With apologies to my many patient readers who want to cover more challenging subjects.
Many people trying to understand climate science have a conceptual problem.
I have written (too) many articles about the second law of thermodynamics – the real and the imaginary version. Resulting comments on this blog and elsewhere about those articles frequently contain comments of this form:
So if we take bucket A full of water at 80°C and bucket B full of water at 10°C, Science of Doom is saying that bucket A will heat up because of bucket B? Right! That’s ridiculous and climate science is absurd!
Yes, if anyone was saying that it would be ridiculous. I agree. To take one example from many, in The Real Second Law of Thermodynamics I said:
Put a hold and cold body together and they tend to come to the same temperature, not move apart in temperature.
Of course, it could be that I am inconsistent in my application of this principle.
One observation on the many contrary claims resulting from my articles – not a single person has provided a mathematical summary to demonstrate that the examples provided contradict the first or second law of thermodynamics.
It should be so easy to do – after all if one of the many systems I have outlined contravenes one of these laws, surely someone can write down the equations for energy conservation (1st law of thermodynamics) or for change in entropy (2nd law of thermodynamics) and prove me wrong. We aren’t talking complex maths here with double integrals or partial differentiation. Just equations of the form a + b = 0.
And here’s the reason why – the problem that people have is conceptual. It seems wrong so they keep explaining why it seems wrong.
Conceptual problems are the hardest to get around. At least, that’s what I have always found. Until a subject “clicks”, all the mathematical proof in the world is just a jumble of letters.
So with that introduction, I offer a conceptual model to help those many people who don’t understand how a cold atmosphere can lead to a warmer surface than would occur without the cold atmosphere.
And if you are one of those people in the “firmly convinced” camp, let me suggest this reason for making the effort to understand this conceptual model. If you understand why others are wrong you can help explain it to them. But if you just don’t understand the argument of people on “the other side” you can’t offer them any useful assistance.
Model 2 – Two bodies – The Boring One that Everyone Really Does Agree With
Very quickly, to “warm everyone up”, and to once again state the basics – if we have two bodies in a closed system, and body A is at temperature 80°C and body B is at 10°C, then over a period of time both will end up at the same temperature somewhere between 10°C and 80°C. It is impossible, for example, for body A to end up at 100°C and body B at 0°C.
Everyone is in agreement on this point.
Note that the “period of time” might be anything between seconds and many times the age of the universe – dependent upon the circumstances of the two bodies.
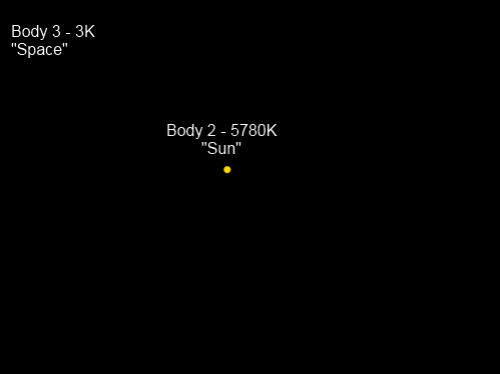
Model 3A – Three Bodies with the Third Body Being Quite Cold
Where’s Body 1? This picture is the view from Body 1, also known as “Chilly Earth”, which is a spherical solid planet.
To make the problem much easier to solve we will state that the heat capacities of Body 2 and Body 3 are extremely high. This means that whether they gain or lose energy, their temperature will stay almost exactly the same. Body 1, “Chilly Earth”, has a much lower heat capacity and will therefore adjust quickly to a temperature which balances the absorption and emission of radiation.
“Chilly Earth” doesn’t have an atmosphere.
However, for the purposes of helping the conceptual model, “Chilly Earth” reflects 30% of shortwave radiation from the Sun but at longer wavelengths absorbs 100% (reflects 0%). This means its emissivity at longwave is also 100%.
“Chilly Earth” has a very high conductivity for heat, and therefore the whole planet is at the same surface temperature. (See note 1).
“Sun” is 150M km away from “Chilly Earth”, and “Chilly Earth” has a radius of 1,000 km (a little different from the planet we call home).
Let’s calculate the approximate equilibrium temperature of “Chilly Earth”, T1
How do we do this? By calculating the energy absorbed from Body 2 and from Body 3, and calculating the temperature of a surface that will radiate that same energy back out.
The method is simple – see below.
Energy Absorbed from Body 2, “Sun”
Radiation from “Sun” at 5780K = 6.3 x 107 W/m² – near the surface of the sun. By the time the sun’s radiation reaches earth, because of the inverse square law (the radiation has “spread out”), it is reduced to 1,369 W/m². Remember that 30% is reflected, so the absorbed radiation = 958 W/m².
The surface area that “captures” this radiation = πr² = 3.14 x 106 m².
Energy absorbed from body 2, Er2 = 958 x 3.14 x 106 = 3.01 x 109 W.
Energy Absorbed from Body 3, “Space”
Radiation from “Space” at 3K = 4.59 x 10-6 W/m². Apart from the very tiny angle in the sky for “Sun”, the entire rest of the sky is radiating towards the earth from all directions in the sky.
The surface area that “captures” this radiation = 4πr² = 1.26 x 107 m².
Energy absorbed from body 3, Er3 = 4.59 x 10-6 x 1.26 x 107 = 57.7W.
So energy from body 3 can be neglected which is not really surprising.
Energy Radiated from Body 1, “Chilly Earth”
For thermal equilibrium (energy in = energy out), “Chilly Earth” must radiate out 3.01 x 109 W, from its entire surface area of 1.26 x 107 m².
This equates to 239 W/m², which for a body with an emissivity of 1 (a blackbody) means T1 = -18°C.
So we have calculated the equilibrium temperature of “Chilly Earth”.
Now, if we change the model conditions – the reflected portion of solar radiation, the emissivity of the earth at longwave, or the conductivity of the planet’s surface – any of these factors would affect the result. They wouldn’t invalidate the analysis, they would simply lead to a different number, one that was slightly more difficult to work out.
But hopefully everyone can agree that with these conditions there is nothing wrong with the method. (I realize that a few people will not agree..)
Model 3B – Three Bodies with the Third Body Being Somewhat Warmer
So now we are going to perform the same analysis with our new Body 1, “Warmer Earth” (a wild stab at an appropriate name).
The only thing that has really changed about the environment is that Body 3, “Crazy Background Radiation”, is now at 250K instead of 3K.
Note that the temperature of Body 3 is higher than before but lower than the equilibrium temperature of 255K calculated for “Chilly Earth” in the last model. As before, body 3 has an emissivity of 1 for longer wavelengths.
Body 1, “Warmer Earth”, still reflects 30% of solar radiation and is the same in every way as “Chilly Earth”.
What are we going to find?
We will do the same analysis as last time. Repeated in full to help those unfamiliar with this kind of problem.
Energy Absorbed from Body 2, “Sun”
Radiation from “Sun” at 5780K = 6.3 x 107 W/m² – near the surface of the sun. By the time the sun’s radiation reaches earth, because of the inverse square law (the radiation has “spread out”), it is reduced to 1,369 W/m². Remember that 30% is reflected, so the absorbed radiation = 958 W/m².
The surface area that “captures” this radiation = πr² = 3.14 x 106 m².
Energy absorbed from body 2, Er2 = 958 x 3.14 x 106 = 3.01 x 109 W.
Energy Absorbed from Body 3, “Crazy Background Radiation”
Radiation from “Crazy Background Radiation” at 250K = 221 W/m². Apart from the very tiny angle in the sky for “Sun”, the entire rest of the sky is radiating towards the earth from all directions in the sky.
The surface area that “captures” this radiation = 4πr² = 1.26 x 107 m². (See note 2).
Energy absorbed from body 3, Er3 = 221 x 1.26 x 107 = 2.78 x 109 W.
In this case, energy from body 3 is comparable with body 2.
Energy Radiated from Body 1, “Warmer Earth”
Body 1 absorbs Etot= Er2 + Er3 = 5.79 x 109 W
For thermal equilibrium (energy in = energy out). “Warmer Earth” must radiate out 5.79 x 109 W, from its entire surface area of 1.26 x 107 m².
This equates to 460 W/m², which for a body with an emissivity of 1 (a blackbody) means T1 = +27°C.
Discussion
Our two cases have revealed something very interesting.
A very very cold sky led to a surface temperature on our slightly different earth of -18°C, while a cold sky (colder than the original experiment’s planetary surface temperature) led to a surface temperature of 27°C.
Well, and here’s the thing, strictly speaking the temperature is actually caused primarily by the bright object in the middle of the picture, “Sun”. The energy absorbed from the sky just changes the outcome a little.
In both cases we calculated the equilibrium temperature by using the first law of thermodynamics (energy in = energy out).
If we do the calculation of entropy change we will find something interesting.. but first, let’s consider the conceptual model and what exactly is going on.
It’s very simple.
In a 3-body problem the temperature of the coldest body still has an effect on the equilibrium temperature of the body being heated by a hotter body.
I could make it more catchy, more media-friendly, but that would go against everything I stand for. I will call this Doom’s Law.
Entropy
The second law of thermodynamics says that entropy can’t reduce. The many cries of anguish that will now arise will claim that Model 3B has broken the Second Law of Thermodynamics. But it hasn’t.
See The Real Second Law of Thermodynamics for more on how to do this calculation. And even clearer, the article by Nick Stokes:
Change in entropy, δS = δQ / T
where δQ = change in energy, T = temperature
We will consider both models over 1 second.
Model 3A
Body 2, “Sun”, δS2 = -3.85 x 1026 / 5780 = -6.66 x 1022 J/K
Body 3, “Space”, δS3 = 3.85 x 1026 / 3 = +1.28 x 1026 J/K
And finally, Body 1, “Chilly Earth”, δS1 = 0 / 255 = 0 J/K
Total Entropy Change = δS1 + δS2 + δS3 = +1.28 x 1026 J/K :a net increase in entropy.
Model 3B
Body 2, “Sun”, δS2 = -3.85 x 1026 / 5780 = -6.66 x 1022 J/K
Body 3, “Crazy Background Radiation”, δS3 = 3.85 x 1026 / 250 = +1.54 x 1024 J/K
And finally, Body 1, “Warmer Earth”, δS1 = 0 / 255 = 0 J/K
Total Entropy Change = δS1 + δS2 + δS3 = +1.47 x 1024 J/K :a net increase in entropy.
Important points to note about the entropy calculation
Both scenarios increase entropy – by transferring heat from a high temperature source, “Sun”, to a low temperature source, “Space” in 3A, and “Crazy Background Radiation” in 3B (which is really also Space at a higher temperature).
The earth-like planet is sitting in the middle and doesn’t have a significant effect on the entropy of the universe.
In both cases the entropy of the system increases, so both are in accordance with the second law of thermodynamics.
The earth cools to space, but just at a slower rate when the background temperature of “space” is higher.
If we replaced “crazy background radiation” by an atmosphere that was mostly transparent to solar radiation, the analysis would be a little more complex but the result wouldn’t be much different.
Reasons Why It Might be Wrong
Just to be clear, these aren’t true..
1. The hotter body can’t absorb radiation from the colder body
a) see Amazing Things we Find in Textbooks – The Real Second Law of Thermodynamics for six textbooks on heat transfer which all say, yes it does. Actually, seven textbooks, thanks to commenter Bryan identifying his “non-cherrypicked” textbook by “real physicists” which also agreed.
b) see The Amazing Case of “Back Radiation” – Part Three which includes the EBEX experiment as well as a brief explanation of fundamental physics
c) see Absorption of Radiation from Different Temperature Sources – clearing up a few misconceptions on this idea
2. It’s not a real situation because the atmosphere isn’t a black body
It is true that the atmosphere is not a blackbody. But look back at model 3B. It doesn’t matter. Body 3 in this model could be a 250K body with an emissivity of 0.1 and the temperature would still increase over model 3A.
In fact, if the claim is that a colder body can never increase the temperature of a warmer body – all we need is one counter-example to falsify this theory. Now, if you want to modify your theory to something different we can examine this new theory instead.
Reasons Why It Has to be Right
1. The First Law of Thermodynamics. This neglected little jewel is quite important. Energy can’t disappear (or be created) or be quarantined into a mental box.
There is a reason why all the people disputing these basic analyses never explain where the energy goes (if it “can’t” go into changing the temperature of the hotter body that might have absorbed it). The reason – they don’t know.
2. The Second Law of Thermodynamics. This law says that in a closed system entropy cannot decrease. Despite angry claims about “no such thing as a closed system” – that’s what the second law says. Entropy is often simple to calculate.
If a solution uses simple radiation of energy (Stefan-Boltzmann’s law) and satisfies the first and second law of thermodynamics, and some people don’t like it, it suggests that the problem is with their conceptual model.
Conclusion
This is a conceptual model that is very simple.
The sun warms up the earth, and the earth cools to space. The colder “space” is, the faster the rate of net heat transfer. The warmer “space” is, the slower the rate of net heat transfer. And because the sun “pumps in” heat at the same rate, if you slow the rate of heat loss the equilibrium temperature has to increase.
The first law of thermodynamics is the key to understanding this problem. It is simple to verify that model 3A & 3B both satisfy the first law of thermodynamics. In fact, more importantly, a different result would contradict the first law of thermodynamics.
It is also easy to verify that in both 3A & 3B entropy increases.
Just to be clear on a tedious point, the earth and space do not have to radiate as a blackbody to have these conclusions. They just make the model simpler to explain, and the maths easier to understand. We could easily change the emissivity of the planet to 0.9 and the emissivity of space to 0.5 in both models and we would still find that Model 3B had a warmer planetary surface than Model 3A.
Many people will be unhappy, but this blog is not about bringing happiness. Clarity is the objective.
One more hopeless note of despair – this article uses simple theory to prove a point, which is actually a very valuable exercise. Next, some will say – “I don’t want that pointless over-theoretical theory, these people need to prove it with some experiments“.
And so I offer the series, The Amazing Case of “Back Radiation” as proof, especially Part Three. Result of Part Three was – “well, that can’t happen because it goes against theory“..
And so the circle is complete.
Notes
Note 1 – These strange conditions that don’t relate to the real world are to make the conceptual model simpler (and the maths easy). This is the staple of physics (and other sciences) – compare simple models first, then make them more complex and more realistic. If you can prove a theory with a simple model you have saved a lot of work and more people can understand it.
Note 2 – Solar radiation is from a tiny “angle” in the sky, and so the radiation is effectively “captured” by the earth as a flat disk in space. This area is the area of a disk = πr². By contrast, radiation from the sky is from all around the planet, and so the radiation is effectively captured by the surface area of the sphere. This area = 4πr². See The Earth’s Energy Budget – Part One for more explanation of this.



Dear Sod. Your series of articles on the 2nd law have promoted a lively discussion. Folks who haven’t had a serious thought for years are comparing your lessons with their own experiences and are asking questions. This is what mature students do and is why so many teachers find them difficult – no instant acquiescence to Authority, see. They’ve been made fools of too many times following that route.
It’s no good getting arsey with guys and gals who are (mostly) doing their best to get their heads round a concept that is simple to you but unclear to them. Steve McIntyre invariably shows infinite patience and good humour when dealing with such people. It works for him, so it might well work for you.
Best of Luck.
Can’t you simply make the point that an object, ANY object, will radiate energy according to the Stefan–Boltzmann law. It does this independently of any other body that may be nearby.
In a nutshell the law says an object loses heat proportional to the fourth power of its temperature so hotter objects lose it really quickly compared to colder objects.
And then concede that with the 10C and 80C bucket example, the 10C bucket may well impart energy to the 80C bucket but the 80C bucket will always be losing energy much faster than it gains because of that very same law?
I think I’m right in saying that the laws of thermodynamics effectively state that you’ll never find in nature two objects that have emissivity/absorbtion characteristics such that the “colder” object will EVER heat the warmer object faster than the warmer object loses heat.
I have no problem with the maths and equations, I just object to the language used to express the results.
The fact that the warmer 3rd body means that body 1 will have a higher equilibrium temperature does not mean body 3 heats body 1. It simply means body 1 will cool more slowly than it would if body 3 radiated less.
You may say (many have) that this distinction ‘doesn’t matter’, but I contend getting it wrong has been responsible for the backasswards way the major climate processes are concieved of by the mainstream scientists. Thus they end up believing that back radiation can significantly heat the bulk of the ocean (it can’t), and that the atmosphere controls the climate despite it’s entirety having less heat capacity than the top two metres of the oceans (it doesn’t).
Absolutely. It’s assumptions like this that inevitably lead to results like positive feedback from clouds.
Tallbloke: Body 1 HAS an equilibrium temperature, it is not “cooling more slowly”. Body 1 IS warmer because of radiation from Body 3.
Any radiative imbalance (from a 2% increase in solar output or from a GHG increase) will eventually warm the ocean. It just takes a long time for the ocean to reach a new equilibrium temperature where incoming and outgoing energy are in equilibrium.
Your plots on upper atmosphere lagging SST are interesting, but I’m not sure the lag is constant back to 1979. Unfortunately the upper atmosphere temperature is tropical while SST is global. The lag could be caused by changes in the tropics requiring time to propagate to the rest of the global. The sunspots and humidity were interesting too.
@Frank “Any radiative imbalance (from a 2% increase in solar output or from a GHG increase) will eventually warm the ocean.”
Agreed in the simplistic case, but the timescale is very long indeed because it still relies on mixing down to actually happen.
However, the earth’s atmosphere is anything but simplistic and by the time you factor actual heating in with SW radiation and how clouds affect that, then suddenly its not so clear how the heating happens with cloud feedbacks.
AGW theory currently says clouds are a positive feedback but thats a joke. The simplest of thought experiments where the earth is surrounded by clouds leads to cooling and hence negative feedback.
Even if clouds have positive feedback right now, they must change to negative at some point and the problem is we simply dont know how they will behave.
TtTM please don’t build strawmen:
“AGW theory currently says clouds are a positive feedback but thats a joke. The simplest of thought experiments where the earth is surrounded by clouds leads to cooling and hence negative feedback.”
I think you need to read up on Stephen Schneider and the IPCC’s view on cloud feedbacks… The current consensus is that they are a negative feedback (but with large uncertainties).
http://www.skepticalscience.com/CO2-is-not-the-only-driver-of-climate.html
@cynicus, Its not a strawman argument. Its true that not all models assume a positive feedback from clouds buy many if not most do. Additionally there are papers that also try to justify it, for example Clement et al’s “Observational and Model Evidence for Positive Low-Level Cloud Feedback”
The IPCC doesn’t specifically say one way or the other but as you say acknowledges the uncertainties.
So if you re-read my comment and look at the following papragraph where I say “Even if clouds have positive feedback right now…” then perhaps you’ll understand the argument.
Likewise there is plenty literature discussing clouds as a negative feedback. Pointing towards one publication which argues positive feedback (for a specific type of cloud) might well be a cherry pick.
You can try to argue that science wants it to be a positive one but if you actually look at the combined science as reported (IPCC) you’ll see the experts consider it to be a negative one. With large uncertainties but negative none the less. Asserting otherwise really is a strawman just like Asserting that most models assume positive feedback.
See: e.g. http://physicsworld.com/cws/article/news/39908
“The team compared their findings with [cloud] feedback predictions made by 18 leading climate models. Only two models predicted a positive feedback and one of these — HadGEM1 from the UK’s Hadley Centre — was particularly good at reproducing the observed relationships between cloud cover, atmospheric circulation and temperature. ”
Lastly, science should go with the best argument. If there is a strong signal that cloud feedback is indeed positive (and signs are it is) then the current position should change accordingly. It’s not a matter of *wanting* it to be so or that it’s a *joke* because you say so.
Ok, enough with assertions, back to your argument.
Let’s assume for the sake of the argument that clouds are a positive feedback. You say that clouds *must* become a negative feedback at some point. Why? Please explain why it must.
@cynicus “according to a study of cloud and temperature records from the north-eastern Pacific Ocean.”
If you’re going to make an argument, at least make it an honest one. The study you pointed to was (according to the map) largely based in the tropics and a bit to the North East as suggested by the cough non-biased journalist.
That squarely supports my argument because the tropics has the most cloud.
“You say that clouds *must* become a negative feedback at some point. Why? Please explain why it must.”
I gave the reason. At the limit, clouds are undeniably a cooling mechanism so at some point even if they’re seen to be a positive feedback right now, they must swap to negative.
Here’s an analogy. If Point A on a road is at 200m elevation and, 1 km down the road, point B is at sea level and you know this to be true, then what faith do you have in someone telling you that because the first 50m of the road rises, then the next 950m will also rise? Or even the next 50m will rise?
Tallbloke,
OK, so, if body 1 is within some equilibrium range, there more or less constant energy coming in to body 1, and the rate of cooling (energy lost) slows down, body one warms up to a new equillibrium range. No?
TimTheToolMan,
I can’t claim complete knowledge of the domain, but I’ve seen enough acknowledgment amongst mainstream scientists that the overall effect of clouds is uncertain, that I don’t think it is accurate to portray the mainstream as being a proponent of clouds being a positive reinforcement. The effects are of different signs between tall clouds and thin clouds, and high clouds and low clouds. What I’ve seen so far is that most scientists admit the sign of the total feedback effect of clouds in a warmer earth is still obscure. Even if the sign of the feedback is negative, the paleoclimate record indicates that any negative feedback that it might have is of less magnitude than the positive feedbacks; else, the magnitude of the recorded climate swings in the past would not have been possible.
You make a very valid point with the emission of energy and Stefan-Boltzmann. It works the other way too, bodies will always absorb some fraction of the energy incident upon them. If they absorb energy, they tend to get warmer than they would be if they did not.
Back to Tallbloke, not to take this to a level totally devoid of science and math, but if the air has so little effect on a body of water beneath it, why is there such a tight correlation between when winter cold fronts move through and when my neighbor’s pond freezes over?
(You know, there was once a scientist or two who ‘proofed’ that heavier-than-air flight was impossible. It’s not clear what they thought about birds.)
Thanks SoD, I agree that the heart of the disagreement lies in the conceptual level. You have to come to an agreement on the concepts before it’s worthwhile to argue about the details.
Perhaps it might be useful to think in terms of just energy rather than radiation, thermal heat, chemical, phase state, etc. Energy can be transformed between various forms readily enough in the presence of matter. The total amount of energy in the earth system, or what the equilibrium will be, is determined very much by the rate of radiative energy in versus the rate of radiative energy out. There are other energy gains and losses, but they are orders of magnitude smaller, and they haven’t been changing much in the last century.
I’m comfortable with probabilities; so, I’d like to propose something at the conceptual level along those lines.
Let’s consider photons in and photons out. Actually, let’s just acknowledge that there is a stream of inbound photons, and focus on outbound photons. Photons are packets of energy. How much energy is within the system depends on how long energy stays within the system before exiting. Since the vast majority of energy leaving the earth leaves in the form of photons, how much energy is within the system depends on how likely it is that any given photon will encounter an obstruction on the way out. The likelihood of any given outbound photon encountering an obstruction is dependent on how many obstructions there are. A CO2 molecule is an obstruction within the wavelengths of photons that the earth emits. Therefore, more CO2 molecules results in more packets of energy being intercepted (absorbed) along their exit path.
Any photon intercepted will go through some transformation and become energy of another nature. It does not really matter at this level whether that is a higher state of excitement within the electron shell, between the bonds that hold atoms in a molecule together, or if it immediately gets re-radiated or if it gets thermalised and stays around a little longer. The energy stays within the earth system, and it does not have another chance to leave the system until it becomes a photon again. The more transitions there are between photon and another form of energy, the longer the energy will stay within the system, and the more energy will be contained within it.
More energy in the earth system leads to higher temperatures of air, land, and sea, more humidity, more convection, changes is Hadley Cell circulation, changes in the thermohaline cycle, etc. There is little point to debate how much change to expect in each of these, and what their feedback effects will be, until there is an understanding that the overall amount of energy will go up when there is more restriction in the outbound flow.
Sorry, this is so basic that I’m embarrassed to post it here, but let’s see where our conceptual differences lie.
Strictly speaking, you can’t prove a theory in science (as opposed to math where the terminology is theorem), you can only disprove it. Simple models or thought experiments are useful in this regard.
tallbloke: “The fact that the warmer 3rd body means that body 1 will have a higher equilibrium temperature does not mean body 3 heats body 1. It simply means body 1 will cool more slowly than it would if body 3 radiated less.”
This is how I, as democratic entity and non-scientist who does actually give a damn, understands the situation. If by “heat” you mean “adds energy to,” then yes it does. It doesn’t necessarily mean that the object will be hotter than it was five minutes ago–unless the object is the recipient of a source of energy that keeps it (all other things having been equal) at a particular temperature.
This is all rather obvious, because if we strip away the atmosphere, the oceans freeze solid (in other words, if the atmosphere is such a small-time player, then why is the ocean liquid?). If we then add our atmosphere, the ice will begin to melt. Does the atmosphere, in this case, “heat” the ocean? Yes, but the energy coming from the atmosphere is puny, but not insignificant, next to the power of the force, err, sun. The sun is–relative to the atmosphere–a stable source of energy. Despite the tremendous capacity of the ocean as a heat sink, the radiative mechanics of the atmosphere ultimately control it. The temperature of the Earth is overwhelmingly determined by the amount of incoming solar energy the Earth receives at any given moment, and by the ability of the atmosphere to delay the radiative exit of that energy. If the sun is a relative constant but the system is heating up, then we have to look for a change in the system. We know what CO2 does, physically, and we know we’re increasing atmospheric CO2 content, so that seems like a useful place to look. Evidence is easy to find: is the top of the atmosphere cooling? Yes. Is downward longwave radiation increasing? Yes. What else could cause these two events and still be consistent with everything else?
“Despite the tremendous capacity of the ocean as a heat sink, the radiative mechanics of the atmosphere ultimately control it. ”
No. It affects it, but it doesn’t control it. If you look at a time series of the changes in ocean temperature and atmospheric temperature side by side, you’ll see that the ocean leads, and the atmosphere follows a few months later.
http://www.woodfortrees.org/plot/uah/from:2000/mean:6/plot/hadsst2gl/from:2000/scale:1.7/mean:6/offset:-0.3
A simple appreciation of the law of cause and effect will inform you that the ocean controls the atmosphere more than the other way round.
And since LW radiation from the atmosphere doesn’t heat the bulk of the ocean, there is no mechanism for the atmosphere to ‘control’ the oceans on a longer timeframe either.
The Sun’s variability controls the atmosphere’s humidity too, unless you think the atmosperes humidity controls the Sun?
Does this correlation strike you as being more convincing than the correlation between the rise of co2 and changes in atmospheric specific humidity at the tropopause?
Can you offer any other explanation?
“A simple appreciation of the law of cause and effect will inform you that the ocean controls the atmosphere more than the other way round.”
Ah! yes that famous physics law.
It is somewhat amusing that SoD gets accused of wandering off course with language, yet some resort to philosophy.
Tallbloke: “No. It affects it, but it doesn’t control it. If you look at a time series of the changes in ocean temperature and atmospheric temperature side by side, you’ll see that the ocean leads, and the atmosphere follows a few months later.”
What I mean is that if you take away the current atmosphere, the oceans freeze, convection is minimized, and albedo increases. The oceans, at that point, aren’t capable of doing much. The oceans contribute to the current composition of the atmosphere, but an atmosphere was in place before the oceans formed. The thing about the ocean is that it’s there. It doesn’t change all that much by itself. It keeps on doing what its doing. As plates shift, the oceans change. As the atmosphere changes, the oceans change. As life within the oceans changes, the oceans change. Ocean temperature is driven by insolation, atmosphere, and warmth coming from the mantle. When those drivers change it, it changes the atmosphere, and in turn, etc. etc. The only way the ocean becomes variable is if it changes composition, and given the size of the ocean, that’s hard to do (unless there’s a massive loss of ocean life or polar ice, leading to a change in albedo).
Yah, sure, the sun is the ultimate driver, but is solar fluctuation the root cause of the current warming? The last 15 years of warming have occurred during a decrease in insolation. As for your graph, I’m not quite sure what you’re saying. How do you describe the relationship between global humidity and global warming?
Nice graphics, Doom. Thanks. I’m increasingly impressed by your patience and by your determination to establish some understanding of and broad agreement on the basic physics before getting onto perhaps more controversial stuff. Keep it up.
I (too) wonder how long it will take realclimate to provide a link. Are they scared or something?
I also think Science of Doom deserves a link at RealClimate.
I can just imagine that they did not pay due attention to SoD’s great work yet.
‘This is how I, as democratic entity and non-scientist who does actually give a damn, understands the situation. If by “heat” you mean “adds energy to,” then yes it does. It doesn’t necessarily mean that the object will be hotter than it was…’
I imagine a democratically minded non-scientist would go by the dictionary definition of the verb ‘heat’, that is: to make hotter.
tallbloke on November 5, 2010 at 1:59 pm:
There’s no confusion that I have ever found in all the textbooks and papers I have read.
After all, this is a very simple subject – the first law of thermodynamics and the equations of heat transfer for conduction, convection and radiation.
The challenges are in other areas.
Why not produce some evidence for your claim that climate science doesn’t understand heat transfer.
“Why not produce some evidence for your claim that climate science doesn’t understand heat transfer.”
I didn’t say they don’t understand it, I said that sloppy linguistic formulations such as yours lead to people making incorrect assumptions about energy flows within the climate system. Like back radiation heating the ocean, rather than merely affecting the rate it cools at.
Tim the Tool man has it right, John Millet has it right, I have it right. You are in a minority here.
Once you (inappropriately) disassociate the DLR “heating” from the radiative cooling then in a complex non-linear system like the atmosphere, you run the risk of stuffing it up.
If scientists and modellers are thinking this way then the risk is very real.
Sod:
I think your entropy calcs are a bit meagre. How do these look?
Entropy calcs across the boundary between Body 3 (space) and Body 1, from the latter’s perspective:
For very very cold space: +57.7/255 – 3.01*10^9/255
The decrease in entropy invalidates radiation from space to Body 1.
For cold space: +2.78*10^9/300 – 5.79*10^9/300
The decrease in entropy invalidates radiation from space to Body 1.
From Body 3’s perspective:
-57.7/3 + 3.01*10^9/3; and
-2.78*10^9/3 + 5.79*10^9/3
In both cases, increasing entropy validates radiation from Body1 to space.
Thus only the sun heats the earth which maintains equilibrium by cooling by radiating to space and the entropy calcs go like:
For very very cold space:
+3.01*10^9/255 – 3.01*10^9/255 + 3.01*10^9/3
That is, increasing entropy and OK
For cold space:
+3.01*10^9/255 – (3.01-2.78)*10^9/255 + (3.01-2.78)*10^9/250
That is, increasing entropy and OK.
The earth cools slower surrounded by cold space than by very very cold space by virtue of the lower temperature potential between them, not by radiation from space to earth.
I second hr’s comment about RealClimate. Science of Doom deserves a link there.
I can only think that they did not pay due attention to SoD’s great work yet.
Warmcast
“A simple appreciation of the law of cause and effect will inform you that the ocean controls the atmosphere more than the other way round.”
Ah! yes that famous physics law.
It is somewhat amusing that SoD gets accused of wandering off course with language, yet some resort to philosophy.
No wonder the warmies have such a hard time working out the significance of the fact that at the end of ice ages, temperature rises 800-2800 years before the co2 level does. 🙂
(Didn’t see a “Reply” link to TTM’s post)
TimTheToolMan:
“AGW theory currently says clouds are a positive feedback but thats a joke. The simplest of thought experiments where the earth is surrounded by clouds leads to cooling and hence negative feedback.”
I’ve noted that on overcast nights, the air temperature is higher (often quite a bit higher) than on clear nights, particularly in the wintertime – at least around here.
Apparently my observations are a “joke” or more simple than “simplest”. How have I gone wrong?
You are correct that overcast nights trap heat. However, if you consider the difference between the amount of Earth converted solar energy being emitted by by a cooler night-time surface and trapped by cloud at night time, in comparison to the amount of solar energy reflected back into space during daytime you can probably see that there will be a bigger cooling effect from cloud cover during daytime, than there will be a warming effect from the same amount of cloud at night-time.
I’d be interested to see some figures from empirical measurements but a priori, that seems right to me. I’m always happy to be corrected by experimental results though!
It seems to me that the reality of in the interaction between the sun, clouds, the air and the surface is quite a bit more complicated than your choice of the words “joke” and “simplest” would indicate.
But I’ll leave it at that.
@Derecho64 : “Apparently my observations are a “joke” or more simple than “simplest”. How have I gone wrong?”
In order to see the ultimate effect of clouds being a negative feedback you need to take cloud cover to the extreme. Completely cover the earth with cloud and then you have much reduced short wave solar radiation reaching the earth. Thats very definitely a cooling effect.
The effect you describe is one of retaining heat. Thats no good if the heat never got here in the first place.
Tim: Venus is covered with highly reflective clouds. By your logic, it must be a really cold place.
Oversimplifications are worthless and a waster of time. All clouds don’t behave in the same way. High clouds are very cold and supposedly can’t radiate much energy to space. Low cloud are warmer and radiate more energy to space.
Derecho64
Clouds – condensing water vapour releases a lot of heat.
This means that the temperature difference between surface and clouds is reduced.
Consequently the convection effect (the main heat transporter) is reduced.
Hence you will notice the temperature is higher.
Do you have calculations to back that assertion up?
Yes, I’d like to see the calculations for the effect of condensation on temperature.
I’ve noticed this overcast “heating” effect most often in the wintertime, when the humidity is very low, and there’s really not much water vapor available. Ergo, it stands to reason that the effect of condensation to change temperature is also low.
Derecho64 cynicus
..”Do you have calculations to back that assertion up?”..
Look up any good set of Physical Tables.
Latent Heat of Vapourisation of water = 2,230,000J/Kg
Bryan, The boiling point of water at sea level is 100 Degrees C. Yes, everyone can loop up a number in a table but that doesn’t make it an calculation.
The question is: how much does the condensing heat up the earth? Therefore you need to know how much water there is per m3 and calculate how much heat that would release. And by how many degrees C would that increase the temperature for that m3?
Another question for your wisdom: if a cloud has already been formed, how much heat does it continue to produce?
You need to know these answers for your assertion to be anything more then a fantasy.
cynicus
….”You need to know these answers for your assertion to be anything more then a fantasy.”….
From Wikipeadia
Gaseous water represents a small but environmentally significant constituent of the atmosphere. The percentage water vapor in surface air varies from a trace in desert regions to about 4% over oceans[10]. Approximately 99.13% of it is contained in the troposphere. The condensation of water vapor to the liquid or ice phase is responsible for clouds, rain, snow, and other precipitation, all of which count among the most significant elements of what we experience as weather. Less obviously, the latent heat of vaporization, which is released to the atmosphere whenever condensation occurs, is one of the most important terms in the atmospheric energy budget on both local and global scales.
I wonder whether the overhead cloud is reflecting radiation from a warmer surface in the proximity. The absence of atmospheric reflection of LW in the K&T energy budget, which includes reflection of SW, puzzles me. Intuitively, lower intensity LW is more likely to be reflected than higher intensity SW?
I don’t understand why sloppy language regarding use of the word ‘heat’ leads to broader confusion about whether or not the oceans participate in global warming due to increased greenhouse gases. Perhaps someone can explain this.
I do think that it is perhaps technically incorrect to say that the atmosphere heats the earth. For example, when you are cold and you put on a sweater the sweater does cause the temperature of your skin (and/or air very close to your skin) to increase. That doesn’t mean that it is proper to say that the sweater is heating your skin.
Mike, there have been a couple of threads on back radiation and its (in)ability to affect the amount of energy contained in the bulk of the ocean recently. have a read.
SoD
I think you missed the problem I set.
Now that you have access to the 8 books on heat transfer and you think yourself quite an expert, try the following simple problem.
Use an Ideal Carnot engine to transfer 200J of heat at 278K up to 200J at temperature of 330K
.
How much extra energy is required to acheive this?
Hint the answer is quite definitely not zero.
This little problem should illustrate the difference between the first law and second law of thermodynamics.
Bryan,
It’s not clear that Joules have a particular temperature associated with them. So, it isn’t clear what you talking about, or how this relates to the problem at hand.
Chris G
This is a standard Physics problem, copied directly from a textbook(without the reference to SOD of course).
We are talking about energy in the form of radiation. It’s not clear how your example involving a piston and convective transfer of energy relates.
*conductive transfer
It is convective… Heat/pressure is still the same basic principle as radiative exchange… But i “think” brian may be missing the point… from what has been discussed here, SoD would be saying, that if you raise the T o the exhaust chamber, you will get less work outta the engine… if you where translating this stuff to engines n all. Smaller differential between expansion and exhaust.
John Millett:
I don’t understand your entropy calculations.
[Model 3A] Consider the exchange of energy between 1 & 3:
Body 1 loses (3×10^19 -57)=3×10^19 at 255K
Body 2 gains (3×10^19 -57)=3×10^19 at 3K
Entropy change for that “system” = 3×10^19 x (1/3 – 1/255) = 3×10^19 x 0.33 = 1×10^19 J/K.
Net gain in entropy.
Strictly speaking it isn’t a closed system but that would be the right way to do the analysis.
And your final comment suggests you are continuing your claim from an earlier article that I have already explained is invented physics.
Sod:
An entropy increase will always result from your method, because it substitutes the qualifying word “net” applied to heat flow for the one that appears in thermodynamic laws, “spontaneous”.
Why can’t I physically reason thus:
I have two bodies and I want to know the direction of spontaneous heat flow between them. I measure their temperatures and do the entropy calculations. These give a positive result for an assumed direction and a negative one for the opposite assumption. Since the entropy law states that spontaneous processes may result only in an increase in entropy, I am able to determine the one direction of spontaneous heat flow.
IEHO, a great deal of this problem comes from the laws of thermodynamics being set in stone before people knew much about thermal radiation. Not to pick on them, but folk such as Gerlich and Kramm keep asking what the “most general statement of the second law is”, without acknowledging that the second law only sets limits on the net interchange of heat and energy by all mechanisms.
As pointed out by many, the fact that a colder body radiates energy is absolutely correct. The fact that when this energy strikes a warmer body, the warmer body will absorb some of it is also absolutely correct. The NET transfer of thermal energy will be from the warmer to the colder body, but, the warmer body will cool more slowly because of the radiation it absorbs from the hotter one.
a great deal of this problem comes from the laws of thermodynamics being set in stone before people knew much about thermal radiation.
Judging by the number of AGW proponents who still believe longwave back radiation from the atmosphere can heat the bulk of the ocean, a ot of people still don’t.
The fact that when this energy strikes a warmer body, the warmer body will absorb some of it is also absolutely correct.
Unless the warmer body is opaque to that radiation, as the global ocean is to the wavelengths emitted by the atmosphere.
the warmer body will cool more slowly because of the radiation it absorbs from the hotter one.
This statement is just nonsensical. You’d be better off sticking with trying to teach chemistry.
“IEHO, a great deal of this problem comes from the laws of thermodynamics being set in stone before people knew much about thermal radiation.”
That pretty much sums up the situation.
According to Doom’s Law the warmer body absorbs all of the colder one’s radiation, not just some of it.
TimTheToolMan on November 6, 2010 at 9:21 am:
The great Ramanathan, for example, says:
Trace Gas Greenhouse Effect and Global Warming, V. Ramanathan, Ambio (1998).
By the way, it is an excellent review article that is well worth reading and I’m sure it used to be accessible for free, but every link I found just now when I checked is behind a paywall. For those who have $16 to spare, it is well worth it: link here.
I would encourage everyone to take the opportunity to find out what climate scientists actually think by reading their work, rather than reading what other people think that they think.
Lots of papers are available free. Even more aren’t which is a travesty..
For example, if you want to find out about “ocean diurnal cooling” –
go to scholar.google.com
type ocean diurnal cooling into the search box
You will see a number of articles with a pdf link on the right hand side – these are usually, but not always, free.
For example – “Diurnal cycling: Observations and models of the upper ocean response to diurnal heating, cooling, and wind mixing” by Price and Weller (1986) – well worth a read, and many others.
But I digress.
tallbloke:
The challenge of understanding past climate change is definitely a difficult one.
See Ghosts of Climate Past for a brief introduction not yet followed up.
However, as you are so free with claims about “what other people think”, I also offer the following link: CO2 Lags Temperature in the Ice-Core Record. Doesn’t that prove the IPCC wrong?
SkepticalScience also has a good discussion on the CO2 lags temperature meme: http://www.skepticalscience.com/co2-lags-temperature.htm
There is a great deal of research in that discussion, which isn’t unsurprising as mystery of ice ages were the primary reason why pioneers in the 18th century discovered the greenhouse principle and CO2 link with climate change. Spencer Weart provides a peek in history: http://www.aip.org/history/climate/
Tallbloke assertions just seem to originate from ignorance.
tallbloke
“No wonder the warmies have such a hard time working out the significance of the fact that at the end of ice ages, temperature rises 800-2800 years before the co2 level does”
And of course the same thing that happened in the past, has to always be what happens now or in the future, right?
So, are saying that because CO2 may have followed initial warming in the past, there is no greenhouse effect? You know that 150 year old theory? What logic says that because some other past forcing initiated warming which in turn increased atmospheric CO2, (which of course would increase warming additionally due to the greenhouse effect) CO2 cannot initiate warming at another time, like now. I have never understood the point of this skeptic argument. It makes no sense.
So, are [you] saying that because CO2 may have followed initial warming in the past, there is no greenhouse effect?
No. If you do the calcs in purely radiative physics terms, the misleadingly named ‘greenhouse effect’ would make the Earth around 60C hotter than a no atmosphere scenario.
The fact that the Earth is estimated (tentatively) to be only around 33C hotter than the no atmosphere scenario shows there are strong processes at work which oppose the radiative processes. Albedo, the hydrological cycle, thunderstorm activity etc.
You know that 150 year old theory?
I’m guessing you are referring to Arrhenius’ co2 warming theory? The one he watered down considerably later on after the heavy criticism it recieved from his peers?
What logic says that because some other past forcing initiated warming which in turn increased atmospheric CO2, (which of course would increase warming additionally due to the greenhouse effect) CO2 cannot initiate warming at another time, like now.
So that would be the Sun then?
What logic says that because the sun was able to warm the climate in the past it didn’t in the second half of the C20th when it’s activity was well above the long term average? (Most active in 8000 years according to the cheif solar physicist at the Max Planck Institute), but instead a trace gas occupying 0.039% of the volume of the atmosphere took over the role of forcing the climate? Even though a quick look at co2’s inter-annual rise and fall shows that changes in it’s atmospheric level still lag behind changes in temperature, just like it always has at all timescales over the last several millions of years?
Extra-ordinary claims require extra-ordinary proof as Leif Svalhgaard is fond of saying.
Tallbloke: “What logic says that because the sun was able to warm the climate in the past it didn’t in the second half of the C20th when it’s activity was well above the long term average? (Most active in 8000 years according to the cheif solar physicist at the Max Planck Institute)”
Here you refer to Solanki 2004 whose results are controversial (judging by the comments), but there is much more research on this topic. Does that show the same results, i.e. is there agreement on this?
And by your own logic: What about the hottest 11 years in the measurements (2000-2010) while the sun was at it’s quietest since 1940? http://skepticalscience.com/solar-activity-sunspots-global-warming.htm
Lastly, even if solar activity was the highest in several millennia does it automatically follow that the current warming is the result? Well, the following is a conclusion one sees often in the literature: “The (TSI) variations measured from spacecraft since 1978 are too small to have contributed appreciably to accelerated global warming over the past 30 years.”
Now the burden is on you to prove that the correlation inplies causation (and explain the obvious discrepancies).
Tallbloke: “but instead a trace gas occupying 0.039% of the volume of the atmosphere took over the role of forcing the climate?”
Now consider the height of the atmosphere and calculate with how many meters you can blanket the earth if you put all of the CO2 in the column in a band of pure CO2. Then watch this video: http://www.youtube.com/watch?v=SeYfl45X1wo
Tallbloke: “Even though a quick look at co2′s inter-annual rise and fall shows that changes in it’s atmospheric level still lag behind changes in temperature”
Wow! You have just discovered that trees in the NH start inhaling more CO2 when spring comes. Genius! Who ever knew that.
But, tell us, what does this yearly cycle have to do with the start of the fossile emissions in 1750, the exponential averaged increase of CO2 since then and the emergence of the AGW signal from the noise in 1980?
And exactly what temperature rise is CO2 supposed to be following when the current levels have not been seen in well over 400.000 years?
It seems like your extra-ordinary claims aren’t very well rooted in extra-ordinary proof. One may even ask: what proof?
Eli Rabett said:
tallbloke responded:
I think that there is an obvious typo in Eli’s statement and probably everyone can see what it is (including Eli when he looks back at it), so rather than being insulting it would be better to ask for clarification “did you really mean that?”
I believe Eli meant to type:
If we consider only radiation, a body radiates at:
j = εσT^4
If that body absorbs no energy from other sources the body will cool down at a rate determined by:
ΔT = ΔQ/mc
where ΔT is the change in temperature, ΔQ = change in energy, m is mass and c is specific heat capacity.
We can solve this quite easily.
If the body absorbs radiation from a colder source, the net outflow of energy will be:
Eout = εσT^4 – Ein
Clearly it will cool more slowly. Therefore, clearly the temperature will reduce more slowly.
Is anyone in doubt about this?
Eli is never shy of dishing out insults himself, so I’m sure he can handle my jibe. 😉
Your equations are fine. Do you take my point about the situation being modified by the question concerning the ability of the warmer body to absorb the wavelength of radiation being emitted by the cooler body?
There does not seem to be much left of the claimed “greenhouse effect”.
Most commentators see that night time conditions are best to test what remains of the effect.
What appears to be the overwhelming consensus is that the heat flow is from Earth Surface to a somewhat cooler atmosphere.
Is there anyone left that disputes that?
The atmosphere insulates the Earth involving all 4 processes of heat transfer(including radiation)
……”.I believe Eli meant to type:
..the warmer body will cool more slowly because of the radiation it absorbs from the cooler one.”………
Arthur Smith said something similar elsewhere.
Now this “insulating effect” is the same as we notice when we wear a vest.
Incidentally the vest has even better radiative properties than the atmosphere.
However no one would describe the insulating effects of the vest as due to the “greenhouse effect”.
I don’t know about the effectiveness of insulting Eli Rabett, but physics has no feelings so calling a GHG a “trace gas” is ineffective. There has been a substantial increase in GHGs, regardless of the amount of nitrogen in the air.
Just out of interest, here is a simple comparison.
Two bodies in a closed system, one starts at 1000K and the other at 500K, both of equal thermal mass.
The first graph shows the change in temperature over time with the “real second law” and the second graph shows the change in temperature over time with the “imaginary second law”.
The mathematical description of these two cases can be seen in Amazing Things we Find in Textbooks – The Real Second Law of Thermodynamics
The first case is what people would expect.
In the second case we see that the temperature of body 1 drops somewhat lower- because it isn’t absorbing energy from body 2.
The temperature of body 2 therefore doesn’t rise as much – because body 1 is radiating a lower value at any given time.
Once the two bodies reach the same temperature suddenly body 1 will absorb the energy from body 2 – and as they are at the same temperature, they stay at the same temperature.
Of course, everyone reading this blog who has read Amazing Things we Find in Textbooks – The Real Second Law of Thermodynamics knows that the first case is what actually happens, whereas the second case is fiction..
tallbloke:
What exactly is your claim?
It’s not a claim, it is well established physics. Longwave radiation cannot penetrate water far enough to heat it. The extent to which it will slow down the rate it cools at will depend on many other processes and forms of energy transfer which are not amenable to a consideration of radiation only.
You repeatedly claim that longwave radiation’s inability to heat water is long established physics? You’ve got to be kidding…in what universe is that?
Sure, longwave radiation does not reach deep into water, but mixing of the water will transport the water to deeper levels. If energy is absorbed then it’s absorbed and the energy is added to the receiver.
An analogy: The same goes for the human body. The longwave radiation from a stove will not penetrate deep into the flesh but it does warm you up. This should not be possible because it’s long established physics?
The longwave heating of the top ocean layer has even been measured as reported by RealClimate: http://www.realclimate.org/index.php/archives/2006/09/why-greenhouse-gases-heat-the-ocean/
So, why is that so difficult to accept, you’ve been told that before even by fellow skeptics. Is such a simple idea blocked by your belief that CO2 cannot be a greenhouse gas because that would mean humans are responsible for the current warming?
If this is not just your belief system but really is long established physics, can you provide some literature to back up your claims?
Derecho64 @ November 7, 2010 at 7:15 pm Derecho64
Are the low clouds a sign of a warmer more humid air mass, or the air mass warmer and more humid because of clouds?
Obviously a frost forms on still nights,with low humidity(less opaque), when the atmosphere at the surface becomes stratified, so less DLR compensating for radiative losses from the surface. A bit o wind will also stop a frost, by mixing the air, resulting in more homogeneous surface atmosphere/temps. But i think clouds would work to stop a frost, by absorbing LW and heating at this higher level, preventing convective losses/slowing stratification from the levels below the clouds.
But thats just my take on it, Dewitt and SoD have commented on this before, but it was a long time ago, and i cant recall what thread.
preventing convective losses/slowing stratification
slowing convective and radiative losses.
preventing convective losses/slowing stratification from the levels below the clouds.
should be slowing convective and radiative losses.
SoD, maybe you can add a simple graphic to this article explaining the word ‘heating’ in scientific understanding?
Allow me to suggest two circles with two different temperatures and two arrows with different thickness between them indicating the different amounts of energy flow, both with the word ‘heat flow’ so that readers would be educated that the scientific meaning of heating is energy flux (transfer of energy).
It seems to cause confusion to some lay-people who think it stands for the net flow of energy instead and then start accusing you and science in general of sloppy linguistic formulations …
Apparently up is down and left is right for some although Occam Razor disagrees.
heat, is the net flow of energy…. that is its definition, the problem is that people think that energy exchange and heat are one and the same. But heat is the net flow, in radiative exchanges, the product o T1-T2.
But heat in everyday english, o course can also be referring to the thermal state, or a change in the thermal state of something. In the english language there are many words with dual meaning, its the context in which they are used that donates the definition. I dont care how people use it, for comunicating to as wide an audience as possible its probably best to use it as it is commonly used as opposed to the TD definition.
To elaborate, why thinking energy exchange and heat being the same thing, can lead to misunderstanding the second law.
“the problem is that people think that energy exchange and heat are one and the same.”
The problem is, that because the second law is often stated as heat flows from hot to cold, people assume it means energy cannot be exchanged between a cooler to warmer body, thus proving that there is no GHE. When the second law is really that chaos(entropy/energetic excitement) increases(spreads) or stays the same in an enclosed system. So chaos will disperse from a greater concentration to a lesser concentration unless work is performed on it, and then it would take as much energy in work as can possibly be gained from the decrease of energy, its saying there is no such thing as free energy.(entropy stays the same, when energy is locked in molecular bonds, until it is released then it will spread)
But thats not to say that the boundary conditions dont effect the rate at which chaos spreads. Because obviously it cant increase the concentration of chaos above its sources state, limiting the rate at which entropy increases(spreads)
And it does this, with radiation, by exchanging energy, but more will be flowing from the hotter body to the cooler body at all times. It certainly dosnt stop the chaos from spreading.
Exchange, energy for entropy where appropriate in above comment 😉
err, and T1-T2 with T1^4 – T2^4…. i really got to get in the habit o proof reading stuff!
Apparently cooling means heating for you.
tallbloke:
That’s why I am asking exactly what you are claiming.
Because earlier you said:
This could mean just about anything.
No one is claiming that you only consider radiation in a heat transfer system. So that’s not clear.
The “situation being modified” = ??
This could be a statement of the obvious that everyone agrees with or a contradiction of some fundamental physics.
If you are precise I can respond.
I agree wholeheartedly that any change in material properties or system properties will “modify the result”.
That’s why we have maths. So people have to make a claim that can be falsified.
scienceofdoom
….”That’s why we have maths. So people have to make a claim that can be falsified.”…..
Why then don’t you use some of your maths to do the simple problem I gave you earlier?
OK, I try to make it precise and simple.
The graph you posted on November 7, 2010 at 9:56 am is OK for a pair of bodies which freely absorb the radiation each other emits, but it doesn’t represent what happens between the atmosphere and the ocean, bcause as we already know, water does not absorb the longwave radiation emitted by the atmosphere.
Do you accept this?
TB: water does not absorb the longwave radiation emitted by the atmosphere.
Where does the energy go?
Bryan:
As regular readers of this blog will know, I have demonstrated the flaws in many of your claims and not once have you actually accepted that your earlier claim was flawed. You simply, “move on”. And then make new claims.
Not a complete list:
1. DLR is “Rayleigh-reflected radiation”
2. DLR is “mostly reflected” by the surface
3. Stefan Boltzmann law is problematic
4. Kirchhoff’s law is problematic
5. Warmer bodies can’t absorb radiation from colder bodies
6. The six textbooks proving that item 5 is wrong were cherry picked (“you must have discarded 99.99% of the ones that said the opposite”) and here’s my recommended textbook that.. oh, says the same thing. Oops.
Except you didn’t say “oops”.
SoD
Are you not getting a bit embarrassed with your method of half truth and sheer invention.
If anyone is still in doubt about SoDs claim to be objectively looking at the “science” all they have to do is pick any one of the above list and ask SoD to “prove it”.
For my part I have come to realise that SoD is more interested in pushing CAGW propaganda than investigating objective science.
Before finding the John Nicol paper(excellent) on tallbloke’s site, by coincidence I was reading a paper by Physisist Roy Clark.
He uses the methods familiar to climatologists but comes to very different conclusions to the IPCC.
Both use all the methods of heat transfer (including radiative transfer) in the atmosphere and find that there is very little to support the current IPCC view of CO2 as a real and present danger to mankind.
Gerlich & Tscheuschner came to the same conclusion following fundamental thermodynamic reasoning.
Now we have 4 Physisists of good standing coming to the same conclusion by different methods.
Their results confirm the practical experimental evidence that radiative effects are very small at atmospheric temperatures.
The simple Woods experiment has now been confirmed by more advanced theoretical considerations.
The Woods experiment has been vindicated for larger structures by the following study.
Click to access penn_state_plastic_study.pdf
This study fits in with Wood and also with practical experience known to hillwalkers.
http://windowoutdoors.com/WindowOutdoors/Staying%20Warm%20-%20Interesting%20Observations%20on%20Heat%20%
SoD claims he has not seen the Woods paper even though it is included in the Gerlich & Tscheuschner paper which he claims to have read.
SoD refuses to do the simple problem in thermodynamics that I gave him.
In short SoD refuses to look at anything that might contradict his AGW views.
The fact that an ostrich will stick its head in the sand does not alter the world around them.
As regular readers of this blog will know, I have demonstrated the flaws in many of your claims and not once have you actually accepted that your earlier claim was flawed. You simply, “move on”. And then make new claims.
Ah, the ol’ Gish Gallop. An all-time [————] favorite. (moderator’s note, please read the etiquette)
Generally with this blog I like to keep roughly on the subject at hand.
That doesn’t mean interesting digressions aren’t welcome – they are.
But it is all too easy for discussion on each article to become a random walk through every argument that rages in the blog world on climate.
And I would especially like this blog to be one that avoids comments that generalize the flaws of “the other side”.
Plenty of better blogs for that. For new visitors, please have a read of About This Blog.
@Bryan:
Look up any good set of Physical Tables.
Latent Heat of Vapourisation of water = 2,230,000J/Kg
True, but apply that to the atmosphere and compare it to the other factors that change temperature, so we can see if it’s relevant or not.
As the ocean heating issue is getting some coverage, I repeat a comment that I recently added at Does Back Radiation Heat the Ocean – Part Two:
—-
Just a comment that I will write a followup article on this, to cover the idea that because convection doesn’t work “upside down” DLR can’t penetrate the ocean.
Two introductory ideas:
1. Heat flows are caused by temperature differentials. If more DLR “appears” and adds more heat to the surface then less heat will flow between the deeper level and the surface.
Likewise for the reverse case.
2. Convection does work “upside down” because the winds stir up the ocean.
During higher winds the temperature differential between the surface and a few meters down is non-existent.
At night, due to cooling of the top layer, the ocean also mixes.
Therefore, heat is able to be transferred from the surface to the layers below.
I just note as well that so far no one has actually offered any experiments or analysis to demonstrate that DLR can’t “heat” the ocean (=”change its temperature). Just their “conceptual model” repeated as “proof”.
All for the followup article.
A couple of points to consider while writing your new post:
1) On average the ocean has a higher temperature than the atmosphere. Therefore, on the average, according to the second law of thermodynamics, heat flows from the ocean into the atmosphere. Heat doesn’t flow from the cooler to the hotter as you know, so maybe you are referring to energy rather than heat.
2) DLR can’t penetrate the surface of the ocean beyond ~0.06mm. This is well known physics.
3)“Convection does work “upside down” because the winds stir up the ocean.”
That is stirring, or as Nick Stokes has it, a form of advection, not convection.
4)“During higher winds the temperature differential between the surface and a few meters down is non-existent.”
I look forward to your provision of experimental evidence which demonstrates the quantities of DLR originated energy mixed into the top few meters of the ocean by high winds.
Hint: High winds greatly increase the rate of evaporation.
I think a major part of the problem comes from confusion of the term heat vs energy. Heat can only go one way; hot to cold. However, energy (photons) can go either way. The net energy balance is changed with nearby warm bodies and the heat transfer is reduced. This is effectively like a form of insulation. In the case of atmospheres, there is some radiation insulation, but convection heat transfer is not constrained (as it would be in a glass covered structure). The net effect does not violate any laws, and can, like any form of insulation, affect equilibrium temperatures. However, for the atmosphere, the main effect is to move the location of outgoing radiation to space to a higher altitude, and the atmospheric adiabatic lapse rate results in the heating. Saying the back radiation causes the heating is misleading because it doesn’t tell the who story.
whole story , not who story
tallbloke:
You have made it simple. But you haven’t made your claim precise.
Water does absorb longwave radiation. It absorbs it in the top few microns. The question is what happens subsequently.
You haven’t provided an analysis of what happens – or doesn’t happen – that can be tested.
For example, I could equally well claim that because the ocean only absorbs solar radiation in the top 10m it can’t mix into the deeper ocean. The ocean is on average 4km deep.
Are you claiming that convection works “upside down”?
In any case I will provide an analysis and I hope it will be interesting and lead to much discussion.
“I hope it will be interesting and lead to much discussion.”
Bring it on, and thanks for your interest in debating the topic.
First — a nitpick. You said:
“The Second Law of Thermodynamics. This law says that in a closed system entropy cannot decrease.”
I think you mean this to say: “…that in an isolated system entropy cannot decrease”. Closed systems can exchange energy, but not matter with their surroundings. Isolated systems do neither. The interior of refrigerators are able to jettison entropy with work being done.
Second — In helping out some of your readers who are having difficulty understanding how the 1st and 2nd laws of thermodynamics are not violated in climate science, here are a couple of questions to pose:
a) Have you ever noticed that on a cold, clear (dry) night that the surface temperature will rise for *no other reason* than the arrival of clouds overhead (even thin, high cirrus)? (or why is it that dew and frost never form on cloudy nights?)
b) How cold do you think it would become at the surface at night, if at night the atmosphere suddenly became transparent to all IR-excited (aka “greenhouse) gases?
“When a cloud absorbs longwave radiation emitted by the Earth’s surface, the cloud reemits a portion of the energy to outer space and a portion back toward the surface.
The intensity of the emission from a cloud varies directly as its temperature and also depends upon several other factors, such as the cloud’s thickness and the makeup of the particles that form the cloud. The top of the cloud is usually colder than the Earth’s surface.
Hence, if a cloud is introduced into a previously clear sky, the cold cloud top will reduce the longwave emission to space, and (disregarding the cloud albedo forcing for the moment) energy will be trapped beneath the cloud top.
This trapped energy will increase the temperature of the Earth’s surface and atmosphere until the longwave emission to space once again balances the incoming absorbed shortwave radiation. This process is called “cloud greenhouse forcing” and, taken by itself, tends to cause a heating or “positive forcing” of the Earth’s climate. Usually, the higher a cloud is in the atmosphere, the colder is its upper surface and the greater is its cloud greenhouse forcing.”
Source: http://earthobservatory.nasa.gov/Features/Clouds/
cynicus —
Thanks for posting the resource. I hope others found it useful. (Note, that I wasn’t posing questions for myself, but for others to consider.)
I was also trying to point out that there is no new or otherwise “unusual” physics pertaining to climate change as it relates to greenhouse gases. This seems to be the sticking point for a lot of readers. The most important concepts are already encountered in day to day life — with a little bit of observation of the world around us.
You lost me spaceman, ive always taken it that the isolated system part, is just so as that part of a process taken in isolation cant be taken as proof that the second law is flawed. And to my mind, a closed system is the same thing. Refrigerators/compressors/expander s etc can be used to decrease, or increase entropy, but the energy required to decrease entropy, will never be less than what is gained from the compression. So long as you dont look at part of a process, the second law holds true. whether its a closed or isolated system.
I could be wrong 😉 but im failing to see the relevance in the difference between a closed or isolated system. Both are just implying, that both sides of a process(compression/work required for the compression for eg) need to be taken into account.
Mike Ewing:
As I said, my first comment was mostly a nit-pick. I am not suggesting anything non-standard regarding the 2nd Law. It’s just that “closed” and “isolated” systems have their own definitions.
As I said, “closed” systems can exchange energy (in the form of heat transfer Q and work W in the 1st law), but not matter (in the form of the integral{mu*dN} in the more general first law, where mu is the chemical potential and N is the number of particles) with their environments, and “isolated” systems can do neither. Open systems do both.
1st Law: dU = dQ – dW + mu*dN
(allowing for possibility of an open system)
Although, if one were to get really nitpicky, one might argue that no truly/precisely isolated systems exist, due to the existence of long-range forces, especially curved space-time 🙂 .
tallbloke,
Then you haven’t understood what Nick Stokes actually says. Horizontal movement causes turbulence which leads to vertical eddies which increase heat transfer by what is called eddy diffusion. It isn’t exactly convection, but it isn’t pure diffusion either. It can be orders of magnitude faster than diffusion. Eddy diffusion is what allows you to mix sugar into your coffee by, as you say, stirring. It would take a very long time for that to happen by diffusion alone.
Hi De Witt,
I fully understand the mechanisms and the motions, I’m the son of a water engineer and I studied fluid dynamics at degree level. I also spend some time as a contracting machinist re-machining centrifugal pumps which were heavily corroded by pumping seawater at high pressures and velocities. In my younger days I also crewed and skippered cargo vessels in the Humber tideway and down the river Trent navigation. So I know quite a lot about the power of eddy currents, cavitation and understand turbulent flows better most people do.
And I also know the difference between convection and fluid motions driven by external forces.
I have written it off as a terminological difference between S.o.D. and myself, though once again, I find the sloppy use of language runs the risk of misleading people into thinking energy transfer from the atmosphere to the ocean surface achieves something it does not.
Interesting review. Essentially correct.
Time (second) is not a thermodynamic variable.
Gibbs.
G = H – TS
delta (G) = delta (H) – T (delta S)
My conclusion is: Some scientists are (unsuccessfully) seeking heat (energy) lost.
tallbloke:
Impressive claim. However, despite the outstanding appeal to authority, I feel the need to get some clarity.
Location A is at temperature Ta.
Location B is at temperature Tb.
Ta > Tb
Water moves quickly from A to B. What is the correct terminology?
1. With mass transfer, heat is transferred and this process of heat transfer is called convection
2. a) It is only called convection when the water moves via “free convection” (buoyancy driven)
b) And so when it is forced by an external force it is called “_____”
3. The heat has to stay behind.
4. ___________ (an option I haven’t thought of).
What do you choose?
My choice is no 1.
Following which, my own less impressive appeal to authority will come from a number of textbooks. I can only claim that heat (in traditional thermodynamics) moves by one of three methods:
a) conduction
b) convection
c) radiation
In climate science, for “sensible reasons”, heat transfer is described as taking place by:
i) sensible heat = conduction and convection without phase change
ii) latent heat = movement of heat via phase change (e.g. water to water vapor) plus transport (covered under “convection” in the standard terminology)
iii) radiation
The reason for this – that I surmise – is conduction of heat is mostly irrelevant in the real climate. And heat transfer by phase change of water to water vapor and then back –latent heat – is very significant and needs to be considered on its own.
SoD
What have we here!!!!
…..”This means that whether they gain or lose energy, their temperature will stay almost exactly the same”………
Is this not the very assumption you will not allow me?
Why do you ask me to account for every last photon from the cooler body when you boldly free yourself of this detail!
I think we should be told!
SoD actually told you, but you didn’t read:
“To make the problem much easier to solve we will state that the heat capacities of Body 2 and Body 3 are extremely high.”
This is quite a good approximation if you consider that bodies B2 and B3 are “Sun” and “Space”.
jon
What about the Earth then jon, its pretty big too!
From Fundamentals of Heat and Mass Transfer, by Incropera and Dewitt, 6th ed. (2007):
The first two chapters of this book on convection are, without any shame or apology, about “forced” convection. Then they move onto a chapter about “free” convection.
Heat transfer handbook: Volume 1, by Bejan & Kraus (2003)
Chapter 5: Forced Convection (Internal Flows)
Chapter 6: Forced Convection (External Flows)
Chapter 7: Natural Convection
And they have the audacity (or is it “hubris”?) to state:
And go on to say:
Fair enough, perhaps this has become more the convention to separate out phase change as a separate method.
Later, they say:
I have a feeling a very large part of the disagreements come from undefined terms used in the arguments. For example, I’ve always understood convection to be a movement of particles/mass (something you can associate kinetic energy to) and heat transfer by convection is only a biproduct of this as “heat has a tendency to stick to particles”.
“Convection is a method of heat transfer” – is not a defintion of convection but a statement that heat can be transfered through convection. For example you can have isothermal body of gas which is ‘convectioning’ (I made that word up just now, but you know what I mean) like a madman without any heat being transfered (at least by most common definition of heat).
PS. SoD – have you thought about adding something of a glossary somewhere here where more used terms are defined. It would be useful to have a definition to have a definition to refer to as the same words tend to be used in quite different meanings in different contexts.
“I have a feeling a very large part of the disagreements come from undefined terms used in the arguments.”
I think the main reason for the disagreement is that in the face of reality and the second law of thermodynamics, warmists have to argue that the cooler atmosphere somehow heats the warmer ocean. Because if they can’t , their theory is dead in the water.
tallbloke
Its strange how similar SoD and our old friends Gerlich & Tscheuschner are.
Gerlich & Tscheuschner argue that most IPCC supporters “greenhouse theories” involve a violation of the second law.
Namely that they argue that heat moves from a colder to a warmer body.
More perceptive people who support the IPCC position like Nick Stokes and Arthur Smith say its better to think of the atmosphere reducing the heat loss from the Earth Surface , but SoD will have none of it.
In post after post SoD plugs away at at “heat”, or heat, or heating effect from cold to hot.
I think a number of regulars like Leonard Weinstein are getting tired of the repetitions and would like to move on.
There was a good example of a more constructive dialog when Leonard Weinstein and Arthur Smith and others discussed the thermodynamics of the atmosphere of Venus.
Remarkably, ocean heat content keeps increasing.
Well I’m glad that at least they distinguish between good old fashioned no qualifying adjectives required unfettered “free” convection and the other kinds they elucidate. it’s a pity you didn’t do the same to start with, or we could have saved all the bother.
Remembering where our diagreement about convection started, the key point is that additional longwave radiation coming down from the dreaded co2 monster in the sky isn’t able, all by itself, to rend the surface of the ocean asunder with it’s nasty sharp little claws and bury itself in the deeps where it can do its dastardly work of heating up the interior of the ocean. The ocean which has by the way been cooling down since 2003 while atmospheric co2 has been rising.
So the LW radiation heated water molecules on the surface, assuming they don’t get sufficiently warmed by the several hundreds of watts/metre2 of back radiation energy concentrated in a film thinner than a human hair to evaporate, has to rely on eddy currents to somehow push or suck them down where they can share their heat with their cold buddies in the deep, against their natural tendency to be more bouyant due to their lower density.
So where is the experimental evidence for this?
Any published papers?
Any data on the magnitude of the effect?
Hmmm? 🙂
Thinking about it, how difficult would it be to set up an experiment to determine this question?
A wave tank, a thermometer, a source of short wave radiation, a source of longwave radiation, and a stopwatch.
Why hasn’t it been done already?
http://eesc.columbia.edu/courses/ees/climate/lectures/o_atm.html
“Fluxes across the sea-atmosphere interface: Heat exchange between ocean and atmosphere is a product of a number of processes: solar radiation heats the ocean; net long wave back radiation cools the ocean; heat transfer by conduction and convection between the air and water generally cools the ocean as does evaporation of water from the ocean surface”
You should have highlighted the “net long wave” as well(im just not that blog savvy)
The reason being, what is being said, is that if you increase atmospheric T’s you reduce net long wave cooling(as well as conductive) , resulting in an increase in ocean energy content… so the atmosphere is a variable that can and does effect the movement of energy from the ocean up… If the atmosphere cools, more energy will be lost from the ocean, it is the climates torque converter 🙂
The thing is Mike, this has the tail wagging the dog. It’s the ocean that heats the atmosphere. Increased solar activity heated the ocean bulk in the later C20th. This raised SST, and that increased atmospheric temperature.
Look at the woodfortrees graph I linked. Increases in SST are followed several months later by increases in lower tropospheric temperature. Just like interannual inflexions in co2 levels lag behind interannual inflexions in surface temps by around 9 months.
As usual, the warmies have this backasswards.
From the same lecture:
“The infrared radiation emitted from the ocean is quickly absorbed and re-emitted by water vapor and carbon dioxide and other greenhouse gases residing in the lower atmosphere. Much of the radiation from the atmospheric gases, also in the infrared range, is transmitted back to the ocean, reducing the net long wave radiation heat loss of the ocean. ”
And the same mechanism but a bit more detailed from RealClimate: http://www.realclimate.org/index.php/archives/2006/09/why-greenhouse-gases-heat-the-ocean/
“…how can a forcing driven by longwave absorption and emission impact the ocean below since the infrared radiation does not penetrate more than a few micrometers into the ocean? Resolution of this conundrum is to be found in the recognition that the skin layer temperature gradient not only exists as a result of the ocean-atmosphere temperature difference, but also helps to control the ocean-atmosphere heat flux.
(The ‘skin layer‘ is the very thin – up to 1 mm – layer at the top of ocean that is in direct contact with the atmosphere).
Reducing the size of the temperature gradient through the skin layer reduces the flux. Thus, if the absorption of the infrared emission from atmospheric greenhouse gases reduces the gradient through the skin layer, the flow of heat from the ocean beneath will be reduced, leaving more of the heat introduced into the bulk of the upper oceanic layer by the absorption of sunlight to remain there to increase water temperature.”
Ah yes, the infamous realclimate ‘experiment’with ‘ocean skin’.
I wonder why P.J. Minnetts symposium paper never got published in a journal.
And of course, the idea would only work if an increase in the increased downward flux from co2 were not offset by a decreased flux from h2o. But a study of the radiosonde measurements found just that, didn’t it?
Which of course led to Ben Santer claiming the radiosonde measurements were unreliable, because they didn’t show a tropospheric hotspot predicted by the models.
I think the radiosonde measurements probably aren’t all that bad. If they were, it’s unlikely the NCEP re-analysis of the radiosonde data would show such a neat correlation as this one I discovered between specific humidity and solar activity at the altitude of the tropopause:
Which of course raises questions about the reasons the IPCC only considers TSI when estimating the solar effect on climate.
Please don’t be so condescending towards Millet. I don’t see your theories available for scrutiny in the literature as well if you want to play it that way.
But you are right at least on one point: Millet gave essentially the same presentation on a Geophysical meeting (fall 2007 afaik) but he hasn’t published it.
Your professor Gordon, on the other hand, on a more general note, said essentially the same thing:
“Much of the radiation from the atmospheric gases, also in the infrared range, is transmitted back to the ocean, reducing the net long wave radiation heat loss of the ocean. ”
How exactly may be up for dispute, but essentially Gordon and Millet agree on that point and together they rank over 200 oceanography publications, if you want to appeal to authority. 😉
Ben Santer is certainly not the only one noting problems with the radiosonde readings (see the link below). So perhaps all of them are biased or maybe your faith in the results is unjust. How familiar are you with the intimate nitty-gritty of those instruments?
http://www.skepticalscience.com/Climate-cherry-pickers-Falling-humidity.html
By the way S.o.D, that lecture was given by prof. Arnold Gordon, who has over 130 oceanography publications stretching back to the mid ’60s.
How’s that for an appeal to authority? 🙂
The second law of thermodynamics determines the direction of the evolution of thermodynamic processes. This law can be formulated in terms of entropy.
The entropy of an isolated system never decreases. The entropy does not change the processes and reversible increases in irreversible processes that occur within the system.
The state of thermodynamic equilibrium of the system is the state of maximum entropy compatible with the conditions that the system is subjected.
The increase of entropy in irreversible processes is very important to give meaning to the concept of entropy. The energy and entropy of an isolated system does not vary if the system evolves reversibly.
Sorry..for the bad english.
DSL:
“What I mean is that if you take away the current atmosphere, the oceans freeze,……”
I have never understood this concept. Less than half of the solar flux at TOA reaches the surface, a result of attenuation in the atmosphere. That is, the atmosphere is a cooling agent and removing it would result in warming, not freezing. The effective radiating temperature of the solar flux is 121 degrees Celsius, sufficient to boil the ocean surface during the day. The ocean’s thermal inertia, not to mention that of the planet as a whole, would make the surface unlikely to freeze overnight.
The temperature on the Moon does indeed swing between extremes, which is the point you make.
I think a better point to have made is that you would have extremes. eg. it would certainly be cold enough in the night to freeze water, ignoring the fact that the water may have gained enough energy during the day to prevent freezing at night.
The presence of green house gases and an atmosphere moderate those extremes, but if ghg levels went to high they would exasperate the retention of energy causing just as much of a problem as would be caused if there were no atmosphere.
So what happened while co2 levels were at 7000 (seven thousand) ppm a few hundred million years ago?
John Millett, DSL
“What I mean is that if you take away the current atmosphere, the oceans freeze,……”
What sceptic ever suggested taking the atmosphere away?
The true subtraction test for GAGW is much simpler.
Everything remains the same except that CO2 does not absorb/radiate in the infra red.
What difference would that bring about?
I would think very little if any.
tallbloke on November 9, 2010 at 5:05 pm:
Pity I didn’t do the same to start with?
From Does Back Radiation “Heat” the Ocean? – Part Two:
“Pity I didn’t do the same to start with?”
Yeah, back over on my blog. When I pointed out that what you now call “free” or “natural” convection doesn’t work upside down, you then invoked mixing forces as adifferent kind of convection. Fair enough, but when i pointed out to you, several times, that studies on wave mixing show that the eddy currents are well below the surface where the back radiation is trapped, you failed to acknowledge. And still do.
tallbloke:
Citing an article:
Well, I don’t want to misunderstand the question you have originally posed..
Who would disagree with this statement? Not me.
The question I thought you were asking is: “if there is more back radiation due to more “greenhouse” gases, can that increase the temperature of the ocean?“.
The statement you cite above is not profound or controversial. Heat flows from hotter to colder, as I have stated many times.
Perhaps you haven’t understood that I agree with this comment.
If you interpret this uncontroversial physics 101 statement to mean that a change in “back radiation” will have no effect then that’s something I would like to address.
Just the same as the fact that (in this article) model 3A and model 3B have different equilibrium conditions.
And yet in both cases, heat is flowing from hotter to colder. So how can the temperature of body 1 in model 3B be higher?
I look forward to your response on this.
Hi S.o.D.
the point I really want to make goes beyond the ‘remit’ of this post. It is that the ocean really did warm during the warming period ~1980-2003, but since atmospheric water vapour has been falling since 1948, it isn’t any ‘extra greenhouse gases’ that caused it, despite rising co2 levels. The factors that caused it were a well above average activity levels Sun, and diminished cloud cover, as the ISCCP data shows. It was the sun heating the ocean through lessened cloud cover heating the ocean that was the cause of the global warming, not increased greenhouse gases causing the ocean to cool down slower.
I don’t have any problem with your theoretical physics, except there is always a danger of extrapolating a wrong conclusion about the actual climate by a failure to include real world factors, and that your choice of subjects seems to be tilting in the direction of trying to validate the AGW hypothesis, which as far as I can tell, is falsified by the non-existence of the predicted tropospheric hotspot.
“…the AGW hypothesis, which as far as I can tell, is falsified by the non-existence of the predicted tropospheric hotspot.”
How is AGW falsified? You mean the greenhouse theory is just a house of cards that disintegrates if you pull one card away?
Well, that seems too much a simplifications given the uncertainties in upper tropospheric measurements and that models aren’t the only proof for the greenhouse theory.
From Brengtsson and Hodges 2009:
“The present 30-years of tropospheric temperature
observations are still insufficient to identify robust
trends as the internal variability of realistic climate
models is larger than the observed trends.”
And:
“An evaluation of the tropospheric lapse rate trend
taken as the difference between TLT and TMT
suggests that models warm the upper troposphere
more than the lower troposphere suggesting a systematic bias. However, these lapse rate errors are
modest but point nevertheless to a scientific issue that
needs further attention.”
Click to access fulltext.pdf
Ocean – Atmosphere. Which is more important?
It’s like asking – “for the sun-earth gravitational interaction – which one is the most important?”
They are both quite useless questions. The atmosphere affects the ocean. The ocean affects the atmosphere. With either one changed the “equilibrium” and the “dynamics” would change.
You still don’t seem to have a handle on the scale of effects here. Take a look at the relative heat capacities, that should give you a clue as to which is the waggy tail and which is the dog.
tallbloke:
Perhaps you make these kinds of statements to try and be amusing.
Yes, I understand the difference in heat capacity between the two.
If your aim is to score cheap points by pointing out stuff that most people already know it doesn’t reflect well on you. Pointing out the heat capacity comparison isn’t analysis.
If you want to demonstrate something of substance go ahead.
Is heat capacity of the oceans and atmosphere the only factor of importance? No.
Enter cheap points scoring mode:
You don’t seem to have a handle on the type of complexity we are dealing with here. Hint, check out where the heat comes from.
See what I mean, easy work isn’t it? I’m sure you have something interesting to say. Put some work in.
S.o.D:
You don’t seem to have a handle on the type of complexity we are dealing with here. Hint, check out where the heat comes from.
It only seems to be complex to the point of view of proponents of the co2 driven global warming hypothesis because they have to do complex contortions to try to make it seem like the energy is coming from the atmosphere and heating the ocean.
It’s actually pretty simple and the other way round. the energy is coming from the sun, into the ocean which then emits energy into the atmosphere which causes the atmosphere to warm.
During the global warming period ~1980-2003 cloud cover was reduced, according to isccp data, and the sun was well above average activity levels, as the sunspot number records show. There was no tropospheric hotspot as predicted by the co2 driven model, as the radiosonde records show, so the extra heat must be due to excess energy coming from the ocean. This thesis is backed up by the ohc records and the sst records and the fact that changes in atmospheric temperature lag behind changes in sst.
All the empirical evidence points to the sun being responsible for global warming.
That’s where the energy came from.
I don’t know how to make it any clearer than that.
tallbloke,
Well for short wave radiation you’d need a tank 100 m deep. But I have a better variation. Take an aquarium tank with a thin polyethylene film cover to minimize evaporation and a circulation pump because it’s probably too small for eddy diffusion to be significant and put it in a room at constant temperature.. Put a heating element in the tank and run it at constant power to simulate absorption of short wave radiation. Measure the temperature. Now put a heat lamp above the tank. Measure the temperature again. Do you really believe the heat lamp won’t increase the tank temperature?
If you put salt water in the tank, you might not need the circulating pump as evaporation from the surface and condensation on the film would create local density gradients, especially if you arranged the cover such that the condensation all drained off at one point.
DeWitt Payne
…..”Now put a heat lamp above the tank. Measure the temperature again. Do you really believe the heat lamp won’t increase the tank temperature?”…….
But in the Earth Surface(water)/atmosphere example the “lamp” i.e. “greenhouse gases” have to get their energy for the half up/half down radiation from the water below.
So all we have once again, is the flow of heat from the water to the atmosphere, mitigated to some extent by the insulative effect of the back radiation.
My request to tallbloke for clarification:
In answer which Tallbloke said:
You can surmise all you like what direction I am “tilting in”.
Why not answer the question at hand?
Your “analysis” instead appears to be “the last X years of measurement of value Y in the real world demonstrates that AGW can’t be correct, therefore your analysis is wrong because it appears to be trying to support AGW“.
Your original question appeared to be about whether more back-radiation could affect the ocean temperature.
Clarity is important.
Notice at this point all I am trying to do is ensure I have understood your question. Why is this so difficult to extract?
In any case, when I do some analysis, please this time don’t claim I am trying to set up a strawman to knock down rather than dealing with your “real (secret) point”.
My original question to you was:
“do you accept that back radiation doesn’t heat the oceans to any significant degree?”
I’m still awaiting an unambiguous reply.
Yes or no would be fine…
But if you like, we can have a debate about what the word ‘heat’ means. Again.
[…] Scientist .of .Doom says: You don’t seem to have a handle on the type of complexity we are dealing with here. Hint, check out where the heat comes from. […]
Bryan,
Exactly. So you admit that DLR does cause the ocean to be warmer than it would be in its absence.
The atmosphere is heated not just by radiation from the surface, but also by convection and absorption of incoming solar radiation.
“The atmosphere is heated not just by radiation from the surface, but also by convection and absorption of incoming solar radiation.”
And also to a large extent by the latent heat of condensation. And to a small extent by conduction.
Of course the atmosphere is cooled by the LW energy absorbed by water when it is evaporated by the back radiation for the ocean surface first, but since condensation tends to occur quite high in the atmosphere, this is one of the main mechanisms whereby the bouncing to and fro of radiative energy from the ocean surface to the atmosphere is short circuited, as the condensation and release of heat takes place above most of the greenhouse gas where the energy has a freer path to space.
DeWitt Payne
…”The atmosphere is heated …. absorption of incoming solar radiation.”…..
Yes I think everyone agrees with that, during daytime.
….”So you admit that DLR does cause the ocean to be warmer than it would be in its absence.”……
At night, I would express this, so as not to cause further misunderstanding, that backradiation reduces to some extent the rate of heat loss from the ocean.
Tallbloke, Cynicus, SOD, et al: Is there one explanation that could satisfy all? An attempt to reconcile divergent views:
Cynicus wrote (11/7 8:11 pm):
The longwave heating of the top ocean layer has even been measured as reported by RealClimate: http://www.realclimate.org/index.php/archives/2006/09/why-greenhouse-gases-heat-the-ocean/
This RealClimate post contains experimental evidence showing that the temperature difference between the surface of the ocean and 5 cm below the surface varies with net long wavelength radiation. The greater the observed imbalance between upward and downward long wavelength radiation, the colder the surface is compared with 5 cm below the surface. These observations demonstrate ONLY that DLR warms the top few microns of the ocean. This experiment demonstrates just what Tallbloke asserts and nothing else! The RealClimate author (Peter Minnett) SPECULATES that:
“Reducing the size of the temperature gradient through the skin layer reduces the flux” [of energy from the ocean to the atmosphere].
This statement ASSUMES that we understand the main mechanism of heat flux through the skin layer of the ocean. IF conduction – which varies with the temperature gradient – is the main mechanism of heat transfer, then increasing DLR will: lessen the gradient, decrease heat flux through the skin layer, and warm the ocean. If heat transfer occurs by what SOD called forced convection (by wind, waves, eddies?), the steepness of the temperature gradient could be irrelevant. Free (buoyancy-driven) convection could increase with the steepness of the gradient, but surface tension might need to be overcome first. Therefore, Minnett has not PROVEN that the DLR warms anything other than the “skin layer” (top 10 um) of the ocean.
Where Tallbloke Gets It Right (with some caveats): Does it make any difference if DLR only warms the top 10 um (hereafter called the “skin layer”) of the ocean? The ocean is still being warmed, isn’t it? No, Tallbloke believes that the energy from DLR is immediately returned to the atmosphere – before that energy can be transferred to deeper layers of the ocean. Tallblock is certainly correct; the surface of the ocean is exchanging long wavelength photons with regions of the atmosphere that are colder (and have lower emissivity) AND losing additional photons directly to space when it isn’t cloudy. Therefore, long wavelength radiation MUST BE a net loser for the top 10 um of the ocean because this skin layer both absorbs AND EMITS ALL long wavelength radiation. There is not “too much” DLR being absorbed in the skin layer (with its miniscule heat capacity). There isn’t ENOUGH DLR to replace the energy lost through upward long wavelength radiation from the skin layer. In a previous post, I showed that shortwave radiation from the sun could deposit enough energy in the skin layer to negate the net loss at long wavelengths for a few hours around noon, but not when averaged over a whole day. This explains why Minnett’s data shows the skin temperature to be an average of 0.2 degC COLDER than 5 cm below the surface. There are a few data points on Minnett’s graph showing that the temperature difference is occasionally zero, which is [remarkably] consistent with my back-of-the-envelop calculations showing short periods of net warming via the small fraction of short wavelength radiation that is directly absorbed by the skin layer.
Equilibrium Considerations (aka Tallbloke’s Waterloo): Although the ocean’s temperature varies with day and night and the local weather, we can define an equilibrium temperature for the ocean as the temperature at which the AVERAGE net upward loss of energy (which increases with ocean temperature by oT^4 and evaporation) is exactly balanced by the AVERAGE downward energy input from radiation (both solar and DLR). When the ocean maintains an equilibrium temperature, the average net energy loss from the skin layer via long wavelength radiation must be supplied by energy from average short wavelength radiation. Short wavelength radiation is absorbed mostly in the top 10 m, with only a small fraction of that energy actually being directly deposited in the skin layer itself. (On the average, DLR directly deposits far more energy in the skin layer than does sunlight). We have been debating the mechanism of (and proper name for) the energy transfer from the top 10 m of the ocean to the colder skin layer, but most readers should recognize that such a transfer must take place: If it didn’t, the skin layer would continue to lose energy through long wavelength radiation to the colder atmosphere and space and the 10 m immediately below would get hotter and hotter. I don’t know whether this energy transfer occurs by conduction (Millett’s assumption), free convection, or forced convection – but the exact mechanism is irrelevant when we are discussing EQUILIBRIUM temperature. The mechanism IS relevant to how fast temperature returns to equilibrium after it is disturbed (for example by night and day or by GHGs), but it doesn’t change the equilibrium temperature needed to balance downward and upward flow of energy.
CONCLUSION: When increasing DLR from GHGs reduces the net loss of energy by long wavelength radiation from the skin layer, the EQUILIBRIUM temperature of the 10 m immediately below MUST warm: If some of the energy that previously went to the skin layer (and then to the atmosphere) doesn’t go anywhere else, it must increase the temperature of the 10 m immediately under the skin layer.
What Part of the Ocean Warms in Response to GHGs and How Fast? (Some of our disagreements may be arise because we are thinking differently about these parameters.)
a) The top 10 um: The best fit equation on Millett’s graph shows that a 4 W/m^2 increase in DLR – the forcing for 2X CO2 – is expected to reduce the difference in temperature between the skin layer and the water immediately below by a negligible 0.008 degC. So the temperature of the skin layer and the next 10 m should change in parallel.
b) The top 100 m (or the physically “mixed layer”): We know that seasonal changes in insolation cause seasonal changes in the temperature of roughly the top 100 m of the ocean. If increasing DLR reduces the need for energy flux from the top 10 m to the skin layer, the extra warmth in the top 10 m clearly can spread throughout the physically mixed layer within a year – even if the total warming is as small as the 0.02-0.05 degC/year projected by the IPCC.
c) The next few hundred meters: We are principally worried about climate change over the next century. Presumably some warming in the mixed will slowly penetrate to these depths by conduction or, more likely, by vertical mixing as ocean currents pass over obstructions on the sea floor.
d) Deeper: If we concern ourselves with only the next century, warming in the mixed layer of the ocean is probably not going to reach the deep ocean. With a circulation time of about 1000 years, the meridional overturn current (or thermohaline circulation) that conveys surface water to and from the deep ocean appears to be too slow to convey more than a small fraction of surface warming to the depths.
In summary, absorption of increasing DLR by the skin layer will reduce the need for energy flux from the top 10 m to the skin layer. This will warm the top 10 m and this warmth will be distributed throughout the top 100 m in less than one year. Increased DLR will not significantly warm most – but not all – of the remaining ocean in the next century.
Frank
We need to include the effects of evaporation at the boundary of atmosphere/ocean.
If you dip your finger in ethanol and then hold it in the air it will feel cold as heat is drawn from your body to supply the energy for evaporation.
The more energetic molecules leaving reduces the average speed of the remaining molecules at the boundary.
Similarly for hill-walkers the “wind chill” is a well known hazard.
From Wikipedia
..”a The human body loses heat largely by evaporation and convection.[1][2] The rate of heat loss by a surface depends on the wind speed above that surface: the faster the wind speed, the more readily the surface cools.”….
So its comes as no surprise to me that the top skin of the Ocean is slightly cooler and we don’t need any reference to a radiative effect to explain it.
Yes, evaporation will increase the amount of energy the “skin layer” needs to get from the 10 m immediately below the skin layer – where most of the short wavelength energy is absorbed. And the energy lost through evaporation will mean that the equilibrium temperature will be a little lower than without energy loss through through evaporation. Looking at the numbers, the planetary average of 78 W/m2 of energy transported upward via latent heat is comparable to the net loss in long wavelength radiation (planetary average 66 w/m2). Numbers for specific ocean situations may be somewhat different.
However, evaporation doesn’t change the fact that: 1) Increasing DLR will decrease the amount of energy the skin layer needs to obtain from the 10 m below heated by short wavelength radiation. 2) The extra energy remaining in the top 10 m will eventually warm parts of the ocean during the next century. Right?
Bryan and others have been asking about energy transfer by latent heat. According to Wikipedia (and a textbook reference therein), 505,000 km^3 of rain falls on the earth every year. With a surface area of 5.1*10^8 sq km, we get an average rainfall of 0.00099 km/yr or roughly 1 m/yr. (Although this value appears somewhat high is certainly more reasonable than 0.1 m/4 inches or a ridiculous 10 m.) Since there are 10^3 kg in one m^3 of water, rainfall amounts to 1000 kg/yr/m^2.
Accepting Bryan’s value for the latent heat of vaporization of water (which actually varies modestly with temperature), we multiply 1000 kg/yr/m^2 times 2,230,000 J/kg, we get 2.23*10^9 J/yr/m^2. Dividing by 3.16*10^7 s/yr gives us 71 J/s/m^2 or 71 W/m^2 average over the whole year.
This value is similar to Trenberth’s value of 78W/m^2 used by the IPCC. http://www.ipcc.ch/graphics/ar4-wg1/jpg/faq-1-1-fig-1.jpg If you want to believe that latent heat is far more important than the IPCC thinks, then you must believe that average rainfall is much greater than 1 m.
Some precipitation returns to the earth as snow, which adds the heat of fusion 334,000 J/kg to the heat of vaporization. The latent heat release by snow is 15% higher than the latent heat released by rain. If the 1000 kg of precipitation that falls on every m^2 every year were all snow, 81 W/m^2 of latent heat would be released.
Frank
The reference I made to the latent heat of vaporization of water was to do with the temperature of clouds.
I was not referring to the full cycle back to Earth that you have worked out.
The clouds are maintained at a significantly higher temperature than open sky.
This has the effect of reducing the convective flow from Earth surface.
The convective flow is widely recognised as being the most important heat transporter.
So its little wonder that surface temperatures at night are warmer as a result of clouds.
Bryan:
Now we seem to have agreement on the equations, and disagreement only over the everyday language we use to express, intuitively, what the equations say. I call that progress.
Here’s another bit of interpretive translation that might help:
As I was raised, energy comes in several forms, one of which is heat (the energy of random molecular motion). The amount of heat energy in a body is (to be quick and rough with it) a matter of its temperature and its heat capacity.
From this point of view, heat energy is not just the energy that flows from hotter to colder bodies–a view which obscures the conservation of energy, a principle that is at the root of the time-symmetry of classical physics, since it suggests that once thermal equilibrium is reached there’s no heat energy left…
So if we could either accept this way of putting things, or alternatively agree to disagree on whether to say (in everyday usage) ‘heat’ is just what flows (spontaneously) via radiation, conduction and convection or that ‘heat’ is a form of energy that every body (at above absolute zero) contains some of, it might help to avoid useless back and forth over preferred intuitive formulations that doesn’t make any difference to the maths.
It might help further to notice that the heat energy in an object at some temperature (as I understand it) is equivalent to the heat (as you, I gather, would prefer to put it) that would flow from the object to an infinite heat sink at absolute zero.
Bryson Brown
…..”Now we seem to have agreement on the equations, and disagreement only over the everyday language we use to express”……
I understand what you mean and in a sense I am sympathetic to the idea of using ordinary everyday language.
However if you follow a Physics Text book through the thermodynamic sections they have a very precise meaning for the word HEAT.
There is no doubt in my mind that I am using the correct thermodynamic meaning of heat.
Now we could agree that when you use the word “heat” you are using your own common understanding of the word rather than the thermodynamic precise definition.
However if a third party looked at our communication they might think we were in a muddle.
In some of the textbooks quoted by SoD some of the writers have obviously used “heat” rather than say “thermal energy” because in the context of say a furnace calculation they thought it did not matter too much.
However when the word is used in climate science the stakes are so high that a disciplined use of language is imperative.
Some people advocate that the whole economy of the world should be dislocated.
Do we build more coal fired power stations or is this exactly the wrong thing to do.
In west Scotland there are many unemployed people living in former mining communities.
Steel works have been shut down and production moved to India.
Some think this is on the whole a good thing.
For myself a will examine in fine detail the Science behind the proposals from the IPCC.
I think it would be instructive for SoD to do a post on the refrigerator and the Carnot cycle.
If a precise meaning of heat is not used we would lose all sense of orientation.
[…] Posted: November 14, 2010 by tallbloke in solar system dynamics 0 Frank said: November 11, 2010 at 5:48 pm Tallbloke, Cynicus, SOD, et al: Is there one explanation that could satisfy all? An attempt to […]
Bryan,
You seem to be ignoring the fact that any object with a temperature greater than 0 K can transfer heat to an object at 0 K. That means all objects above 0 K must contain heat. If you have two objects of equal mass and heat capacity with one at 150 K and the other at 100 K, heat will transfer from hotter to colder until both are at 125 K. Does that mean there’s no more heat in the system? What happens if you then introduce another object at 10 K? Does heat magically reappear or was it there all along?
DeWitt Payne
….”That means all objects above 0 K must contain heat.”….
Strictly speaking no object can be said to contain heat.
We might think for instance that a kilogram of iron at 200C contains “a lot of heat ” but if it is placed in a bath of molten iron at 2000C the flow of heat will be from the higher temperature to the lower temperature.
Thermodynamically defined Heat is a PROCESS by which thermal energy flows from a higher temperature to a lower temperature.
Strictly speaking, there is no such thing as heat at all. There’s just energy in different forms and entropy. Conventionally, internal energy content is referred to as heat, as in properties like heat capacity.
See this for example:
Click to access thermo-laws.pdf
It’s quite entertaining and very rigorous.
“0 K must contain heat” But not heat as we know it? At what point does heat become heat? Far IR is described as thermal and near IR as not. Pointing my remote control at myself instead of the TV isn’t going to do anything to make me feel warmer no matter how long I continue pressing it.
DeWitt Payne
Thanks for your reference it looks interesting.
I will print it off an read it carefully when I get the chance.
A quick look at definitions of “heat” shows that the one I use which he calls “3” is the standard textbook definition.
He finds it a bit cramped however until such times as I find a better definition I will continue to use it.
When you use a word that is used in a variety of ways and insist yours is the only correct use I am reminded of Humpty Dumpty in Through the Looking Glass:
“When I use a word,” Humpty Dumpty said in rather a scornful tone, “it means just what I choose it to mean – neither more nor less.”
DeWitt Payne
Would the world make any more sense when we all have individual meanings for scientific definitions?
Where do we start;
Wavelength,frequency,force,mass,work,electric charge,density,heat,atomic number………
When people want to change the perfectly understood meaning of words, beware of snake oil salesmen.
Let’s check the calculation carried out in Model 3A by applying them to the real case of the Moon. The Moon is lacking atmosphere which is just what Model 3A is about. Well, geometric albedo of the moon is 12%. The distance to the Sun is the same as for the Earth. This means that the power density heating the surface of the Moon is 1369*0.88=1205 W/m2. This means that the mean temperature of the surface of the Moon should be 301K or +28°C if following the theoretical calculations applied to Model 3A.
Now, the measurements of surface temperature of the Moon give mean temperature of 107°C at daytime while mean temperature at nighttime is measured to be -153°C . This gives the measured mean temperature over the total surface of the Moon to be -23°C.
Thus, the discrepancy between the theory and reality is 51°C. What about this?
Sorry! The expected theoretical mean temperature at the Moon is 270K (-3 °C) and not 301K (+28 °C) as I wrote erroneously above (I missed the last step in calculations). This makes the difference between the theoretically calculated value (270K) and the measured one (250K) to be 20K, which is also sufficiently large if one aims to estimate correctly the contribution of the atmosphere by means of this type of the theoretical approach.
Ernest:
Check out Lunar Madness and Physics Basics.
Averaging temperature gives a different result from the correct method of averaging radiation.
The discussion in https://scienceofdoom.com/2010/06/03/lunar-madness-and-physics-basics/ concerns the influence of the specific heat to the temperature of the surface of the Moon. However, what I am pointing on is that the experimental measurements do not confirm the results of calculations described in Model 3A when these calculations are applied to the case of the Moon. And how it can be otherwise if one approximates the real objects such as the Earth or the Moon by a body that “has a very high conductivity for heat, and therefore the whole planet is at the same surface temperature”?
But I assume that the main problem this blog is about is to estimate the influence of the atmosphere on the climate of the Earth. And no doubts, if we could supply the Moon with atmosphere, tranport water to create oceans and lakes then the climate of the Moon would start to resemble that on the Earth as soon as the surface of the Moon had become covered by vegetation. And can we create one and two nice volcanoes then we should harry us to line up for buying a summerhouse at the coast of the Copernicus Ocean.
Now, we are not God Father to be able to sport such an achievement. However, the dream of a summerhouse at the Moon allows us to identify the crucial elements responsible for the climate on the Earth. And these elements are:
• Irradiation from the Sun
• The properties of atmosphere such as heat capacity, composition (and thus the absorptivity, reflectivity and emissivity of the different agents in the atmosphere), heat conductivity, etc.
• The properties of the soils and water (such as heat capacity and heat conductivity)
• The distribution of vegetation (Fauna and Flora represent a rather important storage of radiation energy being converted into the chemical one)
• The additional heat coming from the inner parts of the Earth
• Velocity of rotation and the tilting of the axis of rotation
• And, of course, the position on the surface of the Earth, which is due to the spherical form of the Earth (this indicates that one should not talk about the climate of the Earth but rather about the different climate zones).
In principle, if radiation into the system is equal to radiation out of it, then the total temperature of the Earth should remain unchanged, though the redistribution of temperatures within the system might be expected due both to the complexity of the system and to the changes of the different elements listed above. However, this is true only if the conversion of radiation energy into heat remains constant. Otherwise, the total temperature of the Earth system might increase or decrease even if the total irradiation from the Sun is unchanged. The first law of thermodynamics is, namely, related to heat and not to radiation energy (radiation becomes equivalent to heat only when radiation is absorbed by the given element constituting the system). is the number of moles, is the molar heat capacity (which depends both on the kind of the thermodynamic process and on the internal structure of the given material), is internal energy of the system and is work. Thus only under the conditions that and , the total temperature of the system will be constant, i.e. . It is also obvious that any climate model must involve heat capacity and heat transport properties when discussion the changes of temperature map within the system. The model that takes into account only the exchange of radiation will be inadequate.
Well, it seems that the mathematical expressions typed in Math Type are not coming through. Here is the text at the end of the letter written in the “normal” mode.
“The first law of thermodynamics Eint = dQ – dW = nCdT is, namely, related to heat Q and not to radiation energy (radiation energy becomes equivalent to heat only when radiation is absorbed by the given element constituting the system). n is the number of moles, C is the molar heat capacity (which depends both on the kind of the thermodynamic process and on the internal structure of the given material), Eint is internal energy of the system and W is work. Thus only under the conditions that dQ = 0 and dW = 0, the total temperature of the system will be constant, i.e. dT = 0. It is also obvious that any climate model must involve heat capacity and heat transport properties when discussion the changes of temperature within the system. The model that takes into account only the exchange of radiation will be inadequate.”
Ernest:
Originally you said:
Then corrected with:
The reason I pointed you to the article on the moon was to explain the basic problem with your calculation.
If the total radiation from a surface is Rtot, and so the average radiation is Rtot/A, what is the average temperature? Let’s assume we know the emissivity.
Rtot/A = εσT^4 [eq 1]
Therefore, T = (Rtot/(A εσ))^1/4 [eq 2]
Equation 2 gives you a value of T which unfortunately can be a meaningless number.
One of the main points of the lunar article is with exactly the same total radiation being emitted from the planet’s surface you can have quite different average temperatures.
The reason is that eq 1 and 2 are valid for one location.
Here is the absorbed and radiated energy as heat capacity changes in the lunar model I created:
And here is the corresponding “average temperature” for each of those cases:
The detailed explanation about the problems of averaging temperature is given in the article.
So you have calculated a number which has no meaning. Average temperature can be a whole range of numbers with exactly the same total (or average) radiation.
Ernest:
To comment on your other point:
Every climate model does take into account heat capacity, apart from the zero dimensional, or “billiard ball” model of the earth.
It is quite valid to calculate an approximate steady state model for the earth in equilibrium with the sun. This is the zero dimensional model.
The steady state model is.. steady state. In which case, the idea is that sufficient time has elapsed that the earth has reached its equilibrium temperature. Therefore, for this simplest of models you don’t need to know the heat capacity.
The equilibrium temperature can still be calculated.
The specific problem you have identified is that average temperature can have a range of values.
So being strictly accurate we can say that the equilibrium surface radiation can be calculated for the earth (or the moon). From this equilibrium surface radiation I can say that the “maximum average temperature” will be the value calculated in eq 2 in my last comment.
But if you are claiming that a steady state equilibrium cannot be calculated without knowing the heat capacity then you are wrong.
Some systems would rely on knowing the heat capacity. These would be systems where there was an initial energy in a number of bodies and no new heat. The final condition would depend on the heat capacity of each body as well as some other parameters.
But in the case of the earth and sun, where the sun is continuously supplying energy, an equilibrium condition can easily be calculated without reference to knowledge of the heat capacity. (Dynamics calculations would need this knowledge of course). These kind of problems are the staple of any introductory text on heat transfer.
It is also my opinion that the steady state calculations in the case of the dynamic system give a meaningless number. And I agree with you partially on the subject of heat capacity.
Namely, we can start with investigation of (dynamic) equilibrium in the case of a thin surface volume element dSi*dz that is the subject to the direct irradiation from the Sun during the period of time. At some period of time, temperature of this area volume might follow the variation of irradiation from the Sun. The condition is that this variation is due to the rotation of the Earth, exclusively. The surface volume element dSi*dz absorbs the incoming radiation from the Sun, which is equivalent to the inflow of heat amount dQ = (Fo*Ta*dSi*dt*As*cosP + Fd*dSi*dz*As) into the volume element if no work is done by or over the element. Fo is the power density [W/m2} of radiation from the Sun reaching the upper part of the atmosphere of the Earth, Ta is the transmissivity of atmosphere, As is the absorptivity of matter constituting the surface volume element, P is the angle of incidence counted in relation to z being normal to dSi and Fd is the downward radiation coming from air (the so called diffuse radiation that is the subject of many controversies but I will not take up it since it is not relevant at this moment). Heat dQ is then divided in three components: dQo = mcdT heating the surface volume element dSi*dz itself (this heat is stored within the surface volume element); dQ’ = McdT’ that is flowing into the ground/water, rising its temperature and being thus stored there; and dQ’’ being loosed to air i.e. transported out by vertical and horizontal convection and radiation. A part of dQ’’ will heat air in accordance to nCdT’’ (but also this is a story for another time).
So now back to the actual discussion. Achievement of the (dynamic) equilibrium requires that the heat relation between dQo, dQ’ and dQ’’ remains the same, which might occur during some period at daytime. It will certainly break down when the Sun disappears below the horizon.
Another situation when one can talk about the (dynamic) equilibrium is when studying the temperature variations within dSi*dz during a longer period of time, for example during a year. One can talk then about variation of temperature of dSi*dz within some temperature interval. In this case we can disregard from specific heat of material on the both sides of the absorbing layer and say simply that due to power density Fo temperature of the given surface volume element varies within the range Ti plus minus DT. Ti is the time average of the temperature of the surface volume element dSi*dz. We may take the next step and integrate over all dSi calculating thus the total mean temperature Ttotal over the whole area of the Earth both in time and space. If the climatologists are interested in establishing the relation between Fo and Ttotal, exclusively, then specific heats of the different agents is of less interest. Here I agree with you.
However, I will strongly warn for using the calculations presented in Model 3A for estimation of Ttotal and using afterwards such an obtained factious mean temperature for finding the contribution of the gases in air to the temperature of the Earth system.
Dear Sir “scienceondoom”!
I have read through a couple of your blogs and admire your pedagogical talent and humor (I have come over the blog for a couple of days ago and had no time yet to go through all of them and the comments).
In one of the blogs you wrote something like “If you don’t like the theory then you are welcome to produce your own one”. Now, I don’t think that it is possible to produce a new theory that will differ from the existing one in any considerable degree. The reason to this is that the main elements are such as they are, namely, irradiation, atmosphere, oceans, continents, etc., as well as the lows of physics and chemistry and there is nothing to do about this. So the dispute might arise mainly concerning some minor details (which might show to be not so minor if economy, politics or scientific prestige become involved). Other reasons for the disputes might be either how one is presenting the theory (if the presentation is given in a “non-scientific” manner for the sake of popularization) or be due to the semantic confusions.
The use of the term “greenhouse” gases makes many physicists to react as bulls seeing a matador with the complete requisites. Another one is the explanation of 255K business. Well, the application of the steady – state model to our rotating beautiful Earth causes many people to start to bang their crania at the desks with the risk to demolish those irreplaceable pieces of furniture. By the way, my desk has shown to be a rather solid object which I cannot say about my own forehead.
Thus in order to prevent a rapid decease of the number of persons with some basic knowledge within the physical science, I would suggest that the explanation to the number 255K (i.e. to the theoretical temperature of the Earth without atmosphere) is changed to (or completed with) the diagram showing the variation of S times squared cosine of the angle of incidence of radiation, S*(cosP)^2, where S = 1367 W/m^2. The squaring of cosine is due to the averaging of S over the longitudes and latitudes in the case of the daytime irradiation from the Sun. At nighttime S = 0. This will be a diagram that resembles the first one in the “Lunar madness and physics basis” but with the S axis taken instead of the T one. The averaging over one complete period gives in the case of the squared cosine function the number 0.25. By taking then the fourth root of 0.25*S*0.7 divided by the Stefan-Boltzmann constant you will get 255K. Some people will object even to this presentation but hopefully without the risks to their health.
Ernest,
You can see an explanation of the energy balance for the planet at Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part One.
Calculation of the annual global average of energy absorbed from the sun does not require any cosines or diurnal calculations. The sun is a long way away and 1367 W/m^2 is incident at top of atmosphere (measured) with about 30% reflected (measured). To calculate the absorbed energy in one year is therefore an easy calculation. More in the link provided.
Dear Sir “scienceondoom”!
I have the following reflection after reading Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part One, which might explain the controversy about “the colder body heating the warmer one”. The question is “do we talk about what happens at the daytime or at the nighttime”. At the daytime the downward radiation from the colder air adds to the irradiation from the Sun, which rises additionally surface temperature (i.e. above that which surface would have had without the downward contribution). Hence, colder air heats the warmer surface. At nighttime we have no irradiation from the Sun. The only exchange of radiation is between the warmer surface and the colder surrounding. The surface is cooling but due to the downward radiation the cooling is slowed down. In both cases there is no violation of the established laws of physics.
By adding an insulating layer to the walls of the house we can increase the inner temperature of the house without changing the total input of heat. This is of the common knowledge, of course. The insulating layer has a low thermal conductivity and throttles thus temporary the heat flow out from the inner parts of the house to the surrounding. The inside temperature increases, the heat flow increases until the new steady state is achieved. And of course the inner temperature will vary if the power input is not constant, the temperature response to the changes of the input being dependent on the heat capacity of the house as a whole, i.e. inclusively the heat capacity of the walls.
The addition of the trace gases to the atmosphere seems to act on the heat transport by radiation in the similar way as the insulating layer acts on the heat transport by thermal conductivity. But in this case, it is due to the increase of the downward radiation. Simultaneously, we get the (temporary) decrease of the total upward radiation. During the transition period before the new steady state is established, the mean temperature of the lower parts of the atmosphere is increasing while the mean temperature of the stratosphere is expected to go temporary down. The increase of the trace gases puts thus the total thermodynamic system of the Earth into the transition period, where we seems to be now.
Well, such an explanation of the observable changes of the temperature of the atmosphere given by the climatologists makes sense. Or is it too simple?
Dear Sir ”scienceofdoom”.
I have a question in connection to the average temperature measured experimentally and the average temperature calculated from the estimated average power density over the total area of the Earth.
In the “Lunar madness and physics basics” you have stressed that these two different average temperatures mean two different things.
However, when presenting the climate theory you are comparing the fictive “average power” temperature of -18 Celsius for the Earth without atmosphere with the experimentally measured average temperature of +15 Celsius.
What is your comment on this question? Please observe that I do not question the correctness in finding both these parameters.
I think I will found the answer to my question in “Why Global Mean Surface Temperature Should be Relegated, Or Mostly Ignored”.
[…] Things we Find in Textbooks – The Real Second Law , The Real Second Law of Thermodynamics and The Three Body Problem. And for real measurements of back radiation, see The Amazing Case of “Back Radiation” -Part […]
[…] However, the sun does actually exist and the question is simply whether the presence of the (colder) atmosphere affects the surface temperature compared with if no atmosphere existed. It is The Three Body Problem. […]
[…] However, the sun does actually exist and the question is simply whether the presence of the (colder) atmosphere affects the surface temperature compared with if no atmosphere existed. It is The Three Body Problem. […]
“…this blog is not about bringing happiness.”
Maybe not, but it’s fun. Then come the comments. The most impressive thing here is your patience!
[…] The Three Body Problem […]
[…] The Three Body Problem […]