In previous articles in this series we looked at a number of issues first in Miskolczi’s 2010 paper and then in the 2007 paper.
The author himself has shown up and commented on some of these points, although not all, and sadly decided that we are not too bright and a little bit too critical and better pastures await him elsewhere.
Encouraged by one of our commenters I pressed on into the paper: Greenhouse Effect in Semi-Transparent Planetary Atmospheres, Quarterly Journal of the Hungarian Meteorological Service (2007), and now everything is a lot clearer.
The 2007 paper by Ferenc Miskolczi is a soufflé of confusion piled on confusion. Sorry to be blunt. If I was writing a paper I would say “..some clarity is needed in important sections..” but many readers unfamiliar with the actual meaning of this phrase might think that some clarity was needed in important sections rather than the real truth that the paper is a shambles.
I’ll refer to this paper as M2007. And to the equations in M2007 with an M prefix – so, for example, equation 15 will be [M15].
Some background is needed so first we need to take a look at something called The Semi-Gray Model. Regular readers will find a lot of this to be familiar ground, but it is necessary as there are always many new readers.
The SGM – Semi-Grey Model or Schwarzschild Grey Model
I’ll introduce this by quoting from an excellent paper referenced by M2007. This is a 1995 paper by Weaver and the great Ramanathan (free link in References):
Simple models of complex systems have great heuristic value, in that their results illustrate fundamental principles without being obscured by details. In particular, there exists a long history of simple climate models. Of these, radiative and radiative-convective equilibrium models have received great attention..
One of the simplest radiative equilibrium models involves the assumption of a so-called grey atmosphere, where the absorption coefficient is assumed to be independent of wavelength. This was first discussed by Schwarzschild [1906] in the context of stellar interiors. The grey gas model was adapted to studies of the Earth by assuming the atmosphere to be transparent to solar radiation and grey for thermal radiation. We will refer to this latter class as semigrey models.
And in the abstract they say:
Radiative equilibrium solutions are the starting point in our attempt to understand how the atmospheric composition governs the surface and atmospheric temperatures, and the greenhouse effect. The Schwarzschild analytical grey gas model (SGM) was the workhorse of such attempts. However, the solution suffered from serious deficiencies when applied to Earth’s atmosphere and were abandoned about 3 decades ago in favor of more sophisticated computer models..
[Emphasis added]
And they go on to present a slightly improved SGM as a useful illustrative tool.
Some clarity on a bit of terminology for new readers – a blackbody is a perfect emitter and absorber of radiation. In practice there are no blackbodies but some bodies come very close. A blackbody has an emissivity = 1 and absorptivity = 1.
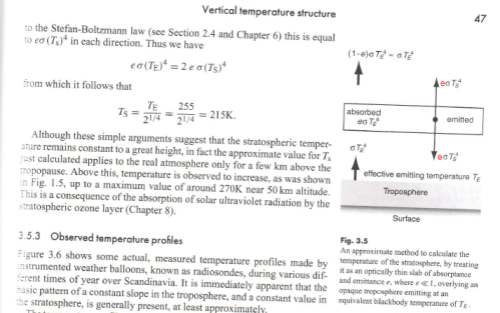
In our atmosphere, the gases which absorb and emit significant radiation have very wavelength dependent properties, e.g.:
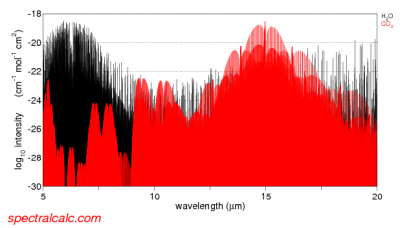
From spectralcalc.com
Figure 1
So the emissivity and absorptivity vary hugely from one wavelength to the next (note 1). However, as an educational tool, we can calculate the results for a grey atmosphere – this means that the emissivity is assumed to be constant across all wavelengths.
The term semi-grey means that the atmosphere is considered transparent for shortwave = solar wavelengths (<4 μm) and constant but not zero for longwave = terrestrial wavelengths (>4 μm).
Constructing the SGM
This model is very simple – and is not used to really calculate anything of significance for our climate. See Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations for the real equations.
We assume that the atmosphere is in radiative equilibrium – that is, convection does not exist and so only radiation moves heat around.
Here is a graphic showing the main elements of the model:
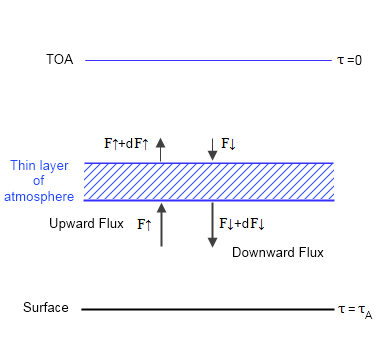
Figure 2
Once each layer in the atmosphere is in equilibrium, there is no heating or cooling – this is the definition of equilibrium. This means energy into the layer = energy out of the layer. So we can use simple calculus to write some equations of radiative transfer.
We define the TOA (top of atmosphere) to be where optical thickness, τ=0, and it increases through the atmosphere to reach a maximum of τ=τA at the surface. This is conventional.
We also know two boundary conditions, because at TOA (top of atmosphere) the downward longwave flux, F↓(τ=0) = 0 and the upwards longwave flux, F↑(τ=0) = F0, where F0 = absorbed solar radiation ≈ 240 W/m². This is because energy leaving the planet must be balanced by energy being absorbed by the planet from the sun.
We also have to consider the fact that energy is not just going directly up and down but is going up and down at every angle. We can deal with this via the dffusivity approximation which sums up the contributions from every angle and tells us that if we use τ*= τ . 5/3 (where τ is defined in the vertical direction) we get the complete contribution from all of the different directions. (Note 2). For those following M2007 I have used τ* to be his τ with a ˜ on top, and τ to be his τ with a ¯ on top.
With these conditions we can get a solution for the SGM (see derivation in the comments):
B(τ) = F0/2π . (τ+1) [1] cf eqn [M15]
where B is the spectrally integrated Planck function, and remember F0 is a constant.
And also:
F↑(τ) = F0/2 . (τ+2) [2]
F↓(τ) = F0/2 . τ [3]
A quick graphic might explain this a little more (with an arbitrary total optical thickness, τA* = 3):
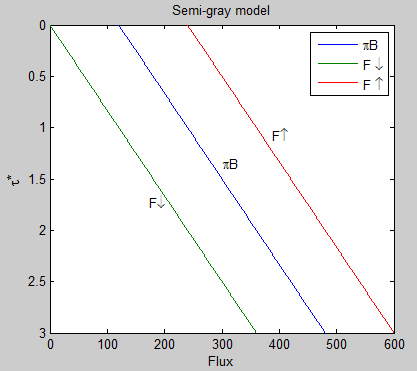
Figure 3
Notice that the upward longwave flux at TOA is 240 W/m² – this balances the absorbed solar radiation. And the downward longwave flux at TOA is zero, because there is no atmosphere above from which to radiate. This graph also demonstrates that the difference between F↑ and F↓ is a constant as we move through the atmosphere, meaning that the heating rate is zero. The increase in downward flux, F↓, is matched by the decrease in upward flux, F↑.
It’s a very simple model.
By contrast, here are the heating/cooling rates from a comprehensive (= “standard”) radiative-convective model, plotted against height instead of optical thickness.
Heating from solar radiation, because the atmosphere is not completely transparent to solar radiation:

From Grant Petty (2006)
Figure 4
Cooling rates due to various “greenhouse” gases:
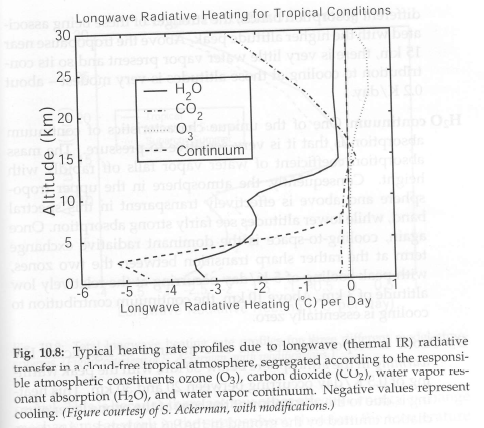
From Petty (2006)
Figure 5
And the heating and cooling rates won’t match up because convection moves heat from the surface up into the atmosphere.
Note that if we plotted the heating rate vs altitude for the SGM it would be a vertical line on 0.0°C/day.
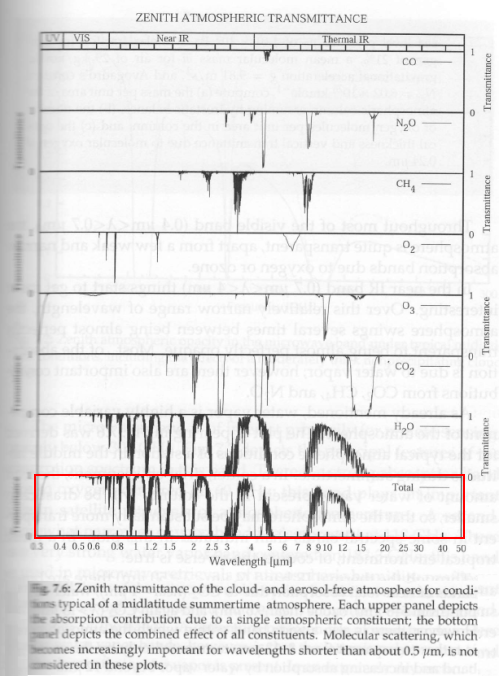
Let’s take a look at the atmospheric temperature profile implied by the semi-grey model:
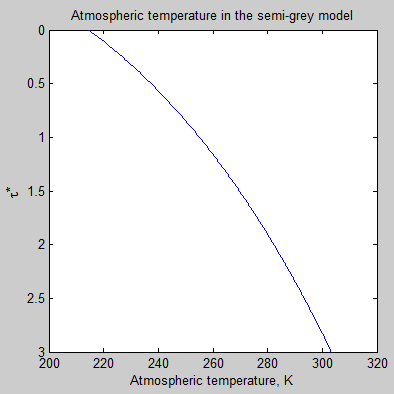
Figure 6
Now a lot of readers are probably wondering what the τ really means, or more specifically, what the graph looks like as a function of height in the atmosphere. In this model it very much depends on the concentration of the absorbing gas and its absorption coefficient. Remember it is a fictitious (or “idealized”) atmosphere. But if we assume that the gas is well-mixed (like CO2 for example, but totally unlike water vapor), and the fact that pressure increases with depth then we can produce a graph vs height:
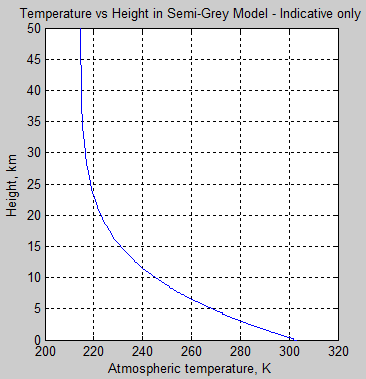
Figure 7
Important note – the values chosen here are not intended to represent our climate system.
Figure 6 & 7, along with figure 3, are just to help readers “see” what a semi-grey model looks like. If we increase the total optical depth of the atmosphere the atmospheric temperature at the surface increases.
Note as well that once the temperature reduction vs height is too large a value, the atmosphere will become unstable to convection. E.g. for a typical adiabatic lapse rate of 6.5 K/km, if the radiative equilibrium implies a lapse rate > 6.5 K/km then convection will move heat to reduce the lapse rate.
Curious Comments on the SGM
Some comments from M2007:
p 11:
Note, that in obtaining B0 , the fact of the semi-infinite integration domain over the optical depth in the formal solution is widely used. For finite or optically thin atmosphere Eq. (15) is not valid. In other words, this equation does not contain the necessary boundary condition parameters for the finite atmosphere problem.
The B0 he is referring to is the constant in [M15]. This constant is H/2π – where H = F0 (absorbed solar radiation) in my earlier notation. This constant B0 later takes on magical properties.
p 12:
Eq. (15) assumes that at the lower boundary the total flux optical depth is infinite. Therefore, in cases, where a significant amount of surface transmitted radiative flux is present in the OLR , Eqs. (16) and (17) are inherently incorrect. In stellar atmospheres, where, within a relatively short distance from the surface of a star the optical depth grows tremendously, this could be a reasonable assumption, and Eq. (15) has great practical value in astrophysical applications. The semi-infinite solution is useful, because there is no need to specify any explicit lower boundary temperature or radiative flux parameter (Eddington, 1916).
[Emphasis added]
The equations can easily be derived without any requirement for the total optical depth being infinite. There is no semi-infinite assumption in the derivation. Whether or not some early derivations included it, I cannot say. But you can find the SGM derivation in many introductions to atmospheric physics and no assumption of infinite optical thickness exists.
When considering the clear-sky greenhouse effect in the Earth’s atmosphere or in optically thin planetary atmospheres, Eq. (16) is physically meaningless, since we know that the OLR is dependent on the surface temperature, which conflicts with the semi-infinite assumption that τA =∞..
..There were several attempts to resolve the above deficiencies by developing simple semi-empirical spectral models, see for example Weaver and Ramanathan (1995), but the fundamental theoretical problem was never resolved..
This is the reason why scientists have problems with a mysterious surface temperature discontinuity and unphysical solutions, as in Lorenz and McKay (2003). To accommodate the finite flux optical depth of the atmosphere and the existence of the transmitted radiative flux from the surface, the proper equations must be derived.
The deficiencies noted include the result in the semi-gray model of a surface air temperature less than the ground temperature. If you read Weaver and Ramanathan (1995) you can see that this isn’t an attempt to solve some “fundamental problem“, but simply an attempt to make a simple model slightly more useful without getting too complex.
The mysterious surface temperature discontinuity exists because the model is not “full bottle”. The model does not include any convection. This discontinuity is not a mystery and is not crying out for a solution. The solution exists. It is called the radiative-convective model and has been around for over 40 years.
Miskolczi makes some further comments on this, which I encourage people to read in the actual paper.
We now move into Appendix B to develop the equations further. The results from the appendix are the equations M20 and M21 on page 14.
Making Equation Soufflé

The highlighted equation is the general solution to the Schwzarschild equation. It is developed in Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations – the equation reproduced here from that article with explanation:
Iλ(0) = Iλ(τm)e-τm + ∫ Bλ(T)e-τ dτ
The intensity at the top of atmosphere equals..
The surface radiation attenuated by the transmittance of the atmosphere, plus..
The sum of all the contributions of atmospheric radiation – each contribution attenuated by the transmittance from that location to the top of atmosphere
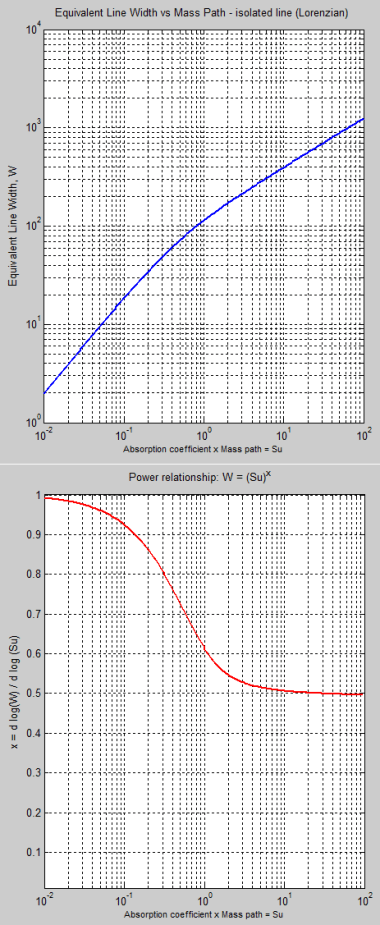
For those wanting to understand the maths a little bit, the 3/2 factor that appears everywhere in Miskolczi’s equation B1 is the diffusivity approximation already mentioned (and see note 2) where we need to sum the radiances over all directions to get flux.
Now this equation is the complete equation of radiative transfer. If we combine it with a simple convective model it is very effective at calculating the flux and spectral intensity through the atmosphere – see Theory and Experiment – Atmospheric Radiation.
So equation B1 in M2007 cannot be solved analytically. This means we have to solve it numerically. This is “simple” in concept but computationally expensive because in the HITRAN database there are 2.7 million individual absorption lines, each one with a different absorption coefficient and a different line width.
However, it can be solved and that is what everyone does. Once you have the database of absorption coefficients and the temperature profile of the atmosphere you can calculate the solution. And band models exist to speed up the process.
And now the rabbit..
The author now takes the equation for the “source function” (B) from the simple model and inserts it into the “complete” solution.
The “source function” in the complete solution can already be calculated – that’s the whole point of the equation B1. But now instead, the source function from the simple model is inserted in its place. This equation assumes that the atmosphere has no convection, has no variation in emissivity with wavelength, has no solar absorption in the atmosphere, and where the heating rate at each level in the atmosphere = zero.
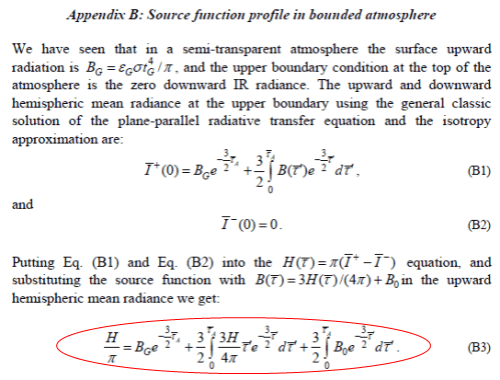
The origin of equation B3 is the equation you see above it:
B(τ) = 3H(τ)/4π + B0 [M13]
Actually, if you check equation M13 on p.11 it is:
B(τ) = 3H.τ/4π + B0 [M13]
This appears to be one of the sources of confusion for Miskolczi, for later comment.
Equation M13 is derived for zero heating rates throughout the atmosphere, and therefore constant H. With this simple assumption – and only for this simple assumption – the equation M13 is a valid solution to “the source function”, ie the atmospheric temperature and radiance.
If you have the complete solution you get one result. If you have the simple model you get a different result. If you take the result from one and stick it in the other how can you expect to get an accurate outcome?
If you want to see how various atmospheric factors are affected by changing τ, then just change τ in the general equation and see what happens. You have to do this via numerical analysis but it can easily be done..
As we continue on working through the appendix, B6 has a sign error in the 2nd term on the right hand side, which is fixed by B7.
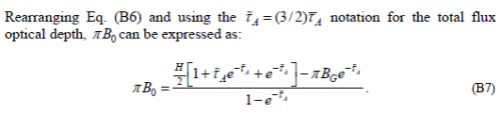
This B0 is the constant in the semi-gray solution. The constant appears because we had a differential equation that we integrated. And the value of the constant was obtained via the boundary condition: upward flux from the climate system must balance solar radiation.
So we know what B0 is.. and we know it is a constant..
Yet now the author differentiates the constant with respect to τ. If you differentiate a constant it is always zero. Yet the explanation is something that sounds like it might be thermodynamics, but isn’t:
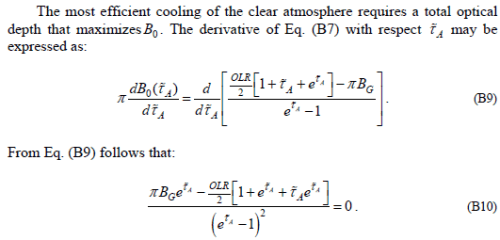
If someone wants to explain what thermodynamic principle create the first statement – I would be delighted. Without any explanation it is a jumble of words that doesn’t represent any thermodynamic principle.
Anyway B0 is a constant and is equal to approximately 240 W/m². Therefore, if we differentiate it, yes the value dB0/dτ=0.
Unfortunately, the result in B10 is wrong.
If we differentiate a variable we can’t assume it is a constant. The variable in question is BG. This is the “source function” for the ground, which gives us the radiance and surface temperature. Clearly the surface temperature is a function of many factors especially including optical thickness. Of course, if somewhere else we have proven that BG is a constant then dBG/dτ=0.
It has to be proven.
[And thanks to DeWitt Payne for originally highlighting this issue with BG, as well as explaining my calculus mistakes in an email].
A quick digression on basic calculus for the many readers who don’t like maths – just so you can see what I am getting at.. (you are the ones who should read it)
Digression
We will consider just the last term in equation [B9]. This term = BG/(eτ-1). I have dropped the π from the term to make it simpler to read what is happening.
Generally, if you differentiate two terms multiplied together, this is what happens:
d(fg)/dx = g.df/dx + f.dg/dx [4]
This assumes that f and g are both functions of x. If, for example, f is not a function of x, then df/dx=0 (this just means that f does not change as x changes). And so the result reduces to d(fg)/dx = f.dg/dx.
So, using [4] :
d/dτ [BG/(eτ-1)] = [1/(eτ-1)] . dBG/dτ + BG . d [1/(eτ-1)]/dτ [5]
We can look up:
d [1/(eτ-1)]/dτ = -eτ/(eτ-1)² [6]
So substituting [6] into [5], last term in [B9]:
= [1/(eτ-1)] . dBG/dτ – eτ.BG /(eτ-1)² [7]
You can see the 2nd half of this expression as the first term in [B10], multiplied by π of course.
But the term for how the surface radiance changes with optical thickness of the atmosphere has vanished.
end of digression
Soufflé Continued
So the equation should read:
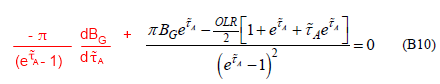
Where the red text is my correction (see eqn 7 in the digression).
Perhaps the idea is that if we assume that surface temperature doesn’t change with optical thickness then we can prove that surface temperature doesn’t change with optical thickness.
This (flawed) equation is now used to prove B11:
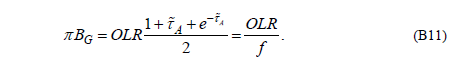
Well, we can see that B11 isn’t true. In fact, even apart from the missing term in B10, the equation has been derived by combining two equations which were derived under different conditions.
As we head back into the body of the paper from the appendix, equations B7 and B8 are rewritten as equations [M20] and [M21].

Miskolczi adds:
We could not find any references to the above equations in the meteorological literature or in basic astrophysical monographs, however, the importance of this equation is obvious and its application in modeling the greenhouse effect in planetary atmospheres may have far reaching consequences.
Readers who have made it this far might realize why he is the first with this derivation.
Continuing on, more statements are made which reveal some of the author’s confusion with one part of his derivation. The SGM model is derived by integrating a simple differential equation, which produces a constant. The boundary conditions tell us the constant.
Equation [M13] is written:
B(τ) = 3H/4π + B0 [M13]
Then [M14] is written:
H(τ) = π (I+ – I-) [M14]
So now H is a function of optical depth in the atmosphere?
In [M15]:
B(τ*) = H (1 + τ*)/2π [M15]
Refer to my equation 1 and you will see they are the same. The only way this equation can be derived is with H as a constant, because the atmosphere is in radiative equilibrium. If H isn’t constant you have a different equation – M13 and 15 are no longer valid.
..The fact that the new B0 (skin temperature) changes with the surface temperature and total optical depth, can seriously alter the convective flux estimates of previous radiative-convective model computations. Mathematical details on obtaining equations 20 and 21 are summarized in appendix B.
Miskolczi has confused himself (and his readers).
Conclusion
There is an equation of radiative transfer and it is equation B1 in the appendix of M2007. This equation is successfully used to calculate flux and spectral intensity in the atmosphere.
There is a very simple equation of radiative transfer which is used to illustrate the subject at a basic level and it is called the semi-grey model (or the Schwarzschild grey model). With the last few decades of ever increasing computing power the simple models have less and less practical use, although they still have educational value.
Miskolczi has inserted the simple result into the general model, which means, at best, it can only be applied to a “grey” atmosphere in radiative equilibrium, and at worst he has just created an equation soufflé.
The constant in the simple model has become a variable. Without any proof, or re-derivation of the simple model.
One of the important variables in the simple model has become a constant and therefore vanished from an equation where it should still reside.
Many flawed thermodynamic concepts are presented in the paper, some of which we have already seen in earlier articles.
M2007 tells us that Ed=Aa due to Kirchhoff’s law. (See Part Two). His 2010 paper revised this claim as to due to Prevost.. However, the author himself recently stated:
I think I was the first who showed the Aa~=Ed relationship with reasonable quantitative accuracy.
And doesn’t understand why I think it is important to differentiate between fundamental thermodynamic identities and approximate experimental results in the theory section of a paper. “My experiments back up my experiments..”
M2007 introduces equation [M7] with:
In Eq. (6) SU − (F0 + P0 ) and ED − EU represent two flux terms of equal magnitude, propagating into opposite directions, while using the same F0 and P0 as energy sources. The first term heats the atmosphere and the second term maintains the surface energy balance. The principle of conservation of energy dictates that:
SU − (F0) + ED − EU = F0 = OLR
Note the pseudo-thermodynamic explanation. The author himself recently said:
Eq. 7 simply states, that the sum of the Su-OLR and Ed-Eu terms – in ideal greenhause case – must be equal to Fo. I assume that the complex dynamics of the system may support this assumption, and will explain the Su=3OLR/2 (global average) observed relationship.
[Emphasis added]
And later entertainingly commented:
You are right, I should have told that, and in my new article I shall pay more attantion to the full explanations. However, some scientists figured it out without any problem.
Party people who got the joke right off the bat..
M07 also introduces the idea that kinetic energy can be equated with the flux from the atmosphere to space. See Part Three. Introductory books on statistical thermodynamics tell us that flux is proportional to the 4th power of temperature, while kinetic energy is linearly proportional to temperature. We have no comment from the author on this basic contradiction.
This pattern indicates an obvious problem.
In summary – this paper does not contain a theory. Just because someone writes lots of equations down in attempt to back up some experimental work, it is not theory.
If the author has some experimental work and no theory, that is what he should present – look what I have found, I have a few ideas but can someone help develop a theory to explain these results.
Obviously the author believes he does have a theory. But it’s just equation soufflé.
Other Articles in the Series:
The Mystery of Tau – Miskolczi – introduction to some of the issues around the calculation of optical thickness of the atmosphere, by Miskolczi, from his 2010 paper in E&E
Part Two – Kirchhoff – why Kirchhoff’s law is wrongly invoked, as the author himself later acknowledged, from his 2007 paper
Part Three – Kinetic Energy – why kinetic energy cannot be equated with flux (radiation in W/m²), and how equation 7 is invented out of thin air (with interesting author comment)
Part Four – a minor digression into another error that seems to have crept into the Aa=Ed relationship
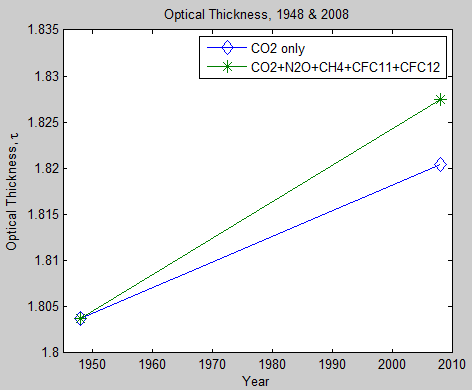
Part Six – Minor GHG’s – a less important aspect, but demonstrating the change in optical thickness due to the neglected gases N2O, CH4, CFC11 and CFC12.
Further reading:
New Theory Proves AGW Wrong! – a guide to the steady stream of new “disproofs” of the “greenhouse” effect or of AGW. And why you can usually only be a fan of – at most – one of these theories.
References
Greenhouse Effect in Semi-Transparent Planetary Atmospheres, Miskolczi, Quarterly Journal of the Hungarian Meteorological Service (2007)
Deductions from a simple climate model: factors governing surface temperature and atmospheric thermal structure, Weaver & Ramanathan, JGR (1995)
Notes
Note 1 – emissivity = absorptivity for the same wavelength or range of wavelengths
Note 2 – this diffusivity approximation is explained further in Understanding Atmospheric Radiation and the “Greenhouse” Effect – Part Six – The Equations. In M2007 he uses a different factor, τ* = τ . 3/2 – this differences are not large but they exist. The problems in M2007 are so great that finding the changes that result from using different values of τ* is not really interesting.










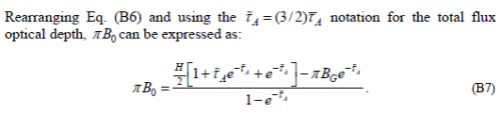
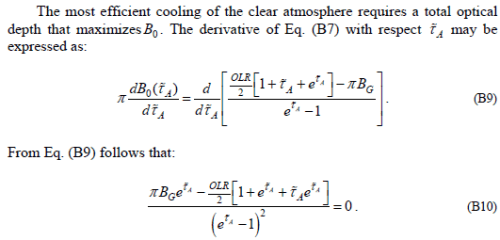
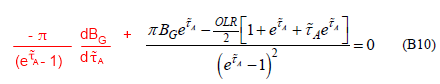
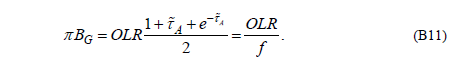

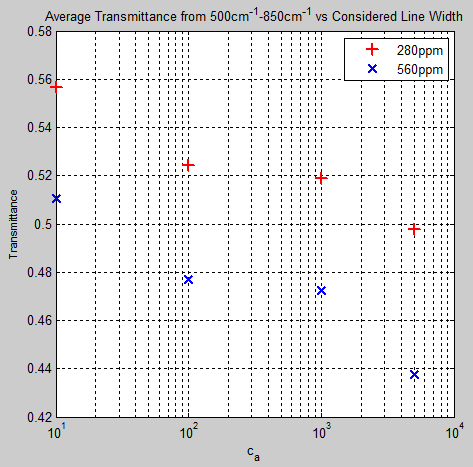
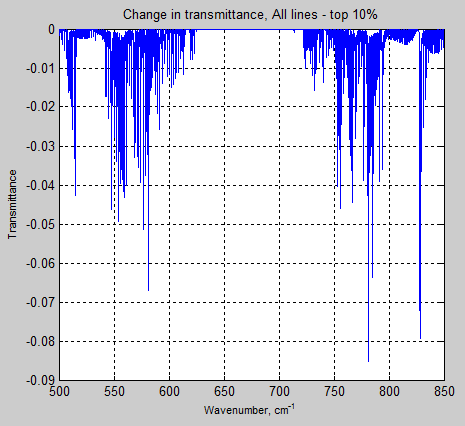
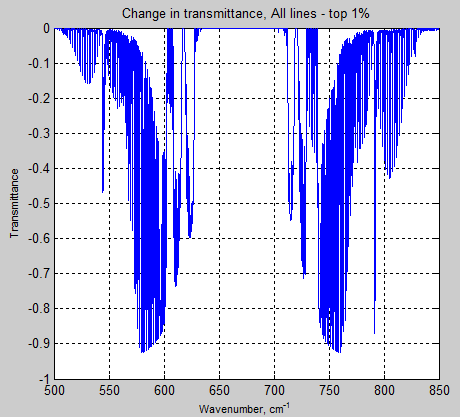
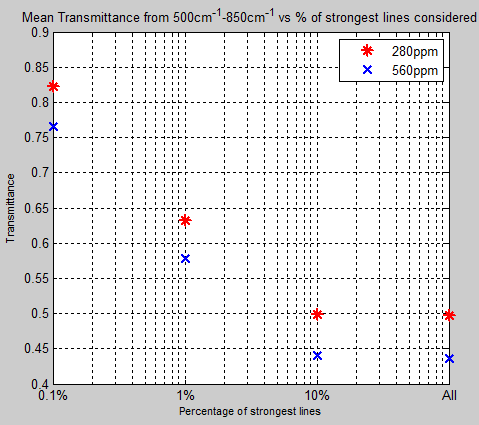


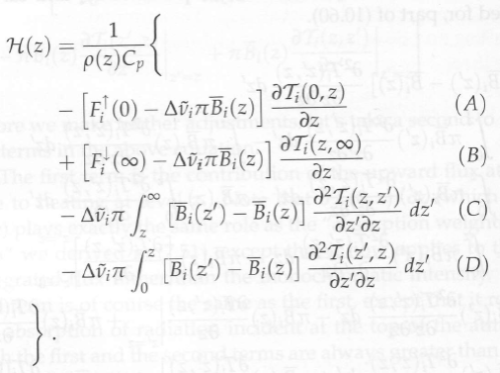



The Mystery of Tau – Miskolczi – Part Four – Emissivity
May 1, 2011 by scienceofdoom
In Part Two we looked at the claimed relationship ED=AA in Miskolczi’s 2007 paper.
I showed that they could not be exactly equal. Ferenc Miskolczi himself has just joined the discussion and confirmed:
That is, there is not a theoretical basis for equating AA=ED as an identity.
There is a world of difference between demonstrating a thermodynamic identity and an approximate experimental relationship. In the latter situation, it is customary to make some assessment of how close the values are and the determining factors in the relationship.
But in reviewing the 2007 paper again I noticed something very interesting:
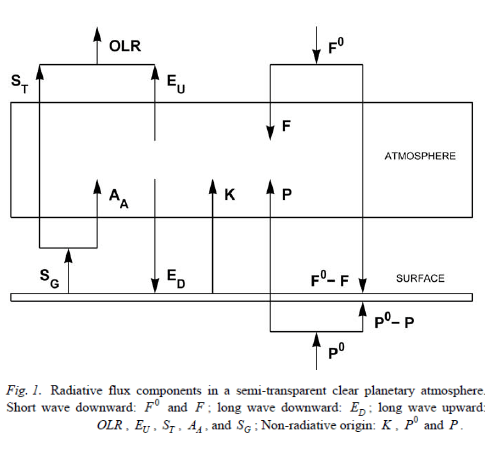
From Miskolczi (2007)
Figure 1
Now the point I made in Part Two was that AA ≠ ED because the atmosphere is a little bit cooler than the surface – at the average height of emission of the atmosphere. So we would expect ED to be a a little less than AA.
Please review the full explanation in Part Two to understand this point.
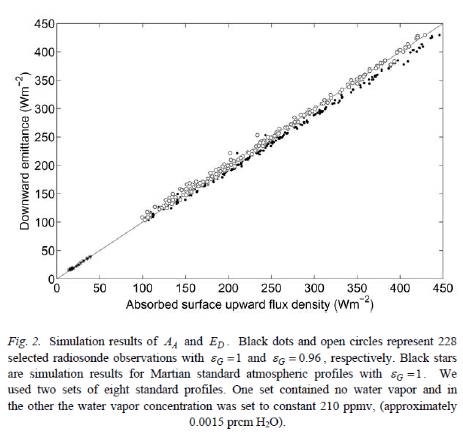
Now take a look at the graph above. The straight line drawn on is the relationship ED=AA.
The black circles are for an assumption that the surface emissivity, εG = 1. (This is reasonably close to the actual emissivity of the surface, which varies with surface type. The oceans, for example, have an emissivity around 0.96).
In these calculated results you can see that Downwards Emittance, ED is a little less than AA. In fact, it looks to be about 5% less on average. (And note that is ED = Absorbed Downwards Emittance)
Of course in practice, εG < 1. What happens then?
Well, in the graph above, with εG = 0.96 the points appear to lie very close to the line of ED=AA.
I think there is a calculation error in Miskolczi’s paper – and if this is true it is quite fundamental. Let me explain..
Here is the graphic for explaining Miskolczi’s terms:
From Miskolczi (2007)
Figure 2
When the surface is a blackbody (εG =1), SU = SG – that is, the upwards radiation from the surface = the emitted radiation from the ground.
The terms and equations in his 2007 are derived with reference to the surface emitting as a blackbody.
When εG < 1, some care is needed in rewriting the equations. It looks like this care has not been taken and the open circles in his Fig 2 (my figure 1) closely matching the ED=AA line are an artifact of incorrectly rewriting the equations when εG < 1.
That’s how it looks anyway.
Here is my graphic for the terms needed for this problem:
Figure 3
As much as possible I have reused Miskolczi’s terms. Because the surface is not a blackbody, the downward radiation emitted by the atmosphere is not completely absorbed. So I created the term EDA for the emission of radiation by the atmosphere. Then some of this, Er, is reflected and added to SG to create the total upward surface radiation, SU.
Note as well that the relationship emissivity = absorptivity is only true for the same wavelengths. See note 4 in Part Two.
Some Maths
Now for some necessary maths – it is very simple. All we are doing is balancing energy to calculate the two terms we need. (Updated note – some of the equations are approximations – the real equation for emission of radiation is a complex term needing all of the data, code and a powerful computer – but the approximate result should indicate that there is an issue in the paper that needs addressing – see comment).
And the objective is to get a formula for the ratio ED/AA – if ED=AA, this ratio = 1. And remember that in Figure 1, the relationship ED/AA=1 is shown as the straight line.
First, instead of having the term for atmospheric temperature, let’s replace it with:
TA = TS – ΔT [1]
where ΔT represents the idea of a small change in temperature.
Second, the emitted atmospheric downward radiation comes from the Stefan-Boltzmann law:
EDA = εAσ(TS – ΔT)4 [2]
Third, downward atmospheric radiation absorbed by the surface:
ED = εGEDA [3]
Fourth, the upward surface radiation is the emitted radiation plus the reflected atmospheric radiation. Emitted radiation is from the Stefan-Boltzmann law:
SU = εGσTS4 + (1-εG) EDA [4]
Fifth, the absorbed surface radiation is the upward surface radiation multiplied by the absorptivity of the atmosphere (= emissivity at similar temperatures):
AA = εASU [5]
So if we put [2] -> [3], we get:
ED = εGεAσ(TS – ΔT)4 [6]
And if we put [4] -> [5], we get:
AA = εGεAσTS4 + EDεA(1-εG)/εG [7]
We are almost there. Remember that we wanted to find the ratio ED/AA. Unfortunately, the AA term includes ED and we can’t eliminate it (unless I missed something).
So let’s create the ratio and see what happens. This is equation 6 divided by equation 7 and we can eliminate εA that appears in each term:
ED/AA = [ εGσ(TS – ΔT)4 ] / [ εGσTS4 + ED(1-εG)/εG ] [8]
And just to make it possibly a little clearer, we will divide top and bottom by εG and color code each part:
ED/AA = [ σ(TS – ΔT)4 ] / [ σTS4 + ED(1-εG)/εG2 ] [8a]
And so the ratio = blackbody radiation at the atmospheric temperature divided by
( blackbody surface radiation plus a factor of downward atmospheric radiation that increases as εG reduces )
We didn’t make a blackbody assumption, it is just that most of the emissivity terms canceled out.
What Does the Maths Mean?
Take a look at the green term – if εG = 1 this term is zero (1-1=0) and the equation simplifies down to:
ED/AA = (TS – ΔT)4 / TS4
Which is very simple. If ΔT = 0 then ED/AA = 1.
Let’s plot ED vs AA for a few different values of ΔT and for TS = 288K:
Figure 4
Compare this with figure 1 (Miskolczi’s fig 2).
Note: I could have just cited the ratios of ED/AA, which – in this graph – are constant for each value of ΔT.
And we can easily see that as ΔT →0, ED/AA →1. This is “obvious” from the maths for people more comfortable with equations.
That’s the simplest stuff out of the way. Now we want to see what happens when εG < 1. This is the interesting part, and when you see the graph, please note that the axes are not the same as figure 4. In figure 4, the graph is of ED vs AA, but now we will plot the ratio of ED/AA as other factors change.
Take a look back at equation 8a. To calculate the ratio we need a value of Ed, which we don’t have. So I use some typical values from Miskolczi – and it’s clear that the value of Ed chosen doesn’t affect the conclusion.
Figure 5
You can see that when εG = 1 the ratio is almost at 0.99. This is the slope of the top line (ΔT=1) in figure 4.
This is clear from equation 8a – as εG reduces below 1, the second term in the denominator of equation 8a increases from zero. As this increases, the ratio must reduce.
In Miskolczi’s graph, as εG changed from 1.0 → 0.96 the calculated ratio increased. I believe this is impossible.
Here is another version with a different value of ΔT:
Figure 6
Conclusion
Perhaps I made a mistake in the maths. It’s pretty simple – and out there in the open, so surely someone can quickly spot the mistake.
Of course I wouldn’t have published the article if I thought it had a mistake..
On conceptual grounds we can see that as the emissivity of the surface reduces, it absorbs less energy from the atmosphere and reflects more radiation back to the atmosphere.
This must reduce the value of ED and increase the value of AA. This reduces the ratio ED/AA.
In Miskolczi’s 2007 paper he shows that as emissivity is reduced from a blackbody to a more realistic value for the surface, the ratio goes in the other direction.
If my equations are correct then the equations of energy balance (for his paper) cannot have been correctly written for the case εG <1.
This one should be simple to clear up.
Update May 31st – Ken Gregory, a Miskolczi supporter appears to agree – and calculates ED/AA=0.94 for a real world surface emissivity.
Other articles in the series
The Mystery of Tau – Miskolczi – introduction to some of the issues around the calculation of optical thickness of the atmosphere, by Miskolczi, from his 2010 paper in E&E
Part Two – Kirchhoff – why Kirchhoff’s law is wrongly invoked, as the author himself later acknowledged, from his 2007 paper
Part Three – Kinetic Energy – why kinetic energy cannot be equated with flux (radiation in W/m²), and how equation 7 is invented out of thin air (with interesting author comment)
Part Five – Equation Soufflé – explaining why the “theory” in the 2007 paper is a complete dog’s breakfast
Part Six – Minor GHG’s – a less important aspect, but demonstrating the change in optical thickness due to the neglected gases N2O, CH4, CFC11 and CFC12.
Posted in Basic Science, Commentary | 293 Comments »