physics explain the inverse square law completely differently from you.
Your enthusiasm and determination to make everyone see things your way are inspiring but sadly massively outweigh your competence in this field.
My only comment on this subject.
SOD,
Wikipedia’s graphic is accompanied by the following text:
“The lines represent the flux emanating from the source. The total number of flux lines depends on the strength of the source and is constant with increasing distance. A greater density of flux lines (lines per unit area) means a stronger field. The density of flux lines is inversely proportional to the square of the distance from the source because the surface area of a sphere increases with the square of the radius. Thus the strength of the field is inversely proportional to the square of the distance from the source”
All I have done is equate “r” to the radius of the radiating molecule in the same way you do in respect of the sun when explaining the absence of wavelengths less than 4 microns at earth’s distance from it.
If this is prohibited, it would be good to know why.
John Millett
“The radius of a molecule is calculated approximately at 10^-10 m. At a distance of one metre, energy density would be a quite small fraction of that at source. ”
It’s probably OK to think of this as true for every individual model.
However, the atmosphere is not *one* molecule, it’s many.
Think of a black wall, extending infinitely in all directions.
Put a single light bulb on it – analogous to your single molecule.
Now, as you move back from the wall, the light intensity falls as the square of your distance from it.
Now, put an infinite number of light bulbs, equally spaced at (say) 1m intervals on the infinite wall.
There is NO difference in light intensity as you move back from the wall.
However, the intensity from any SINGLE bulb DOES still decrease as the square of distance from that single bulb.
This is because the emissions from each individual bulb overlap – and the geometry means you see more from more distant bulbs as you step back from the wall.
Does this help?
VeryTallGuy,
I thank you for making the effort to help with my “problem”. But I don’t think many light sources in combination can overcome the reduced intensity with distance each experiences. I can read a book by the light of a 100W bulb one metre away; but not from one 100 metres away; nor from 100 bulbs at that distance.
As SOD says, locate the radiating surface – a sphere with radius centred on the radiating entity – and then apply the inverse square rule. The sun’s radius is ill-defined, but it is taken to be the radius of its radiating surface which is well defined. The question is where is the radiating surface of a vibrating CO2 molecule in the atmosphere. Is it the smallest sphere containing the molecule? That would give the well-remarked absurd result. So, it must be at some multiple of the molecular radius; but what multiple and why?
Or is the result as absurd as it appears to be? Could it be that these individual radiators diffuse energy into the surrounding electromagnetic field over a distance which is short to a human observer but not so short relative to the radiating molecule?
John,
well, I tried with one analogy which hasn’t helped you.
Try and have another think about it – if the wall is *infinite* then there is an absolutely constant flux from it regardless of distance of the observer.
In the same way, the atmosphere completely surrounds the surface. Moving further away makes essentially no difference to the received flux (as long as the depth of the atmosphere remains small cf the diameter of the earth)
All of this is totally independent of a notional radius of a molecule.
If you really want to think of a molecule as a very small sphere emitting radiation, then you could get that to work. But note that it doesn’t matter what size you set the sphere to be (as it’s not a physical reality anyway) – you simply need to set the flux at the boundary of the sphere to be the appropriate level.
But note that to simulate the greenhouse effect you’d have to consider the effect of many trillions of molecules acting together in a gas. This summation could be presented in propoerties of the bulk gas such as the optical depth.
It would, of course be much easier to start with these already known properties.
In all honesty, you appear absolutely committed to a view that the greenhouse effect doesn’t exist, so I doubt I’ve been able to help your (mis)understanding.
But good luck building that understanding, and please, try a textbook.
John Millett,
“All I have done..” is ignore the theory completely. And randomly associate another variable in place of the one used in the original theory.
The flux from a surface is needed. The units of flux are W/m2.
Once you calculate, or measure, flux then you can use the inverse square law.
The simplest way to think about it – the “Inverse Square Law” lecture 101 – is a small sphere of radius r, emitting a flux f.
Then at a radius of r’, the flux f’ incident on a body at this radius will be:
f’ = f.(r/r’)2
It is simple to see geometrically. In fact, it is proven geometrically.
Take a sphere with r=1m emitting a total energy E per second. The flux, f = E/A = E/(4πr2).
Now picture a sphere with r’=10m encompassing the 1m sphere.
All of the energy E must be incident on the 10m sphere. (Conservation of energy).
So the total energy per second incident = E.
And f’ = E/A’ = E/(4πr’2).
So E = fA = f’A’
So f’/f = (r/r’)2.
Therefore f’/f = (1/10)2 = 1/100.
The flux incident on the 10m sphere is 1/100th of the flux emitted from the 1m sphere.
Super basic. Why am I explaining this ??
The photon from a molecule is.. not a flux. It’s energy is in units of Joules.
Find the flux in W/m2 from a surface and then you can use the inverse square law. You can’t take the radius of the molecule that emits the photon and insert it into the equation. And you can’t take your shoe size and insert it into the equation.
I will probably ignore your further questions on this topic.
And so the radius in question has to be the radius of the body emitting the flux.
If you have a surface with radius = 1m but each molecule has a radius of 10-10m what are you to do?
The crazy unenlightened physics books suggest that the correct value = 1m and never entertain the idea that it should be 10-10m.
(Flat surfaces come with more challenging geometrical calculations).
SOD,
Believe me, I understand everything you have written – from a previous article.
“The photon from a molecule is.. not a flux. It’s energy is in units of Joules.
Find the flux in W/m2 from a surface and then you can use the inverse square law.”
Skywatcher introduced the photon into the conversation. My response (to DeWitt Payne) was:
“radiation intensity, with dimensions Wm-2, may be expressed {number of photons*energy per photon} per square metre. While the inverse square law doesn’t apply to energy per photon, it does to the number of photons and, hence, to intensity.”
Is there anything wrong with that?
VeryTallGuy,
A probing mind is a teacher’s delight (Confucius, or maybe not). Today’s probe is: Which physical principle transforms a directionless electromagnetic field, reducing in intensity with distance from source, into a one-directional constant-intensity one?
If an EM field has a single source, it isn’t directionless. Don’t confuse a photon flux with an EM field. An EM field is generated by charges or magnets or both. In fact, special relativity is implied directly by Maxwell’s equations. The field an observer sees depends on the relative motion of the observer and the field. If the observer is at rest with respect to a point charge, only an electric field is observed. If there is relative motion between the observer and the charge, an EM field is observed.
As to the number of light bulbs at 100m, It wouldn’t be 100, it would be 100,000 or more. Assume an infinite plane with 100W bulbs spaced at 1m intervals horizontally and vertically. No matter how far you get from the plane, the light intensity you observe will be constant. Strictly speaking, the plane should have a uniform flux density rather than a bunch of point sources or the distance from the plane should be large compared to the spacing between the sources. With a 1m spacing, the intensity close to the plane would vary somewhat with position.
Precisely
Thank you, both.
“Don’t confuse a photon flux with an EM field”.
I’ll rephrase the question: Which physical principle transforms a directionless photon flux, reducing in intensity with distance from source, into a one-directional constant-intensity one?
It appears that the principle is firstly and secondly geometric approximation: firstly, because the vertical range of the moecular radiators is small compared to the horizontal range, the radiators are assumed to be located on a single horizontal plane; and, secondly, because of the large number of radiators they are assumed to coalesce into a radiating plane surface. Enter, physics. A plane radiating surface generates a constant-intensity, one-directional flux perpendicular to the plane, as per S-B.
If this is how the transformation works, it’s a bit of a stretch IMO. Has it ever been validated experimentally?
Once again, if there’s a single source, the flux cannot be directionless. But inside the atmosphere one is surrounded by a very large number of sources. Even then, though, the flux may not be directionless. The intensity of the flux could well vary with direction. It certainly does at the surface of the Earth, especially if the sun is above the horizon. But traveling horizontally, the total flux won’t vary much and it certainly won’t decrease as the inverse square of the distance from the starting point. Because the Earth’s orbit isn’t perfectly circular, the solar flux varies seasonally as the inverse square of the distance from the sun. But the sun is a distant single source.
Semantics must be tripping me up. Directional and directionless I take to have opposite meanings. If a flux from a single source cannot be directionless, it must be directional. But which direction? and how would the molecule know to choose that direction?
The molecule doesn’t choose a direction. Why do you think it would need to? A single molecule emits in a random direction. Lots of molecules means lots of different directions amounting to a uniform flux which, seen from a distance, decreases with the inverse square of the distance.
There is definately a lot to learn about this subject. I really like all
of the points you made.
Bryan,
Carried here from “Temperature profile…” at SOD’s request.
“What the second law states is that HEAT cannot flow spontaneously from a lower to a higher temperature. In the case of a purely radiative exchange, the HEAT would be the net radiative flux”.
In the point at issue – increasing CO2 leading to a warmer surface via increased down-welling radiation from the atmosphere (aka back-radiation) – the net radiative flux (aka HEAT) must be into the surface. That is, back-radiation is HEAT flowing spontaneously from a lower to a higher temperature, violating 2LoT. The continuing confusion rests ultimately in the theoretical conflict between 2LoT which prohibits back-radiation and the theory of radiative exchange which mandates it. Terminology is a contributing factor.
My question: When and by whom has Prevost’s 1792 theory of radiative exchanges been demonstrated experimentally?
Bryan,
Carried here from “Temperature profile…” at SOD’s request.
“Three objects separated by a vacuum can ‘see’ each other.
Object A is at 320K
Object B is at 300K
Object C is at 280K
All three objects will emit 10um photons as part of their BB spectrum.
All would agree that object B will absorb a 10um photon from A.
However a 10um photon from C is identical in all respects.
Photons are also Bosons so it is impossible to differentiate them.
So by logic if B absorbs a 10um photon from A it must absorb one from C”
Neat? Certainly. Does it give sufficient wriggle room to IPCC v 2LoT? I think not.
Your model is an enclosure – with walls at temperature T which fill the enclosure with radiant energy at a density consistent with T. Each of the three objects interacts directly and independently with the enclosed radiant energy stock, importing from it if cooler, exporting to it if warmer. If T = 300K, objects B and C’s 10um photons won’t be emitted; C will absorb one from the surrounding energy soup while A will emit one into the soup; and there will be no interaction between B and the soup.
While this description is in accord with 2LoT theory, it violates the theory of radiative exchanges which requires (under the above formulation) two-way exchange between each body and the surrounding energy soup and (under Bryan’s) among the three bodies each “seen” by the others.
My question: When and by whom has Prevost’s 1792 theory of radiative exchanges been demonstrated experimentally?
John Millett
“My question: When and by whom has Prevost’s 1792 theory of radiative exchanges been demonstrated experimentally?”
I don’t think that there is a definitive experiment.
It is just the most reasonable explanation of radiative transfer between objects given our present knowledge.
www2.ups.edu/faculty/jcevans/Pictet’s%20experiment.pdf
Bryan,
Thanks for the reference.
Pierre Prevost was of a restless nature, abandoning theology and law – the subjects of his early training in Geneva – to publish, in Paris, his translation of the tragedies of Euripides. Translations of the British political economists followed accompanied by publication of original research in the subject from his post as chair in philosophy in the Academy of Berlin. Back in Paris, collaboration in the translation and publication of the Greek classics followed. Returning to Geneva, Prevost turned his attention to the physical sciences, first to the origins of magnetic forces, then to Pictet’s experiment. In the first of two extracts from the paper (which regrettably I lack the skill to show here) Pictet clearly explains that his eperiment was a proof of the radiation and reflection of heat, not “cold”.
In the second excerpt, the authors display a faulty short memory and perhaps a little bias in favour of Prevost – an analytical blind spot. As shown in the first excerpt, which in their paper precedes the second one, Pictet’s explanation was of the reflection of heat, not “cold”. However, the authors respond to the strawman explanation that it was all about radiation and reflection of “cold”
The blind spot led to a fatal internal contradiction: “by the mechanism of the double reflection, the thermometer is more cooled than its mirror”………”Hence….fire cannot pass from the thermometer to its mirror…..and thence to the ice”. Clearly, to Prevost (as interpreted by the authors) “double reflection” referred to “cold”, a straw man’s representation of Pictet’s explanation.
As well, there is in my mind a question mark over Prevost’s assertion that the thermometer would be more cooled than its mirror and therefore unable to double-reflect “fire” to the ice.
Wouldn’t the mirror focus be a local “hot spot”?
The principal difference between Pictet and Prevost centred on Pictet’s concept of thermometric equilibrium in which the “fire” in each body, being of equal “tension”, cancelled each other, preventing flow between them. Prevost argued that “discrete fluids” cannot be stayed in this way and that the equilibrium is dynamic, not static – each receiving from the other as much “fire” as it sends to the other. Prevost doesn’t consider the situation at the boundary between the discrete fluids flux and the solid surfaces that give rise to it. Consequently, he doesn’t wholly rule out the viability of Pictet’s concept.
The boundary could be modeled as a membrane. Consider a flat “U” tube with provision for membranes at each end of the horizontal section. Fill the tube with “discrete fluid”, the levels in the vertical sections coming to a common level. Insert the membranes. There is no flow across them, equal numbers of the constituents of the fluid impacting it on either side. Add fluid to one vertical section. The impact on the membrane now being greater on one side than the other, fluid flows across it. At the other membrane, the same condition applies and fluid flows across it in the same direction.
The property called “pressure” implicit in this description was shown to be a property of radiation around 1900, though Kepler, observing the way comet tails bent away from the sun, had speculated about it 300 years earlier. First Lebedew and then Nichols and Hull showed that the pressure exerted by radiation on anything in its path is equal to its energy density.
Giving a modern explanation of Pictet’s experiment, the authors begin by accepting Prevost’s theory of exchanges (more correctly an unproven/unprovable hypothesis of exchanges?). Therefore, the thermometer receives radiation from the room and radiates to the room equally at equilibrium. Introducing the mirror displaces part of the room’s radiation to the thermometer and substitutes the higher or lower intensity radiation from the accompanying hot or cold body, respectively. Accordingly, the thermometer reading either rises or falls.
The 2LoT explanation would begin by noting that the terms “hot” and “cold” have meaning only in a relative sense – something is hotter or colder than something else, usually the environment or surroundings. Heat flows from hot bodies to the environment and from the environment to cold bodies. The introduction of ice creates a cold region into which heat flows from surrounding regions including from the thermometer which accordingly registers a fall in temperature; and vice versa for the introduction of boiling water.
A close reading of the paper leaves me with the uneasy feeling that the modern radiation transfer theorists have chosen, unlike Newton, to stand on somewhat scientifically diminutive shoulders, eschewing thermodynamic giants.
[…] The Amazing Case of “Back Radiation” – Part Three and Part One and Part Two […]
[…] Quote: Originally Posted by gslack Quote: Originally Posted by flacaltenn Quote: Originally Posted by PMZ Two bodies in a vacuum. One radiates energy. The other is passive. When the passive body reflects as much as it absorbs, it remains at constant temperature. If its reflectance lowers, it must, must, must move to a higher temperature in order to achieve and maintain energy balance. There are simply no other possibilities. Everything else is about the details of the process to restore balance. Greenhouse gas concentration in our atmosphere have the affect of lowering our reflectance. Pretty severely mangled, but better than the combative crap that I've seen on this thread so far.. My gosh folks — its not that hard once you realize there are SEVERAL different text books required here. The laws of EM propagation have NOTHING to do with laws of Thermodynamics. EM energy in the form of light or IR or UV CAN AND DO propagate from cooler to hotter objects. I can't imagine why Polar and SSDD want to deny the role of GHGases in warming the planet. And I abhor your inference that religious people can't comprehend or practice science. It's actually quite simple and most of the errors I've seen here is because of the confusion between EM radiation and heating. They follow different rules. And YOU are only partly right about CO2 "lowering our reflectance". It is not a great reflector reflector of the INCIDENT sunlight which comes into the surface in a broad band of wavelengths, but it does ABSORB the longer wave IR radiation that is generated by the Black Body effect of the earth's surface. Not as good as dominating water vapor, and it does saturate in its ability to convert long wave IR to heat, but nonetheless, this conversion of long wave to heat in the troposphere DOES heat the troposphere. EVEN IF the troposphere is cooler than the surface. I could heat hamburgers with IR thru a vacuum tube with no Thermodynamics involved in the transfer. Just as the Sun heats the earth thru the cold vacuum of space. Furthermore, I COULD lower the emitter temperature of the IR heater to near Zero and still use it efficiently to heat burgers at a distance. You are just spoiled because most EM emitters "self-heat" and end up being quite hot because of the materials involved and the inefficiencies of converting electrical power in EM radiation.. But it's not REQUIRED that an EM emission source be "warmer than the impinged surface" in order to contribute to thermal energy in that material.. No science at all says that… I could blast nitrogen with long wave IR all night long and not raise it's temperature. But because of the absorption bands in GHGases, it will HEAT if radiated at the earth's Black Body frequencies. That's the GreenHouse. It's NOT a material like glass that's preventing convection or conduction heating. That's a disservice in the naming of the effect. It's a change in the THERMAL RESISTANCE of that thin layer of atmosphere caused by the mater4ial composition of that layer. All the rest you need to know comes from the Thermo book which defines that the AMOUNT of heat energy flow is proportional to the Temp diffs between the surfaces. If you raise a thermal barrier ANYWHERE in the trop. , then the heat transfer to space will slow down the THERMAL energy flow towards space. ((Thus the confusion about detecting a cooler or warmer Stratosphere in the presence of warming. It's not clear that a couple degree barrier in the Trop will have a distinct and detectable fingerprint farther up because its too small and the heat paths and mixing are too complex)) That's it.. and there are good and valid reasons to discount the hysteria about CO2 forced heating of the earth surface. But in THEORY, and in real life, it DOES what the "GreenHouse" theory says it does. Only not as a prime driver of the climate as the wacky believers declare… THIMK a little about the diff between your Field and Wave class and your Thermo class and then we'll all be on a better track here.. The issue isn't if GH gases can react to long wave IR, and shed some of that heat back out. The problem is whether or not that IR radiated from the GH Gases can warm the already warmer surface, its source. The second laws states no, but the warmers seem to think it can anyway without violating the 2nd law, or with bending it somehow depending on who you ask. I suspect this is way above your pay grade, but here is why "the warmers" are correct. The Amazing Case of ?Back Radiation? ? Part Three | The Science of Doom […]
“Greenhouse gas concentration in our atmosphere have the affect of lowering our reflectance. Pretty severely mangled, but better than the combative crap that I’ve seen on this thread so far.. My gosh folks — its not that hard once you realize there are SEVERAL different text books required here. ”
And you missed the most important book. The one that teach the difference between reflecting and absorbing. You know, absorption increase with lowered temperature, and since co2 is claimed to increase absorption of heat which is confirmed by experiments and observation, it is a sign of lower temperature. Increased emission is a sign of rising temperature.
I think many people make the assumption that absorption equal rising temperature. It doesn´t. Absorption depend on temperature.
In any of our everyday situations where we add more of a heat absorber to a volume at a certain temperature, has anyone of you seen the temperature increase as the same energy is absorbed by more mass absorbing the heat?
“There’s no alternative – energy can’t be absorbed and just disappear. However, as a technical note, energy can be absorbed into chemical bonds or phase changes of materials. So you can put heat into ice without changing the temperature, while the ice turns into water. Of course, energy is still not lost..”
Then the vacuum of space must heat earth like hell. It absorbs the radiation from earth, and emits energy from an infinite spherical surrounding (infinite energy at 3K) ever more concentrating as it approach the surface. Or, not.
“Therefore, if your current belief is that radiation from a colder atmosphere cannot “change the temperature” of the hotter surface then you have to believe that all of the radiation from the atmosphere is reflected”
No, we have to believe nothing when there is a proven theory of thermal energy and heat transfer. The “net” transfer of energy is the energy that is called heat. Only the “net”. If the rate of transfer is negative, no heat is transferred. If you want to believe in energy that is transferred outside of “net”, that is fine, and it might be true. But the energy that is not included in “net”, is not heat. And energy that is not heat, isn´t involved in rising temperatures. So the term “net” is perfectly useless in the same way that energy that is not “net” is useless for construction of a theory of earth surface temperature.
“Notice that there is no dependence on the temperature of the source. Think of individual photons as anonymous – a 10μm photon from a 2,000K source has exactly the same energy as a 10μm photon from a 200K source.”
The surface of earth emits a photon in the visual range, the same wavelengths as the sun, every 41 second. That doesn´t mean that it is hot like the sun.
There are good reasons to not include photons that are a quantum phenomenon into calculating bulk properties of mass like temperature. Photon counting is not the way to understand temperature in a radiating body.
“No dependence on temperature”, then why does all solids start to glow at the same temperature?
It seems that in this discussion the atmosphere is considered to be a black body. Is it?
Dilettante: The atmosphere is not a blackbody. The spectrum of LWR leaving the Earth for space is not appropriate for a blackbody at one particular temperature. Nor is the spectrum of LWR arriving at the surface (DLR). At some wavelengths and some altitudes the atmosphere behaves as if it were a blackbody and at others it does not. Unfortunately, temperature varies with altitude and therefore the amount of radiation one would expect a blackbody to emit varies with altitude. I recently discussed this at the links below.
It is more useful to think of the atmosphere are something that MODIFIES the radiation traveling through it by absorption and emission. OLR starts as blackbody radiation emitted by the surface and is modified by absorption by and emission from GHGs in the atmosphere. In general, there is more absorption than emission because the atmosphere gets colder with increasing altitude. DLR starts at the top of the atmosphere with effectively zero intensity and grows in intensity at first because there is negligible intensity to absorb and some emission. On the way to the surface, there is more emission than absorption because it is getting warmer. Radiation travels in all directions, but we usually ignore the horizontal components because they doesn’t warm or cool the planet and the net flux in the horizontal directions cancel. This is called the two-stream approximation.
So what you’re saying is that if the ground is frozen and colder air at -10C wafts over it, the ground will warm up almost as much as it warmer air at +10C wafts over it?
Seriously? Do you even believe this yourselves?
Cite please. That’s not my reading of this article at all. I haven’t a clue as to how you reached your conclusion.
The article says:
“If a 0°C surface can absorb radiation from 10°C radiation, it must be able to absorb radiation from -10°C radiation. And yet this would violate the imaginary second law of thermodynamics…DLR is emitted by the atmosphere, reaches the surface and is absorbed by the surface. This absorption of energy changes the surface temperature.”
Sure, two things absorb radiation from each other. But the warmer one will cool down and the cooler one will warm up. The article says “changes the temperature”, implying that the -10C air will warm up 0 C ground.
Mark: The question you pose is fundamentally absurd, because it is based on a gross misconception about what controls temperature.
This post and I are saying that the molecules in the surface emit thermal infrared photons upward and absorb or reflect photons arriving at the surface. Since the surface emits like a graybody with an emissivity near 1, we can say its emission also rises with temperature and obeys Planck’s Law and the S-B equation and their is little reflection. Needless to say, the ground has no idea what the temperature is in the air above.
Likewise, molecules in the atmosphere have no idea of what the temperature of the surface of the Earth or even what direction it is. Those molecules follow the laws of quantum mechanics and emit thermal infrared photons in all directions and absorb at the same wavelengths they emit. They emit more photons as their temperature gets warmer because the excited states that emit photons are almost always created by molecular collisions (not absorption of a photon.) However, molecules in the atmosphere don’t have the appropriate excited states needed to emit photons of all wavelengths – as blackbodies do. So their emission per molecule depends on Planck’s function and a cross-section that applies to both emission and absorption. From a practical point of view, N2, O2 and Ar don’t emit or absorb thermal infrared photons; only GHGs do.
******** The temperature of the surface CHANGES depending on whether it RECEIVES or LOSES more power (energy per unit time per unit surface area) from ALL sources and by all mechanisms. If incoming and outgoing fluxes are in balance, then the temperature doesn’t change. ************
THEREFORE IT IS ABSURD TO DISCUSS HOW OR WHETHER ONE OBJECT CONTROLS THE TEMPERATURE OF ANOTHER.
The same is true for the temperature of the atmosphere (which we often divide into layers with different temperature).
These same physics principles apply everywhere, not just to climate.
We can correctly say that the presence of GHG’s in the Earth’s atmosphere makes both the atmosphere and the surface warmer than they would be otherwise – because GHGs slow down the rate of radiative cooling to space. However, this GHE effect wouldn’t exist if the temperature of the atmosphere remained constant with altitude. The GHE is more complicated than just absorption and emission.
The main mechanisms of energy/power transfer are radiation (absorption and emission of LWR and SWR), convection (within a fluid), conduction (molecular collisions between the surface and the atmosphere) and phase change (latent heat of evaporation from surface water evaporation).
Since emission of thermal IR increases with temperature, there is often a temperature where incoming and outgoing fluxes are in balance and temperature is [temporarily] stable. Temperature CHANGE is the net result of all energy fluxes and so is TEMPERATURE itself – when the net of all fluxes is zero.
The laws of quantum mechanics “conspire” so that the NET flux of thermal radiation between any two objects is always from hot to cold.
This “conspiracy” arises because the absorptivity and emissivity are equal at all wavelengths. (Kirckhoff’s Law.) If CO2 in the atmosphere absorbs a lot of 667 um radiation from the ground it also emits a lot of 667 um radiation, some of which reaches the ground. When the ground emits at 1000 um, a wavelength the atmosphere doesn’t absorb very well, the atmosphere emits little thermal IR at that wavelength too.
In the case of radiation, the 2LoT is a consequence of molecules obeying the laws of quantum mechanics. Individual molecules and photons don’t even obey the 2LoT, because individual molecules don’t have a temperature (though they do have kinetic energy that changes with every collision, about 10^9 times per second). Temperature is proportional to the mean kinetic energy of a large group of rapidly colliding molecules and remains stable unless energy enters r leaves. Heat is the NET energy flux between two large groups of rapidly colliding molecules.
My favorite climate skeptic cartoon has a thermometer sitting next to a block of ice with the caption: Don’t tell me the ice makes the temperature warmer! I laughed suggested the cartoonist should have put the thermometer in an igloo! The host agreed. Then I told him to move the igloo to interstellar space, where the temperature is 3 K, and asked him if the igloo at 0 degC made the thermometer warmer? After the host failed to deal with this dilemma, he deleted my comment!
Frank “GHGs slow down the rate of radiative cooling to space. However, this GHE effect wouldn’t exist if the temperature of the atmosphere remained constant with altitude. The GHE is more complicated than just absorption and emission”
Ah yes, but that is the more sophisticated “top of atmosphere” theory as explained at length by Nullius in the earlier comments i.e. the effective emitting layer moves to a higher altitude etc.
(Somewhere on this site is an article saying that “back radiation” is a red herring and the real explanation is the “top of atmosphere” effect, so can you make up your minds please?)
That’s a nice theory but fails on the facts because the temperature at the tropopause has been falling, not increasing. This is because of ozone depletion, which is a far more plausible candidate for explaining GW since 1980.
Mark replied: Ah yes, but that is the more sophisticated “top of atmosphere” theory as explained at length by Nullius in the earlier comments i.e. the effective emitting layer moves to a higher altitude etc.
(Somewhere on this site is an article saying that “back radiation” is a red herring and the real explanation is the “top of atmosphere” effect, so can you make up your minds please?)
You are confusing several topics. Elsewhere I have discussed why it is simplest to consider the steady-state balance or imbalance at the TOA. An imbalance at the TOA is created by rising GHGs slowing down the rate of radiative cooling to space. DLR is an internal radiation flux that moves heat WITHIN our climate system and therefore isn’t important to the radiative imbalance at the TOA. A “rising effective emitting layer is important to the balance at the TOA.
Since AGW is a very complicated subject, it is often explained with a variety of models paradigms including: Increasing DLR “warms” the surface, CO2 “traps” heat, CO2 acts as a blanket or insulation, GHG’s act as shell or layer around the Earth (Willis promotes a “Steel Greenhouse” Model) and a “rising effective emitting layer” model (promoted here and by Linden among others). IMO – and others probably do disagree – all of these models are flawed. When I first came here (on the advice of Steve McIntyre), I was continuously frustrated and intemperate, because I KNEW that doubling CO2 would double the number of photons emitted by CO2 and usually halve the distance they traveled between emission and absorption. I couldn’t see why doubling CO2 would change anything – which turns out to be an excellent first approximation to the truth. The reduction in radiative cooling space from 2XCO2 is barely more than a 1% change despite doubling the emission of photons by CO2. (As best I can tell, the rising effective emitting layer model doesn’t take into account the doubled emission of photons by doubled CO2.)
My confusion and anger ended when our host stopped talking about models and paradigms for AGW and showed me the real physics: Schwarzschild’s equation for radiation transfer. This is the equation that climate scientist use to predict that a doubling of CO2 will slow radiative cooling to space by about 3.5 W/m2. Unfortunately, this equation is a differential equation that must be numerically integrated over the path radiation takes, and therefore may or may not be meaningful to you. The incremental change in radiation intensity (dI) at a given wavelength (lambda) as incoming radiation of intensity I passes an incremental distance through an absorbing/emittting gas is:
dI = n*o*[B(lambda,T)]*ds – n*o*I*ds
dI = n*o*[B(lambda,T) – I]*ds
where n is the density of absorbing/emitting molecules, o is their cross-section at the wavelength of interest, and B(lambda,T) is Planck’s function for the local temperature T. In the top version of the equation, the term on the left is the emission that GHGs in the layer add to the incoming radiation I. The term on the right is the absorption subtracted from I. In the bottom version of the equation, the sign of the intensity change dI on passing through the layer depends on B(lambda,T) – I. Photons traveling upward through the atmosphere almost always have been emitted where the temperature is warmer, so B(lambda,T) – I is usually negative. When we integrate along a path from the surface to space to calculate OLR crossing the TOA, the sum of all of those negative dI terms reduces upward radiation from an average of 390 W/m2 at the surface to 240 W/m2 at the TOA. And if you increase the density of GHG (n) along the path, radiative cooling to space will slow. When we integrate from the edge of space to the surface, most of the dI terms are positive and result in average DLR at the surface building up to 333 W/m2. Increasing n increases DLR reaching the surface (though not by as much as OLR decreases).
So here we have the fundamental reason rising GHGs slow down the rate at which our climate system radiatively cools more slowly to space as GHGs rise. There are no models or paradigms involved except for the need to know the temperature, pressure and composition (GHG concentrations including humidity) of the atmosphere along the path from the surface to space. And this is why the definition of radiative forcing is the slowdown in radiative cooling assuming nothing else changes or before anything changes.
I was so enamored with the Schwarzschild’s equation (which contains both Planck’s Law and Beer’s Law) that I wrote an Wikipedia article on the subject. No one has ever told me it’s great, so it may not be as meaningful to you as to me.
https://en.wikipedia.org/wiki/Schwarzschild%27s_equation_for_radiative_transfer
Computer programs have been written automate numerical integration of Schwarzschild’s equation, including Modtran which is available at the link below. Climate scientist rarely tell you that they are numerically integrating this equation, but that is what is happening when they talk about “radiative transfer calculations”.
http://climatemodels.uchicago.edu/modtran/
Mark also wrote: “That’s a nice theory but fails on the facts because the temperature at the tropopause has been falling, not increasing. This is because of ozone depletion, which is a far more plausible candidate for explaining GW since 1980.”
Although we can predict rising GHGs will slow radiative cooling to space, we can’t use Schwarzschild’s equation to predict how temperature at the surface and in the troposphere will change because temperature depends on both radiation and convection. Since convection becomes negligible at and above the tropopause, we can use radiative transfer calculation to predict temperature change in the stratosphere with changing GHGs. In the lower stratosphere temperature is RISING with altitude and B(lambda,T) – I is positive. So as OLR passes through the lower stratosphere, it increases in intensity. If CO2 doubles, that increase will be bigger and the lower stratosphere will cool.
Levels of ozone in the stratosphere, but not at the tropopause have been reduced by CFC’s, so this altitude also gets less heat from absorbing UV. The tropopause is at the junction of a warming upper troposphere and a cooling stratosphere. Most climate models show the transition from warming to cooling occurring at too high an altitude.
Frank, thanks for the time and trouble. You have just given the “top of atmosphere” explanation again, with which I am familiar. Nullius repeated this above.
I quite like that one. It took me a couple of weeks of mulling before I spotted the obvious flaws. So at least it[‘s a bit of an intellectual challenge!
PS, you don’t need to take a Schwarzschild Equation out of context and fudge the variable to prove that upper atmosphere emits less radiation than the surface. It’s a lot colder up there (because of the gravity induced lapse rate), so of course it emits less.
Mark writes: You have just given the “top of atmosphere” explanation again, with which I am familiar. Nullius repeated this above. I quite like that one. It took me a couple of weeks of mulling before I spotted the obvious flaws. So at least it[‘s a bit of an intellectual challenge!”
A lot of words have been written above, so the “obvious flaws” you spotted aren’t clear to me. Could you please be kind enough to copy and paste the key passages above describing those flaws in a reply?
What is clear is that, if the rate of radiative cooling to space across the TOA slows and the rate of incoming SWR remains, then the law of conservation of energy demands that it warm somewhere below the TOA until incoming and outgoing radiation are again in balance.
Mark also wrote: PS, you don’t need to take a Schwarzschild Equation out of context and fudge the variable to prove that upper atmosphere emits less radiation than the surface. It’s a lot colder up there (because of the gravity induced lapse rate), so of course it emits less.
I didn’t take Schwarzschild’s Equation out of context – I am applying it to the real context, our atmosphere is it really is today. While Schwarzschild’s Equation is used to calculate radiative fluxes between grid cells in an AOGCM, it can not predict temperature anywhere that convection also transfers a significant amount of heat – in other word in the troposphere. Temperature is an INPUT used in the B(lambda,T) term to calculate how radiation changes in intensity. The other input is the density of GHG(s) – n. The OUTPUT from Schwarzschild’s Equation is a CHANGE in radiative flux, not any prediction about temperature – such as why it is colder in the upper atmosphere.
As you correctly note, it is colder in the upper troposphere. This is mostly, but not solely, because the atmosphere is unstable to buoyancy-driven vertical convection, where the local lapse rate exceeds a moist adiabatic [gravity-dependent] lapse rate. However, as in noted in my first comment, temperature is never the result of a single mechanism of heat flux – it is the net result of all mechanisms. The word “adiabatic” in “moist adiabatic lapse rate means with no gain or lost of heat (for example, by radiation), which isn’t the case in the troposphere. What we observe on the average is close to a moist adiabatic lapse rate because heat transfer by convection is often significantly faster than by radiation.
Furthermore, at wavelengths in the atmospheric window (ca 800-1000 cm-1), the coldness of the upper troposphere has no effect on outgoing radiation, which has the same intensity as it did leaving the surface.
“THEREFORE IT IS ABSURD TO DISCUSS HOW OR WHETHER ONE OBJECT CONTROLS THE TEMPERATURE OF ANOTHER”
Correct.
But I wasn’t doing that (the article was).
I was just asking a question about what the article is trying to say.
I apply common sense and very basic physics and assume that warmer things get cooler and cooler things get warmer until they reach the same temperature.
Mark: You are correct about a warmer and a cooler object coming into equilibrium at an intermediate temperature. However, our climate isn’t that simple and the proper term to use is steady-state, not equilibrium.
The surface temperature of the sun is around 5700 K and has been and will be for millions of years. The temperature of our planet is determined by the rate at which our planet absorbs (rather than reflects) SWR from the sun and the rate at which it emits LWR to space. Since our planet emits more LWR as it gets warmer*, our planet reaches a STEADY STATE temperature when incoming post-albedo SWR is equal to LWR leaving for space (crossing the TOA).
* If this weren’t true, we would experience a run-away GHE.
Radiative transfer calculation indicate that rising GHGs will slow the rate of radiative cooling space – about 3.5 W/m2 for a doubling of CO2 if nothing else changed. So, to reach a new steady state, the climate system needs to warm up enough to increase the emission of LWR and reflection of SWR to space by 3.5 W/m2. This is merely conservation of energy.
We approach this problem by looking for steady state balance at the TOA – rather than the surface – because is it relatively easy to calculate and observe the flux of radiation across the TOA. Surface energy balance involves radiation (including DLR) and convection of latent and sensible. Convection involves fluid flow, phenomena we can’t calculate or observe accurately.
This article has, so far, not attended to the important point. The important question is that approached in the 2nd section, the 11th sentence of the article. If all the IR incident on an object, regardless of the temperature of the source is absorbed and results in a temp change for all sources of IR, as stated there, then is the conclusion that if the IR from 3 objects at 100 deg C is carefully reflected and focused at one object then that target can reach a temp higher than 100 deg C. i.e. the 2nd Law does not apply to IR transmission. Is that part of the physics that S.o.D. is discussing. If that is not the case what is the correct description of what happens when IR from a cooler object arrives at the surface of a warmer object.
Dinero,
Sorry, it doesn’t work that way. For example, you can’t achieve a higher temperature than the sun’s visible surface by simply focusing sunlight on an object. Period. The part you missed is that the object focused on radiates in all directions while only a small fraction of the area being radiated to has a higher temperature.
Well lets make the target object no bigger than that small area of the focus spot of radiation. Then the target object is hotter than the source, not necessary the sun. As SoD says, transfer from cold to hot is not outlawed . He calls outlawing that is “imaginary”. Therefore temperature higher at the target than the source from focused radiation is not outlawed. What do you think.
There has been some increase in DLR (longwave radiation down at surface) over the last 40 years (satellite period).
It may be around 0,8 W/m2. And DSR (shortwave radiation) has increased much more, about 3,6 W/m2. As seen from figures in a paper : Analyzing changes in the complexity of climate in the last four decades using MERRA-2 radiation data. Alfonso Delgado-Bonal et al 2020.
All the change of DLR can be attributed to change of DSR and perhaps some surface warming, which is attributed to change of clouds. A conclusion that can be drawn is that change of CO2 has no effect on back-radiation, in the real world. Or can it be another explanation?
Click to access s41598-020-57917-8.pdf
NK, good find. The extra DSR is probably because of ozone depletion.
Mark and NK: If you go to the Modtran website, you will find that no plausible change in stratosphere ozone will have any effect on DLR.
NK, I didn’t understand much about this paper. In Figure 1 and 2, the changes in reflected SWR and LWR are -0.10 and -0.021W/m2/yr. (Negative values for heat lost by the climate system to space.) If these are SWR and LWR feedbacks to warming over the past four decades (roughly 0.02 K/yr), dividing gives SWR and LWR feedback parameters of -5 and -1 W/m2/K! That would make ECS about 0.5 K/doubling, so something may be wrong.
Looking at the amount of noise in the LWR channel, the slope could easily +/-50%, so this data is not inconsistent with the conventional view that LWR feedback is about -2 W/m2/K (-3.2 W/m2K from Planck feedback plus +1.1 W/m2/K from WV+LR plus weakly positive cloud LWR feedback). LWR has increased from about 237.5 in 1980 to 238.3 W/m2 in 2017 with a STD of about 2.5 W/m2.
If SWR feedback is positive the slope of the reflected SWR vs. time plot in Figure 1 would have to be negative, not positive. SWR feedback is conventionally believed to be about +0.5 to +1.0 W/m2/K. This is way off. However, the spike is reflected SWR is about right (3-4 W/m2) for Pinatubo in 1993 and smaller for El Chichon in 1982. Reflected SWR has grown from about 103 W/m2 in 1980 to 107 W/m2 in 2017 with the value in any 2 hour period varying by +/-5 W/m2.
One hint about the nature the problem is that the data taken every 2 hours was transformed to induce stationarity. This process must have removed the seasonal 8 W/m2 increase in LWR associated with summer in the NH. There are regular seasonal changes in reflected SWR due to the 7% increase in SWR arriving in NH winter in the due to the elliptical nature of the Earth’s orbit and snow cover in the NH winter.
The other thing worth remembering is MERRA is a re-analysis product – changing sources of observations proceeded through a climate model to produce a best fit to all of the data. There are questions about Paltridge’s paper about re-analysis data showing humidity in the upper troposphere falling with time. It would be nice if this data were “right”, but I’m not expecting it to be.
I’m trying to enhance my understanding, as opposed to push an agenda.
If All the “Back Radiation” Was Reflected..
In this section, it is discussed what would happen if all the radiation was reflected by the surface then we explore what would happen. I have a few questions:
1) Why do we use σT^4 to estimate up radiation to the atmosphere, the atmosphere is not at 0K, so should we use P=eσA(T1^4-T2^4)?
2) What % of the back radiation is actually reflected? 100% (unlikely as argued), 0%? somewhere in between?
2.a – If we accept that a certain percentage of the back radiation is reflected upwards; have we created a run-away process whereby this reflected radiation is in turn absorbed by the atmosphere and reradiated back down, only for a percentage to be reflected by the surface again ad-infinitum. When does this stop? Do we have to sum the infinite series to determine the up radiation?
Andrew,
1. There’s a simple formula for emission of thermal radiation from a surface:
εσT4
It’s in all the textbooks. The temperature of the atmosphere isn’t relevant.
2. Very low. It depends on the material. Emissivity is wavelength and direction dependent. Absorptivity is equal to emissivity for the same wavelength and direction.
You can see the graphs of different surface types – the emissivity is very close to 1 across the wavelengths of interest. This means the reflectivity is very close to zero.
2a. It’s hard to explain to people who’ve never done heat transfer equations how to do heat transfer equations.
Try reading Heat Transfer Basics – Part Zero – a few examples of heat transfer, simple stuff but often misunderstood.
There’s an online heat transfer textbook that’s free – Lienhard & Lienhard -Heat Transfer Textbook, 3rd edition, MIT. I downloaded it ages ago.
It’s not for everyone – textbooks usually aren’t. But without understanding what’s in the textbook all kinds of crazy ideas can seem sensible.
Andrew,
I think you can use a thought experiment to determine that reflected radiation will not lead to some sort of thermal runaway, your 2.a. Let’s say we have a plane blackbody surface (opaque by definition) that is being irradiated, has no other energy input and is at steady state at a particular temperature To. It will then emit and absorb σTo^4. If we now change the emissivity to 0.98, then the surface will emit less radiation, 0.98σTo^4 and reflect 2% of the incoming radiation and will absorb 98% of incoming radiation. Since we have postulated that steady state exists, then the temperature of the surface won’t change because it will still emit the same amount of energy that it absorbs. Emission plus reflection, however, is still equal to σTo^4.
This is also why a cavity with a small hole is a good approximation of a blackbody. The radiation flux in the cavity will be almost the same as for a blackbody even for an interior with a relatively high reflectivity, except for gains or losses through the hole.
Obviously things will be more complicated for a surface that’s irradiated by a solar spectrum and radiates to and absorbs radiation from an atmosphere that is not completely opaque, but the principle is the same.
Andrew asked: “Why do we use σT^4 to estimate up radiation to the atmosphere, the atmosphere is not at 0K, so should we use P=eσA(T1^4-T2^4)?”
P = eoT^4 is the formula for the flux of radiation emitted by a gray-body. If the atmosphere and surface both behaved like gray-bodies, then the upward flux emitted by the surface would be eoTs^4 and the downward flux emitted by the atmosphere would be eoTa^4 and the net transfer of energy from the surface the atmosphere (which is called heat in thermodynamics) would be e_s*o*Ts^4 – e_a*o*Ta^4. (The emissivity of the surface e_s and the atmosphere e_a are different.)
By saying “if the atmosphere and surface behaved like gray-bodies”, we are proposing a MODEL for how our climate system behaves. Aside from the problem that temperature varies with latitude and altitude, a gray body is a lousy model for a semi-transparent atmosphere composed of gases that emit and absorb very differently at different wavelength (and negligibly at some wavelengths). So you need to be careful how far you trust this model. The model works great for some phenomena, but not others.
Many skeptics are confused about how radiation can travel from the colder atmosphere to the warmer surface, when the second law of thermodynamics says HEAT flows from hot to cold. They refuse to acknowledge that HEAT refers to the net flux in the case of radiation. Instead, they have proposed an absurd “alternative reality”, where both emitting surfaces and the radiation traveling between those surfaces somehow “know” what their temperatures and emissivities are and magically arrange for a one-way flux of photons from hotter to colder with an intensity of eσ(T1^4-T2^4). This formulation ignores the fact that two different emissivities are involved. Some believe that interference may be involved, but interference occurs only with coherent radiation from a single source. This alternative reality guarantees that heat will flow from the warmer surface to the colder atmosphere. This “alternative reality” is extremely appealing, because it allows skeptics to claim that the presence of more GHGs in the atmosphere doesn’t change the rate at which the surface radiates away the energy acquired by absorption sunlight.
Warning: As soon as I mention an increase in GHGs, we are encountering a situation where a gray-body model for the atmosphere can create confusion. Usually we think of emissivity as parameter that varies from one material to another, but doesn’t vary with the amount or thickness of the material. In the case of our atmosphere, increasing the amount of GHGs in the atmosphere increases the number of thermal infrared photons traveling from the atmosphere to the surface (a higher emissivity in a gray-body model) and decreases the number escaping to space from the atmosphere (a lower emissivity). This happens because photons are both emitted and absorbed at different altitudes with temperatures and densities. Technically, a gray-body model for a non-homogenous atmosphere has an “effective emissivity” based the temperature you assign it in your model and its GHG concentration. Some phenomena, like upward and downward fluxes of radiation, can only be fully understood by using radiation transfer calculations (Schwarzschild’s equation).
Everyone with a technical education learns the physics of the macroscopic world: Newtonian mechanics and the laws of thermodynamics. Most are told that the physics of the microscopic world of atoms, molecules and photons – quantum mechanics – is radically different. Few learn statistical mechanics, a difficult branch of physics and chemistry that explains how large numbers of molecules and photons following the laws of quantum mechanics produce macroscopic phenomena such as temperature, pressure, entropy, and heat flow from hotter to colder. The laws of thermodynamics don’t control the behavior of individual molecules and photons – those laws are a CONSEQUENCE of large numbers of molecules and photons obeying the laws of quantum mechanics. The laws of quantum mechanics say that a single photon can be emitted from a GHG molecule in the atmosphere and absorbed by the surface. The concept of temperature is only defined for a large groups of molecules. Heat flows from a large warmer GROUP of molecules on the surface to a large colder GROUP of molecules in the atmosphere, but individual photons are traveling both ways between individual molecules in both groups. Since warmer groups emit more photons than colder groups (and absorptivity equals emissivity) heat flows in the correct direction.
This doesn’t make any sense.
If I have a room that’s completely insulated and at 40 degrees and I want to warm it up, putting in 30-degree heaters won’t do it no matter how many I put in. Yet we’re to believe that the walls of the room must be affected by the radiation put out by the 30-degree heaters such that the walls will warm up, and hence the room will warm up.
In a sealed room at 40 degrees, a heater that starts at 30 degrees will warm up unless it’s refrigerated. But then it isn’t a heater. Consider a 30 watt heater rather than something that will require energy to stay at or below room temperature.
The point is, we’re told that a colder atmosphere can indeed warm up a warmer surface through back radiation.
If this is so, then it follows that a heater set at 30 degrees can warm up a 40-degree room. How? Because, we’re told, it’s ridiculous to imagine that the heat radiating from the 30-degree heaters has no effect on the walls.
I always thought that for heat transfer to take place, higher-energy molecules would excite lower-energy molecules. But now I’m to understand that 30-degree radiation has more energy than 40-degree walls, and so will warm up the walls.
Let’s take a lump of granite at 50 degrees. Now we’re to understand that in a 40-degree room, the walls radiating IR to the tune of 40 degrees will warm up the 50-degree granite through the magic of IR photons, because it’s ridiculous for us to imagine that the IR photons aren’t affecting the granite.
Makes perfect sense?
The Earth’s surface and atmosphere isn’t a closed system. There is energy coming in from the sun and energy being radiated to space by the surface and the atmosphere. Your sealed room example is irrelevant, not to mention wrong.
nobodysknowledge posted this but it somehow got lost along the way. I received it in an email from wordpress even though I never checked the notify me box:
Let’s say we have a block of ice in a 60F room. The block of ice is receiving and emitting IR from the walls: this is analogous to the colder atmosphere in relation to a warmer surface, regardless of whether the system is open or closed.
Are we to believe that because the ice is emitting IR, then this warms the walls? Or are we to believe that because the ice is emitting IR, this slows the cooling of the walls?
No it isn’t analogous at all. Saying it is doesn’t make it so. You have created a straw man argument that no one who knows anything about atmospheric radiative transfer believes.
Don132: Put your block of ice in a room with walls at -60 DegF. In this case, I think we would agree that the thermal infrared emitted by the block of ice would “warm” the -60 degF walls. So, if you think the ice is not emitting IR radiations that warms the walls of a 60 degF room, how does the ice decide whether it should and shouldn’t emit thermal IR that can warm a wall that absorbs it?
The solution to this dilemma is be much more careful about how we use the terms “warm” and “heat”. In all cases, both the ice and the walls emit thermal infrared ENERGY towards each other regardless of what their temperature may be, but the flux (energy -per unit time per unit surface area) of radiation varies with their temperature and emissivity. Technically, that energy comes in packets called photons. Photons don’t know the temperature of the object that emitted them. They are absorbed or reflection independent of the temperature of the object they encounter. So there is a two-way exchange of radiant ENERGY between the wall and the ice. “Heat” is the net flux of ENERGY from this two-way transfer of energy. HEAT always travels from warmer to colder, but ENERGY travels both ways as radiation (photons).
When photons are absorbed and create an excited state, collisions relax that excited state converting that energy motion – higher internal energy which is proportional to higher temperature.
The colder atmosphere radiates IR that is absorbed by the warmer surface, but the net flux of HEAT is from the warmer surface to the colder atmosphere. However, if no atmosphere were present, there would be negligible radiative flux from above to the surface. A “cold” atmosphere radiates more energy to the surface than empty space at 3 K. The atmosphere doesn’t HEAT the surface, but the energy it radiates to the surface does make the surface is WARMER than it would be without radiation from the atmosphere. And the atmosphere is HEATED by the surface making it warmer too.
The ENERGY needed to make both the surface and atmosphere warmer than they would be without each other comes from the sun. This is possible because most of the photons that escape to space are emitted from a high altitude that is much colder than the surface.
Another reason why your example is not relevant to a planetary atmosphere and surface is that your system is not anywhere close to steady state. The ice is going to melt.
Going from no ice to ice actually reduces the radiation impinging on the walls of the room. The opposite wall area viewed will be reduced by the effective area of the block of ice and the radiation from the ice will be lower than from the wall. So by introducing the ice block, you reduce back radiation. Increasing CO2 increases back radiation from the atmosphere and reduces radiation emitted to space.
You really need to think through your attempt at a counterexample.
And I second Frank’s point about your loose thermodynamic terminology. The term ‘heat’ can mean several different things in different contexts.
Lots of mistakes here.
1. Emissivity numbers here are misleading. The data suggested for water a) restricted in wavelength (no data for far-IR) and b) to surface normal. As rightfully pointed out in “Emissivity of the Ocean” the spectral emissivity to normal of water is 0.96. The hemispheric spectral emissivity however is only 0.91, substantially less than 1.
You do not need to take it from me, also Huang et al 2016 (Fig. 3) have identical results as myself..
Click to access jas-d-15-0355.1.pdf
2. The issue of “back radiation” has not been understood. There are many perspectives to understand it correctly, but none were considered. Essentially surface radiates as much “energy” into the atmosphere, as the atmosphere radiates onto the surface. Note: I am not talking about the total amount of surface emissions, but only those absorbed by the atmosphere. The surface thus emits about 360W/m2 (not 390!, as surface emissivity is NOT 1), about 40W/m2 may pass through the atmospheric window, and about 320W/m2 then get absorbed by the atmosphere. A similar figure, be it 310W/m2 or so, of “back radiation” is emitted onto the surface.
Although there may be some gap between the two figures, essentially they are about even. And that is the point. If I give you one Dollar, and you give me one Dollar, and we repeat this 1000times a day, then both of us will have gotten 1.000 Dollars. And if we do that every day, we might make a lot of money. Or not.
Regrettably we can not make a fortune that way, although a lot of people have believed so. Talking about Ponzi-schemes. Neither is the exchange of radiation at the surface/atmosphere boundary layer anything specific. Rather it happens everywhere. It is equally true for the boundary layer 1m (or 1 inch, or 1 foot..) underneath the soil. Or any arbitrary boundary layer within the atmosphere. These exchanges of radiation are NEVER a source of energy. Rather they are strictly a function of temperature, not the other way.
3. An interesting detail hereto is on how much “back radiation” so to say is getting traded within the atmosphere. According to modtran it should be over 20.000W/m2 for CO2 alone. Including other GH agents it should be well over 50.000W/m2. Imagine that much radiation would do anything..
4. It is well reknown fact that an isothermic atmosphere would not be able to produce any GHE. That is because the atmosphere would emit at the same temperature as the surface, thus could not reduce emissions. And this is not just a theoretical consideration, as since due to inversion, the GHE over the Antarctic is actually negative. From this perspective alone, one can already understand of “back radiation” must be irrelevant. In an isothermic scenario “back radiation” will not disappear, rather it is hardly affected. Only the GHE disappears.
5. The IPCC has spread a lot of bad, or rather misinformation on the subject. Still in AR4 the definition (in the glossary) of the GHE included phrases about “back scattered” radiation. With AR5, and that has not changed with the recently released AR6, “back radiation” is indeed out of the window. Some progress at last..
So no, “back radiation” does not heat anything and never did. And this article spreads misinformation.
You’re nitpicking. Nothing you say changes the fundamentals and the lack of a GHE in an isothermal atmosphere is discussed elsewhere on this site.
Emissivity is less important than you make it out to be. If the emissivity is 0.91 (which, btw, would still be considered by most people to be pretty close to 1) then the absorptivity is also 0.91 by Kirchoff’s Law so less radiation from the atmosphere is also absorbed and more is reflected as thermal IR is not going to be transmitted by water so it must be reflected. That means the sum of the emitted and reflected radiation will still be about the same as given by the published (and measured) energy balances.
John Doe: DeWitt is correct that errors in surface emissivity are almost perfectly compensated for by the decrease in absorptivity of incoming DLR, something the authors of the paper you cited failed to mention. However, the (instantaneous) decrease in OLR at the TOA they calculate for a more accurate surface emissivity averages only about 1 W/m2.
The reason for this is something you hint at – the nearly complete opacity of clear skies to thermal infrared outside the “atmospheric window” – and the fact that cloudy skies are opaque at all wavelengths. This can best be observed using the online Modtran calculator at:
http://climatemodels.uchicago.edu/modtran/
Look up from the surface at a tropical atmosphere (surface 299.3 K which can be offset to exactly 300 K if you want). With non-cirrus clouds, DLR almost perfectly overlays the blackbody spectrum for 300 K and is about 2 W/m2 less than the calculated upward emission from the surface. (This model must be using surface emissions that is near 1.0 , but that is compensated for by using a surface absorptivity near 1.0 too.) Without clouds, at the surface DLR is about 83% of OLR. Outside the tropics (using the US Standard Atmosphere, for example, absolute humidity is much lower, the atmospheric window is clearer and wider, while emission by water vapor at 500-550 cm-1 is a little weaker, making DLR about 70% of OLR. If you look up from 1.5 km, there is less water vapor on the path to space, but emission has the intensity expected for a BB at 280 K at many wavelengths. In the tropics, looking up from 7 km (257 K), fewer wavelengths emit with slightly less than blackbody intensity for 260K. Looking up from 12 km (224 K), only the center of the CO2 band is emitting DLR with the intensity of a blackbody slightly warmer than 220 K.
Muy buen articulo, felicidades.
https://vanbeek.pe/
This article clearly debunks the myth that a colder atmosphere cannot affect a warmer surface, showing that absorbed radiation must impact surface temperature. I appreciate how it links photon energy, Planck’s law, and material properties to explain why the “imaginary second law” is flawed. The legacy ghost writers in scientific literature have long shown the value of clarity in explaining such complex topics.