The atmosphere cools to space by radiation. Well, without getting into all the details, the surface cools to space as well by radiation but not much radiation is emitted by the surface that escapes directly to space (note 1). Most surface radiation is absorbed by the atmosphere. And of course the surface mostly cools by convection into the troposphere (lower atmosphere).
If there were no radiatively-active gases (aka “GHG”s) in the atmosphere then the atmosphere couldn’t cool to space at all.
Technically, the emissivity of the atmosphere would be zero. Emission is determined by the local temperature of the atmosphere and its emissivity. Wavelength by wavelength emissivity is equal to absorptivity, another technical term, which says what proportion of radiation is absorbed by the atmosphere. If the atmosphere can’t emit, it can’t absorb (note 2).
So as you increase the GHGs in the atmosphere you increase its ability to cool to space. A lot of people realize this at some point during their climate science journey and finally realize how they have been duped by climate science all along! It’s irrefutable – more GHGs more cooling to space, more GHGs mean less global warming!
Ok, it’s true. Now the game’s up, I’ll pack up Science of Doom into a crate and start writing about something else. Maybe cognitive dissonance..
Bye everyone!
Halfway through boxing everything up I realized there was a little complication to the simplicity of that paragraph. The atmosphere with more GHGs has a higher emissivity, but also a higher absorptivity.
Let’s draw a little diagram. Here are two “layers” (see note 3) of the atmosphere in two different cases. On the left 400 ppmv CO2, on the right 500ppmv CO2 (and relative humidity of water vapor was set at 50%, surface temperature at 288K):
Figure 1
It’s clear that the two layers are both emitting more radiation with more CO2.More cooling to space.
For interest, the “total emissivity” of the top layer is 0.190 in the first case and 0.197 in the second case. The layer below has 0.389 and 0.395.
Let’s take a look at all of the numbers and see what is going on. This diagram is a little busier:
Figure 2
The key point is that the OLR (outgoing longwave radiation) is lower in the case with more CO2. Yet each layer is emitting more radiation. How can this be?
Take a look at the radiation entering the top layer on the left = 265.1, and add to that the emitted radiation = 23.0 – the total is 288.1. Now subtract the radiation leaving through the top boundary = 257.0 and we get the radiation absorbed in the layer. This is 31.1 W/m².
Compare that with the same calculation with more CO2 – the absorption is 32.2 W/m².
This is the case all the way up through the atmosphere – each layer emits more because its emissivity has increased, but it also absorbs more because its absorptivity has increased by the same amount.
So more cooling to space, but unfortunately more absorption of the radiation below – two competing terms.
So why don’t they cancel out?
Emission of radiation is a result of local temperature and emissivity.
Absorption of radiation is the result of the incident radiation and absorptivity. Incident upwards radiation started lower in the atmosphere where it is hotter. So absorption changes always outweigh emission changes (note 4).
Conceptual Problems?
If it’s still not making sense then think about what happens as you reduce the GHGs in the atmosphere. The atmosphere emits less but absorbs even less of the radiation from below. So the outgoing longwave radiation increases. More surface radiation is making it to the top of atmosphere without being absorbed. So there is less cooling to space from the atmosphere, but more cooling to space from the surface and the atmosphere.
If you add lagging to a pipe, the temperature of the pipe increases (assuming of course it is “internally” heated with hot water). And yet, the pipe cools to the surrounding room via the lagging! Does that mean more lagging, more cooling? No, it’s just the transfer mechanism for getting the heat out.
That was just an analogy. Analogies don’t prove anything. If well chosen, they can be useful in illustrating problems. End of analogy disclaimer.
If you want to understand more about how radiation travels through the atmosphere and how GHG changes affect this journey, take a look at the series Visualizing Atmospheric Radiation.
Notes
Note 1: For more on the details see
- Part Three – Average Height of Emission – the complex subject of where the TOA radiation originated from, what is the “Average Height of Emission” and other questions
- Kiehl & Trenberth and the Atmospheric Window
Note 2: A very basic point – absolutely essential for understanding anything at all about climate science – is that the absorptivity of the atmosphere can be (and is) totally different from its emissivity when you are considering different wavelengths. The atmosphere is quite transparent to solar radiation, but quite opaque to terrestrial radiation – because they are at different wavelengths. 99% of solar radiation is at wavelengths less than 4 μm, and 99% of terrestrial radiation is at wavelengths greater than 4 μm. That’s because the sun’s surface is around 6000K while the earth’s surface is around 290K. So the atmosphere has low absorptivity of solar radiation (<4 μm) but high emissivity of terrestrial radiation.
Note 3: Any numerical calculation has to create some kind of grid. This is a very course grid, with 10 layers of roughly equal pressure in the atmosphere from the surface to 200mbar. The grid assumes there is just one temperature for each layer. Of course the temperature is decreasing as you go up. We could divide the atmosphere into 30 layers instead. We would get more accurate results. We would find the same effect.
Note 4: The equations for radiative transfer are found in Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations. The equations prove this effect.




In the new steady state, after a change in atmospheric CO2, the cooling flux to space (surface + atmospheric) must be exactly the same like before the change in CO2, assuming unchanged absorbed energy. The same with the surface – the sum of the cooling fluxes remains the same in the new steady state.
A doubling of ozone in the stratosphere actually would cool the planet through increased IR to space !
Clive,
Can you explain to readers why that is?
clivebest:
You drew the wrong conclusion.
The lagging/pipe analogy is not bad; or you could understand the GHG effect as analogous to adding another blanket to your bed: By adding more thickness between your body and the cold outside, the temperature gradient is reduced, so the heat flux is reduced. But since the body is releasing heat at the same rate, the heat that can’t escape is trapped, the temperature increases – until the temperature gradient is what it was before.
Ozone is not a well mixed gas and is mostly concentrated in the stratosphere where temperature increases with height. The effective height where photons escape directly to space increases with greenhouse gas concentration. Therefore for increasing ozone concentration, the new height will be at a higher temperature than before and will therefore emit more IR to space due to Stefan Boltzmann.
CO2 is a well mixed gas and most of the emission lines lie within the troposphere where temperature decreases with height. However some of the main lines in the 15 micron band already emit in the stratosphere. The net greenhouse effect for CO2 is positive because there are more emission lines in the troposphere which reduce emission to space than those in the stratosphere which increase emission to space.
This is the reason why CO2 radiative forcing increases only as the logarithm of concentration.
Clive,
You’re forgetting that ozone is not transparent for incident solar radiation. More ozone makes the stratosphere warmer. Less ozone, caused by chlorofluorocarbons, has made the stratosphere cooler. Increased CO2, which is nearly transparent to incident solar radiation, in the stratosphere also causes cooling.
Yes – you’re right. The ‘greenhouse effect’ of increasing ozone acts to cool the planet, whereas the extra UV absorption of sunlight acts to warm the planet !
I agree about increased CO2 in the stratosphere. So all we now have to do is to ‘capture’ all the CO2 emitted by fossil fuels in large balloons mixed with hydrogen and let them float up to the stratosphere.
Problem solved !
The main influence of the upper stratosphere is to block part of the solar radiation from reaching lower altitudes. That’s a purely cooling influence for those lower levels and the surface. The additional heat of the upper stratosphere gets almost totally radiated out as the lower parts of the stratosphere shield effectively the tropopause, troposphere, and the surface from the heat of the upper stratosphere. All mechanisms of heat transfer are suppressed effectively in the stratosphere.
The reasoning with the geometric height I think is not very productive. I consider it rather on the pressure profile.
With more CO2, the optical thickness (optical mass by Schwarzschild) increases, ie the radiative transfer resistance. The increased transport resistance has consequences: while moving a constant temperature difference, the power transported decreases bzw.eine higher temperature difference is necessary for a constant power transported.
The nature takes a middle course: the temperature difference increases slightly and the power carried slightly decreases. The rising temperature difference is divided into an increasing surface temperature and decreasing stratospheric temperature.
Due to this increasing surface temperature and decreasing stratospheric temperature, the total radiation into space remains constant: By the atmospheric windows allow more heat from the warmer surface to outer space and of the cooler stratosphere flows less heat into space.
FWIW, I’ve sometimes thought clear skies as short straight tailpipes out of an engine and various clouds as variously curved longer tailpipes. The analogy does not of course hold water as there are ghg’s even in clear skies, maybe the diameter of the straight or curved pipe would be a better analogy.
When nature will not follow the laws of science:
http://www.news.wisc.edu/23050
““We have been building models and there are now robust contradictions,” says Liu, a professor in the UW-Madison Department of Atmospheric and Oceanic Sciences and the Center for Climatic Research. “Data from observation says global cooling. The physical model says it has to be warming.””
“Over the last 10,000 years, Liu says, we know atmospheric carbon dioxide rose by 20 parts per million before the 20th century, and the massive ice sheet of the Last Glacial Maximum has been retreating. These physical changes suggest that, globally, the annual mean global temperature should have continued to warm, even as regions of the world experienced cooling, such as during the Little Ice Age in Europe between the 16th and 19th centuries.”
“With their current knowledge, Liu and colleagues don’t believe any physical forces over the last 10,000 years could have been strong enough to overwhelm the warming indicated by the increase in global greenhouse gases and the melting ice sheet, nor do the physical models in the study show that it’s possible.”
Perhaps we can call it a scientific dissonance. And as such, it is the stuff we can learn of. I will not argue that it contradicts the SOD calculations, but the empirical consequences are difficult to draw.
The paper has obvious connections to some of the issues SoD has discussed in the series of posts on Ghosts of Climates Past.
I should add my common disclaimer to every article:
Climate is complicated. The above results were with all other things remaining equal (which they don’t).
SOD wrote: “A lot of people realize [2XCO2 doubles emission from CO2] at some point during their climate science journey and finally realize how they have been duped by climate science all along!”
Based on my experience, the feeling of being duped arises from the excessive focus on absorptivity (“trapping heat”), rather than on emissivity (which is generally ignored). You were the first to help me combine my intuitive understanding that 2XCO2 must double emission from CO2 with the obvious fact that it also doubles absorption. The radiative forcing produced is a small net difference between two large opposing phenomena, occurring mostly at a small subset of the wavelengths absorbed by CO2.
SOD continued: “It’s irrefutable – more GHGs more cooling to space, more GHGs mean less global warming!”
If anyone ignorant of absorptivity and knowledgable about emissivity exists, they would logically believe that anthropogenic GHG’s should be causing global cooling, not less global warming. I prefer when you stick to the science and avoid strawmen like this one. After all, this is the blog I read to NOT get duped.
Frank,
Nitpick:
Intuition can be misleading.
Technically, that’s only true for wavelengths where the combination of path length and concentration gives an optical density much less than one so emission and absorption are linear with concentration. At wavelengths, concentrations and path lengths where the optical density is much greater than one, making the absorptivity/emissivity are only slightly less than one, doubling concentration changes emission and absorption only slightly. And that’s ignoring the effect of lapse rate on emission while having little effect on absorption. The actual behavior for real world atmospheric concentration integrated over all wavelengths is logarithmic because while there is little change at the band center, the band width expands.
DeWitt: I could have expressed this thought more accurately using differential form: Per increment of distance (ds), there are twice as many photons being emitted and absorbed (dI). When integrated over longer distances, your comments apply to the radiation that emerges from a layer of atmosphere, but ignore what happens inside. Of course, what emerges is the most important phenomena.
Macroscopically, I like to say that 2XCO2 produces twice as many photons from CO2 that travel half as far before being absorbed, but this is only correct when the optical density is high and the chances to reaching the surface or space are negligible. Most radiative forcing is produced at wavelengths where optical density isn’t high.
I doubt doubling CO2 changes the shape of CO2 lines: Pressure broadening is mostly caused by collisions with nitrogen and oxygen, not CO2. Doppler broadening depends on temperature which doesn’t change in instantaneous RT calculations and changes trivially at equilibrium. The characteristic emission level does rise, but the lines at a given altitude probably don’t change much when CO2 doubles.
@DeWittPayne,
“Which cloud tops provide an isothermal layer in the Earth’s atmosphere, low, mid or high level clouds?”
My intention is to model multiple cloud layers with partial coverage using Finite Element Analysis. That is a tough problem so I am starting with a one cloud layer model of Venus. It seems highly likely that this approach will work well as it will be simply be a more rigorous version of the analyses that Sagan carried out in 1967 and 1968.
@DeWitt Payne,
As I have said several times on this excellent blog, you and Leonard Weinstein have been an inspiration to me.
Your guidance has enabled me to apply engineering software to analyzing the surface temperature of planets and moons. Finite Element Analysis enabled me to model the diurnal variations in the Moon’s equatorial surface temperature with an rms error relative to observations of less than 1 K.
I went on to speculate about the surface temperature of an “Airless Earth”:
Then I turned to the seven bodies in the solar system with significant atmospheres:
Currently I am applying finite element analysis to confirm the Robinson & Catling model mentioned in the above guest post. It may even be possible to extend their work to include the effect of clouds. I plan to meet Tyler Robinson at Duke university in December.
The R&C model explains the temperature gradient on all seven bodies with significant atmospheres but it lacks the ability to define an isothermal layer (aka the “Cloud Tops”). That limitation has serious consequences. Take a look at “Slide 35” here:
Click to access Catling.pdf
In this slide James Hansen is able to explain the “Ice Ages” in terms of CO2 by assuming 16 K/ doubling and CAGW (Catastrophic Antropogenic Global Warming) by assuming 4.5 K/doubling.
Hansen’s slide becomes absurd when an isothermal layer corresponing to the cloud tops is introduced. When the temperature at the cloud tops is defined, the concentration of CO2 has no significant effect on temperature at any altitude in the troposphere. Likewise for Venus with its 100% cloud cover.
Your comments (and Leonard’s) would be appreciated.
galloping camel,
The 255K temperature for an “Airless” Earth is actually quite close to the radiative temperature of the planet as viewed from space. The difference between the average radiative temperature of the surface as it exists now and the radiative temperature viewed from space is the actual greenhouse effect, not the difference calculated for a hypothetical airless Earth.
The surface temperature of a truly airless Earth is not a useful calculation, however interesting it may be to do in terms of the greenhouse effect. The lunar regolith has a very low effective heat capacity compared to the Earth’s surface as it exists now. Heat capacity has a large effect on how the average temperature changes with rotation rate.
Thanks for taking the time to respond.
I don’t understand why you think it is not useful to calculate the average temperature of an airless Earth. Consensus climate scientists have been telling us that the temperature is 255 K so the warming caused by the atmosphere is 33 K.
Likewise mavericks like Nikolov & Zeller claim that the correct temperature of an airless Moon (and hence and airless Earth) is 154 K so the warming caused by the atmosphere is 134 K. Both are demonstrably in error.
You were wrong to say that the lunar regolith has a low heat capacity. Its measured specific heat is ~2,900 J/kg-K. The remarkable thing about lunar regolith is its low conductivity that is more that two orders of magnitude less than the bedrock.
I can supply the data files and the FEA code if you want to check my analysis. It breaks the lunar regolith into 50 layers each 10 mm thick and each with different thermal properties.
Did you not notice that I wrote effective heat capacity? Specific heat capacity is meaningless if the heat isn’t conducted.
Perhaps I should have said low thermal diffusivity rather than low effective heat capacity. The thermal diffusivity of the lunar regolith is on the order of 1E-08 compared to the thermal diffusivity of water at 25C of 1.4E-07. But that’s for pure diffusion. In fact, the upper layer of the ocean is relatively well mixed by turbulent eddy diffusion to a depth of several meters so the effective thermal diffusivity is orders of magnitude higher than for regolith. Plug that into your model.
@DeWitt,
You suggest plugging the ocean thermal properties into my model. That is probably beyond my resources but I might have a crack at it if I manage to improve on the R&C dense atmosphere model.
If my speculations about the temperature of an airless Earth, the oceans were permanently frozen even at the equator so my model (based as it was on the thermal properties of ice) seemed appropriate.
You need the Greenhouse Effect to melt the oceans!
Galloping Camel: Although the GHE is really, the idea of quantifying the earth’s greenhouse effect absurd in practice. First, there is the problem of selecting the albedo, humidity (ice sublimes), and surface heat capacity for an earth lacking GHG’s or an atmosphere. There are many possible models for an earth without a GHE, and all are too different from the real earth to ascribe the resulting temperature difference only to the GHE.
Even worse, however, the real earth’s mean surface temperature is a function of convection as well as the greenhouse effect. The average of T^4 is greater than the fourth power of average T. Since radiative cooling varies with T^4, radiative cooling is minimized by convectively averaging mean surface temperature. This averaging maximizes the average surface temperature for a given incoming irradiance from the sun. If your model earth lacking a GHE also has less convection, this will inflate the apparent GHE.
Consider how the earth “averages” temperature by convection between periods of light and darkness. For most of the ocean, SST drops less than 1 degK during 12 hours of darkness, because convection begins almost as soon as the surface begins to cool. On land, however, surface air temperature drops about 10 degK at night. Now consider what happens at the poles, which receive no radiation for up to six months (100-fold longer). Winter temperature at the North Pole averages about 45 degK colder than summer temperature. At the South Pole, the difference is only 35 degK. Vast amounts of heat are convected by air and ocean from the equatorial region to the poles, reducing average radiative cooling and increasing average temperature. If your model earth without a GHE (a radiative effect) doesn’t have as much convection, some of its relative coolness will be due to a loss of convection.
In my analysis of airless bodies convection and conduction above the surface were set at zero. Above the surface radiation is the sole means of heat transfer. Given that the model corresponds accurately with Diviner LRE measurements I feel that it is superior to the analysis done by “Respectable Climate Scientists”:
The temperature of 255 K derived above is mathematically correct but it assumes a surface at constant temperature which is unrealistic.
Please note that I assumed that the Moon’s emissivity to be a uniform 0.95 in the thermal IR region (outgoing radiation). I assumed that the Moon’s Albedo to be non-Lambertian (not uniform) in the solar spectrum (incoming radiation). This seems to be in close agreement with observations and Vasavada’s one dimensional model.
Below the surface, convection has been set at zero as the surfaces are assumed to be solid (ice or regolith). Thus only conduction was considered.
GC: The uniform case you display and complain about is an attempt to illustrate the principle with a very simple case – steady-state conditions identical over the earth’s surface. As you show, results for this case can be calculated with a couple of lines of math.
As soon as you get even slightly more complicated, the calculations get far, far more involved, as your resort to FEA software demonstrates. But it is easy to demonstrate that any variation in temperature, whether over time (day/night) or over area (equatorial/polar) will lead to a lower average temperature, which means a lower total energy level. So the 255K level is best viewed as the upper limit of an earth-like body with the same albedo but without any radiatively active gases.
Curt,
The rule that 255K is the maximum average temperature for a sphere emitting an average of 239 W/m² over its entire surface is called Hölder’s Inequality and is obtained when the surface is isothermal. Any other temperature distribution radiating with the same total power will have a lower average temperature.
But whether the greenhouse effect results in an average surface temperature increase of, say, 36 instead of 33 degrees C for an isothermal surface is a quite unimportant as far as the actual existence of the greenhouse effect is concerned and that increasing atmospheric greenhouse gas concentration will result, over time, in an increase in the average temperature of the surface.
I find GC’s posts boring and irrelevant. When I see six replies at once, it’s a sign that it’s not worth reading them. So I don’t. RW was fond of that sort of thing too.
DeWitt: I was just explaining Holder’s Inequality without naming it. I almost included an extra sentence to include the name, but decided against it. I am in complete agreement with you that the exact magnitude of the effect is not important if you want to demonstrate whether the effect exists or not.
You’re probably right about GC’s posts, but I fine that if I commit an argument to writing once, I will be able to express it later better and more quickly.
GallopingCamel wrote: “In my analysis of airless bodies convection and conduction above the surface were set at zero. Above the surface radiation is the sole means of heat transfer.”
As I demonstrated above, the more heat that is convected from the equator to the poles, the more uniform the mean global temperature and the higher the average global temperature can be and still be in radiative equilibrium with the incoming solar radiation. When your comparison world has no convection, the GHE you quantify is not caused by radiation alone.
Ooops!
In this slide James Hansen is able to explain the “Ice Ages” in terms of CO2 by assuming 16 K/ doubling and CAGW (Catastrophic Antropogenic Global Warming) by assuming 4.5 K/doubling.
That 16 K/doubling should have been 16 K/halving!
gallopingcamel,
What is the isothermal layer corresponding to cloud tops you refer to? Where do you think that such layers exist?
The results of Hansen are from the paper Climate sensitivity, sea level and atmospheric carbon dioxide, where the authors tell that in their model snowball Earth instability occurs just beyond three CO2 halvings. Ice ages of the past are, however, not the same thing as a snowball Earth and have occurred with CO2 concentrations much closer to the present one.
Cloud tops provide an isothermal layer with a temperature defined primarily by total pressure and the physical properties of the dominant vapor.
On Earth water is dominant; on Venus sulphuric acid and on Titan methane/ethane. I am trying to extend the Robinson & Catling’s model to include cloud layers.
Among other things, that Hansen paper claims to explain the last seven glaciations in terms of [CO2]. Unfortunately this hypothesis does not fit the facts that show temperature driving the [C02] thanks to Henry’s law:
Total pressure does not define the temperature, neither do the physical properties of the dominant vapor. You can find such connections only by making additional assumptions that are usually not true. Thus nether do such connections exist except in some special cases.
In vertical direction the lapse rate is close to the moist adiabatic lapse rate inside tropospheric clouds. In stratosphere the result depends on the radiative balance as you have also stated. Clouds do affect that, but I don’t see any general argument that would lead to the expectation of an isothermal layer at the tops of the stratospheric clouds either.
Variation in CO2 concentration are surely an important factor in glacial cycles true feedbacks, where the falling temperature leads to falling CO2 concentration and the falling CO2 concentration contributes to further falling of the temperature. This feedback loop is not the sole, or the originating, reason for the glacial cycles, but glacial cycles were almost certainly impossible without the help of this feedback cycle.
This is consistent with what Hansen et al state about the glacial cycle. In the abstract they write:
I emphasize in the above quote words that tell on the importance of other factors beside the role of CO2.
I don’t present any endorsement of the paper. As SoD has discussed in several posts, many questions remain unanswered on the glacial cycles. This is one of the many papers that presents results that agree with others on points like the important role of the mechanisms mentioned above, but disagrees on details.
galloping camel,
Which cloud tops provide an isothermal layer in the Earth’s atmosphere, low, mid or high level clouds? Apparently you’ve never flown long distances in an airplane with windows. Cloud top height varies as does the cloud top temperature.
As for pressure alone determining the surface temperature, here’s a thought experiment: Instantaneously transport the Earth from its current orbit at 1 AU to an orbit with a radius of 2 AU. What happens to the surface temperature? If you think it won’t go down because the surface pressure doesn’t change, you’re posting in the wrong place.
@Pekka,
“Total pressure does not define the temperature, neither do the physical properties of the dominant vapor.”
You may be right but please provide some calculations to support your position.
It’s difficult to present calculations to refute a claim that has not been specified exactly. I don’t even know, whether you have in mind part of troposphere, where the lapse rate is definitely not zero or stratosphere, where that’s possible, but not automatically true.
If you mean that the temperature does not change horizontally, that’s certainly possible, but again not always true.
@DeWittPayne,
“As for pressure alone determining the surface temperature, here’s a thought experiment: Instantaneously transport the Earth from its current orbit at 1 AU to an orbit with a radius of 2 AU. What happens to the surface temperature? If you think it won’t go down because the surface pressure doesn’t change, you’re posting in the wrong place.”
I can’t imagine how you extracted that little gem from the links I provided. Why would you construct such a “Straw Man” argument? You can answer your own question by constructing a model which allows you to alter the Total Solar Irradiance.
I will be happy to do it for you when my atmospheric FEA model is running but until then I think the Robinson & Catling model is closest to reality for all seven bodies with dense atmospheres starting with Venus at 0.72 A.U to Neptune at 30.1 A.U.
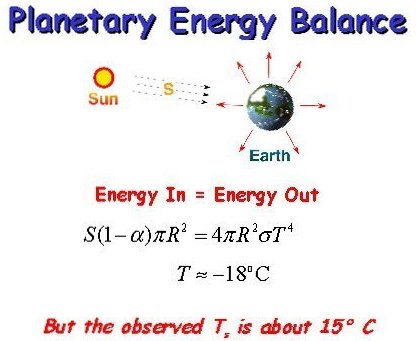
The energy in = energy out does not in any meaningful manner explain the surface temperature of the Earth.
The maximum surface temperature of the Earth’s surfaces are determined by the solar radiation- period. There is ample evidence of this and it is a certainty that the solar radiation could induce far higher surface temperatures than currently observed. I cite the Moon’s daytime temperature and Skylab as just 2 examples of why Wikipedia’s claim that a blackbody in space at Earth’s orbit would be ~5.3 is nonsense – the truth is we really have no idea because a blackbody is simply a theoretical consideration and real grey bodies exceed this theoretical claim all the time. Upon launch some of Skylab’s heat shields were damaged and the interior heated to over 134 degrees F and was uninhabitable until repaired.
It is obvious that our atmosphere reduces the maximum thermal impact of the solar radiation – even the IPCC acknowledge that UV radiation is significantly absorbed by Ozone and !51 % of the solar radiation is IR and there is significant absorption of these wavelengths by water vapour and CO2.
Why would these absorptions of highly energetic wavelengths not contribute to significant atmospheric heating and hence radiative emission while absorption of significantly less energetic terrestrial radiation is supposedly solely responsible ?
Even K & T claim about 23% absorption of the solar radiation – their mistake is the solar radiation is not 341 W/sqm as they claim but closer to a maximum of 1kW/sqm. If this were not true and significant then the solar industry would not exist !!
The thermal effect of 180 degrees C in an oven in deep space for an hour is not the same as minus 18 C for 10 hours even though the total Watts add up to the same value.
Simply reducing the solar radiation by the cosine factor of latitude and 70% albedo – which may not be appropriate for a clear sky – means that the solar radiation between 40 N and 40 S varies from ~958 W/sqm to ~730 W/sqm.
At these latitudes the Earth’s surfaces are predominantly oceans and water can absorb enormous quantities of energy with little thermal impact both by circulation driven by convective forces and evaporation. Earth’s climate would be significantly different at times in the past when the continental masses theoretically predominantly occupied this portion of the globe. The historical record suggests this and may explain the faint young sun paradox.
Imagine an Earth where a land mass similar to the Sahara but significantly larger occupied the majority of the tropics !!
I will not question the validity of the measurement of DLR but will state that DLR can only be radiation from the temperature of the atmosphere – fact.
99% of the atmosphere is supposedly IR inactive – O2 and N2 have only slight absorption spectra in IR wavelengths – fact.
This means they do not radiate significantly at IR wavelengths and are never hot enough to radiate at shorter wavelengths.
So the real question is twofold.
1. Do N2 and O2 increase in temperature by conduction/convection ? I say the answer is an obvious yes. On a day when the temperature is 30 C – like it is here right now – N2 and O2 are at 30 C – nothing else is realistic.
N2 and O2 collect energy from the surface through conduction and the expanding and rising gases also collide with other molecules transferring energy by convection – to argue this does not occur is simply stupid.
Yet the fact that N2 and O2 heat up and cool down seems to be ignored as they have little IR absorption.
2. If N2 and O2 cannot radiate significantly at ambient temperatures how do they lose this energy acquired during the day ?
Surely this is significant and explains how during a relatively short night our atmosphere retains its temperature. Remember according to standard science a gas expanding and rising is not the same as radiating the energy away – it is actually a transformation not a loss – although some work is performed.
If the radiation budget is the be-all and end-all of climate science then N2 and O2 must have a mechanism to lose their acquired energy and this must be relatively rare collisions with GHGs which in turn radiate the energy away.
Professor Wood said something like the energy thus transferred to the atmosphere remains there due to the low radiating power of gases – surely N2 and O2 which constitute ~99% have a low radiating power ?
Some claim the fact that a desert environment cools rapidly after sunset as proof that the atmosphere radiates rapidly. I argue rather that this indicates how transparent our atmosphere really is when the concentration of water vapour is low. The desert air is always convecting whilst the surface radiation rapidly escapes so it is cooling as well because the surface is rapidly cooling.
But how anyone can ignore the power of convection and atmospheric compression and de-compression simply amazes me. The solar radiation drives these phenomena !
PV = nRT may not explain atmospheric temperatures totally – although I suggest you try it with NASA’s Planetary Fact Sheets and you will see the calculation agree rather remarkably leading to the question are these Planetary Fact Sheets real measured data or calculations ?
Anyway, all the powerful weather events on Earth are driven by convection and the interplay of cold and warm air masses with humidity.
All of the energy driving this comes from the Sun !
RoscoRosco,
When you write comments like this, don’t be surprised that most people ignore you.
I don’t know where to start.
Solar radiation at the top of atmosphere (TOA) is about 1360 W/m2. There are two ways to calculate the average TOA over the whole surface area of the earth.
One way – the simple way – is to calculate the total absorbed solar radiation – 1360 impinging on a disc of πr2 and then divide that into the surface area of the earth, 4πr2.
You can see this explained in The Earth’s Energy Budget – Part One.
Or in any climate basics textbook.
The second way – the complicated way – is to integrate over the surface of the earth, taking into account that one half of the planet has zero at any given time, and the integration needs to have the cosine of the solar angle. Well, it comes to the same amount.
So no, K&T are not wrong. It’s simple geometry. And an average is different from a peak.
I’ll leave it there. If you are interested in learning, read a little and then ask a question. If you want to demonstrate to the world your amazing insights probably you should visit other blogs where lack of basic geometry and lack of any knowledge of atmospheric physics will be well received.
If you want to get a sense of Roscoe’s level of sophistication on these matters, please refer to his post from a little while back here:
http://www.principia-scientific.org/an-inconvenient-experiment-that-invalidates-the-greenhouse-gas-theory.html
While he realized he made errors there, it doesn’t appear he has learned much in the time since.
Roscoe asked above: 1. Do N2 and O2 increase in temperature by conduction/convection? 2. If N2 and O2 cannot radiate significantly at ambient temperatures how do they lose this energy acquired during the day?
N2 and O2 molecules gain and lose energy by colliding with other molecules in the atmosphere. In the troposphere, molecules collide about 10^9 times per second and about 0.1% of these collisions (10^6 per second) involve a GHG (usually water vapor or CO2). Some of these collisions excite or relax vibrational and rotational states in these GHGs, providing the most important mechanism by which the thermal energy in N2 and O2 molecules can be gained or lost. Some minor mechanisms are discussed below.
Minor mechanism 1. Some N2 and O2 molecules do collide with the surface and pick up kinetic energy from the surface (more often) or deliver kinetic energy to the surface (less often). The average N2 or O2 travels only 1 um between collisions (mean free path), so only about one in 10^10 N2 and O2 molecules in the atmosphere is exchanging energy with the surface at any moment. (In comparison, all the N2 and O2 molecules in the troposphere collide about 10^6 times per second with a CO2 or H2O molecule. Some of these collisions relax or excite vibrational and rotational states in these GHG – transferring energy to and from N2 and O2 molecules.)
After colliding with the surface, N2 and O2 can’t transfer the energy they pick up very far. The thermal conductivity of air is very low, about 0.025 W/m/degK. A 1 degK difference between the surface and the air can drive a 1 W/m2 heat flux across 0.025 m or 2.5 cm. The KT energy balance diagram estimates that collisions with the surface (simple heat) transfer about 18 W/m2 of energy from the surface to the atmosphere (only 4% of the total flux from the surface). This suggests that thermal energy flows about 1 mm by conduction before convection or radiation takes over. (If these other mechanisms didn’t take over, the difference between surface temperature and air temperature would increase.)
Minor mechanism 2. A small fraction of the N2 and O2 molecules in the atmosphere are gaining energy when condensation of water vapor (latent heat) produces clouds. The mechanism of energy transfer is still collision.
Minor mechanism 3. A very small fraction of N2 and O2 molecules are above the tropopause where they can gain energy from solar UV. They can exchange this energy with other molecules, but this energy leaves only when it is radiated by a GHG molecule. This mechanism is controls temperature above the tropopause (about 10% of the atmosphere), but has little impact on the surface.
N2 and O2 don’t do much in the atmosphere besides collide with GHGs. They are moved by convection, but convection doesn’t transfer energy into or out of these molecules. Their temperature may change according to the ideal gas law and adiabatic lapse rate – but “adiabatic” means that no energy is gained or lost in the process. Turbulent mixing also moves N2 and O2, but doesn’t transfer energy into or out of them.
For information on mean free path, collision frequency and thermal conductivity see Wikipedia and
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/frecol.html
Roscoe wrote: “The energy in = energy out does not in any meaningful manner explain the surface temperature of the Earth.”
Since temperature IS a form of energy, any meaningful explanation for surface temperature must be based on conservation of energy. Perhaps YOU can’t find a meaningful explanation for the earth’s surface temperature based on energy fluxes, but one can be found at the link SOD provided. Read it and explain why it is wrong.
The temperature of the equatorial region of the moon ranges from 200 degK during the night to 400 degK during the day and dips below 50 degK near the poles. We would make an unacceptable error calculating the moon’s mean OLR from eoT^4 using an average surface temperature. The average temperature of the moon can’t be easily understood in terms of average incoming and outgoing flux.
The earth has an average surface temperature (290 degK) that can be roughly understood in terms of average incoming and outgoing fluxes. For half the planet (equatorial regions), the average temperature is about 300 degK. It gradually drops about 250 degK near the poles (a small fraction of the surface). High heat capacity means that SST’s usually change <1 degK between day and night and land surface temperature changes only about 10 degK. So we don't make a big error calculating average OLR from eoT^4 using a single average value (290 degK) for surface temperature. Approximations (averages) that are inappropriate for the moon (50 to 400 degK) are reasonable for the earth (250-300 degK).
The earth is a sphere; Skylab was fairly cylindrical. Most spacecraft are spinning about their long axis fast enough to produce a surface temperature with little difference between the light and dark sides. For earth, divide incoming solar radiation by a factor of four to get the average radiation received per unit surface area. For Skylab, divide by pi. Using the principle of energy in = energy out that you disdain, NASA designed a parasol with the correct emissivity (for LWR) and albedo (for SWR) to replace the micrometeorite shield lost during launch and restored habitable temperature.
The same physics principles apply to surface temperature and energy flux on Venus, Earth, Moon, Skylab (and everywhere else): When equilibrium is reached and temperature not changing, energy in = energy out. That doesn't mean the details of the calculation (especially approximations) will be the same in all cases. On Venus and Earth, however, one needs to worry about an atmosphere – which emits radiation and permits energy flux by convection.
@DeWittPayne,
“I find GC’s posts boring and irrelevant. When I see six replies at once, it’s a sign that it’s not worth reading them. So I don’t. ”
I find that comment most discouraging considering that I finds your posts interesting and insightful. My main incentive for visiting this site is to find out what you and Leonard Weinstein have to say.
There will be no more “gallopingcamel” posts or commnets here but my parting request is that you comment on James Hansen’s 2013 paper (Fig. 7a) that shows Earth’s surface temperature cooling at a rate of 16 k/halving of [Co2] and warming at a rate of 4.5 K/doubling of [CO2]. You can find the relevant slide (#35) here:
Click to access Catling.pdf
It this “Science” or BS?
@Pekka,
“It’s difficult to present calculations to refute a claim that has not been specified exactly.”
Fair enough, so let me more specific.
Earth’s atmosphere is complicated given that cloud cover is partial so one needs to model multiple cloud layers within the troposphere. Venus on the other hand represents a much simpler problem (from a modeler’s point of view) given that it has 100% cloud cover with the altitude of the “Cloud Tops” well defined.
At the Venusian equator the cloud top altitude is 58 km and the temperature is ~260 K (Magellan,1991). Please explain what the cloud top temperature would be if the CO2 was magically transformed into nitrogen at the same pressure. Remember that the Cp of CO2 is almost identical to that of nitrogen.
Carl Sagan made this analysis in 1968. Do you agree or disagree with Sagan? This is not an essay question. I am asking for equations and numerical calculations.
No matter what you say I won’t be commenting here so you will have the last word.
GP,
What you write here contains no mention of the point a commented on. You do not use the word isothermal or anything equivalent to that.
I’m not planning to answer every issue you bring up. I note only that the bond albedo of Venus is 0.90 according to NASA Venus Fact Sheet. The smaller distance from the Sun leads to twice the solar irradiation hitting Venus compared to Earth. Combining both factors Venus absorbs per unit area about 30% of the energy Earth absorbs. Thus the effective radiative temperature of Venus is 184.2 K (value given by NASA). Without any GHG’s outside the clouds, but with dense clouds that cover the whole planet the temperature at cloud tops would be close to this value (184 K, when calculated from the average fourth power of the temperature).
@SOD,
Being kicked off CAGW sites has mostly been an exhilarating experience. It is hard to respect people like John Cook and his team of nincompoops:
http://www.gallopingcamel.info/docs/DeletedCamel.doc
The exception was “Brave New Climate” given that I still repect Barry Bond at Adelaide University in Australia.
While you did not eject me from your excellent site I no longer feel welcome here so I reluctantly bid you farewell. You remain high in my esteem.
Old thread, but…
Why is the greenhouseeffect model designed with an atmosphere that is heated with mainly it´s own emitted radiation?
Doesn´t the sun provide enough Watt/m^2 in the same part of the spectrum that earth emits at any part of the atmosphere. Seems to me as IR-radiation in the atmosphere would be absorbed from solar radiation rather than earth emissions?
Shouldn´t the atmosphere be treated as an extension of the surface layer instead of a separate absorbing and emitting body?
Instead of trying to make the graybody earth heat up by absorbing it´s own emission with a model that uses the atmosphere the same way as windowpanes in a greenhouse, it seems more sensible to add the atmospheric properties as changes in absorptivity and emissitivity of the surface of the graybody.
The outgoing radiation spectrum is then treated as the change in absorbtance and emissitivity of the surface instead of the product of two(or more) separate greybodys interacting.
Doesn´t the earth blackbody graph fit under the solar spectrum at all wavelength? Any rise in mean temperature would cause a drift in emission towards the suns spectral distribution and would still not increase emission in the IR-spectrum above solar spectrum irradiance. Right?
My understanding of greybodys heated by another blackbody is that the temperature decides spectral distribution and intensity, so the earth can´t radiate more IR than incoming radiation by having an atmosphere that reduces it´s absorptance and emittance as a graybody.
If greenhouse gasses absorbs and emitts incoming radiation it means that the atmosphere is heated by the sun instead of it´s own emissions which is more likely if the sun radiates more intensly in the same spektrum. If the net flux doesn´t radiate primarily from the surface, but the lowest part of the atmosphere, the system is more in balance and there is no need to absorb it´s own emission to gain heat, just to retain heat during night.
Of course that would make the atmosphere a cooling property of the earth´s surface instead of warming. Which is nice because that is what we are used to when we add water to hot stuff.
I think that is very backwards, that if we heat a sphere that represents a greybody in vacuum, with a blackbody radiator that provides radiation at 6000K, and then pour water with carbonated acid on it, would add more heat?
And that a gaslayer added to the surface would increase the total absorptance and emittance? without raising the temperature so that the spectrum would drift?
Anyways, doesn´t the sun provide more IR than the earth emitts? And if so, why should the earth wait for it to be absorbed into the surface and absorb it´s own emission again to gain heat?
Isn´t the atmosphere a product of temperature rather than temperature as a product of the atmosphere?
Sure, if you’re the same distance away. But the sun is far enough away that, at the distance of the Earth from the Sun, the effective temperature of solar radiation isn’t ~6,000K, but more like 390K. It’s intensity has been reduced by a factor of 1/215² = 0.0000216. Then there’s the fact that the Earth is a sphere and rotates, so averaged over the sphere and over a full rotation, you have to divide by the solar spectral intensity by another factor of four. So, no, the Earth’s blackbody spectrum does not fit under the solar spectrum at all wavelengths at the Earth’s orbit.
Emil asked: Doesn´t the sun provide enough Watt/m^2 in the same part of the spectrum that earth emits at any part of the atmosphere. Seems to me as IR-radiation in the atmosphere would be absorbed from solar radiation rather than earth emissions?
No. 99% of the sun’s energy is emitted at wavelengths shorter then 4 um and 99% of the earth’s emission is at wavelengths longer than 4 um. GHGs interfere more with outgoing thermal radiation than with incoming solar radiation.
https://images.duckduckgo.com/iu/?u=http%3A%2F%2Fsanjeev.sabhlokcity.com%2Fco2%2Findex_files%2Fimage013.gif&f=1
Emil also asked: “Shouldn´t the atmosphere be treated as an extension of the surface layer instead of a separate absorbing and emitting body?
Instead of trying to make the graybody earth heat up by absorbing it´s own emission with a model that uses the atmosphere the same way as windowpanes in a greenhouse, it seems more sensible to add the atmospheric properties as changes in absorptivity and emissitivity of the surface of the gray body.”
In short, No. The concepts of blackbody radiation, absorptance, and emissivity are difficult to apply correctly to the atmosphere. The temperature of the atmosphere varies from 190 K to 290 K, which would be a 3-fold change if the atmosphere behaved like a blackbody. So thermal emission by the atmosphere should be expected to vary dramatically with altitude.
Planck’s Law for blackbody radiation assumes radiation in thermal equilibrium with the molecules it is passing through: absorption = emission. Solids and liquids have emissivity’s less than unity because some reflection/scattering occurs at the interface between the material and air. Gases in thermal equilibrium with the radiation passing through them have no interface and therefore should have an emissivity of unity. The problem is the radiation passing through the atmosphere is NOT in thermal equilibrium with the atmosphere – the atmosphere is too thin. At some wavelengths and altitudes, absorption does not equal emission. At strongly absorbed wavelengths, thermal equilibrium between absorption and emission does exist, but the density of the gas always drops low enough with increasing altitude that emission and absorption eventually become negligible. So the spectrum of radiative cooling to space is rather complex:
https://images.duckduckgo.com/iu/?u=http%3A%2F%2Fupload.wikimedia.org%2Fwikipedia%2Fcommons%2Fthumb%2F9%2F9c%2FModtranRadiativeForcingDoubleCO2.png%2F800px-ModtranRadiativeForcingDoubleCO2.png&f=1
Some misleading models of the GHE assume an atmosphere that emits blackbody radiation – aka an optically thick slab atmosphere. Accurate models of the atmosphere deal use many optically thin layers of atmosphere where emission depends on the density and temperature of the GHG molecules in that layer. To calculate the absorption by such a layer, you need to know the intensity of the incoming radiation. Software for doing radiation transfer calculations in the atmosphere is available at this website.
http://climatemodels.uchicago.edu/modtran/
“So as you increase the GHGs in the atmosphere you increase its ability to cool to space.”
No, of course not, but it doesn’t change the fact the atmosphere must make its massive cooling push toward radiative balance with the Sun at the TOA (above all else). The primary way it does this is by continuously emitting IR up towards the TOA, everywhere in the atmosphere. Non-stop, never ending — no matter what changes are induced, natural or anthropogenic.
However, energy absorbed by the system from the Sun is a continuous stream of downward flowing radiant energy all flowing towards the surface, i.e. continuously acting to warm the system and ultimately the surface. Moreover, these are all new joules added to the system.
Where as, the IR energy captured by GHGs, aggregate or incremental, is not new energy to the system, and when re-radiated is henceforth non-directional, i.e. occurs with equal probability up or down — no matter where the energy goes or how long it stays in the atmosphere.
Yet, climate science would have us believe the *intrinsic* surface warming ability of additional GHG absorption and additional post albedo solar power entering the system on a W/m^2 are equal to one another.