One commenter asked about CO2 absorption in the solar spectrum.
If CO2 absorbs incoming solar radiation then surely an increase in CO2 will reduce incoming radiation and balance any increase in longwave radiation.
The important factor is the usual question of quantifying the different effects.
Let’s take a look.
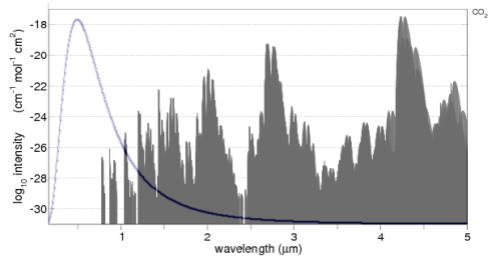
The CO2 absorption spectrum is from the line list browser of the recommended spectralcalc.com. The line list only goes down to 0.17μm (170nm), hence the reason for the graph not starting at 0.0μm.
The solar radiation is overlaid. Well, more accurately, the Planck function for 5780K is overlaid (simply drawn using Excel). Note that the CO2 absorption spectrum is on a log graph, while the radiation is on a linear graph. For those not so familiar with logarithmic graphs, the peak absorption around 4.3μm is 10-18, while the two peak absorptions just below 1μm are at 10-26 – which is 100,000,000 less.
The value of seeing the solar radiation spectrum overlaid is it enables you to see the relative importance of each absorption area of CO2. For example, the solar radiation between 2 – 4μm is only 5% of the solar radiation, so any absorption by CO2 will be quite limited.
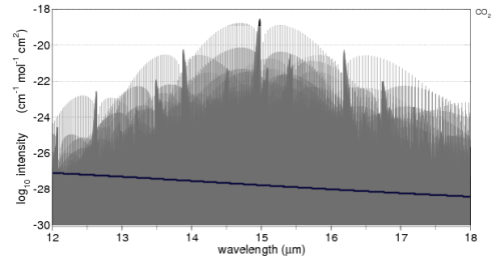
Here’s the comparison with the important 15μm band of CO2. A 6μm width is shown, overlaid (blue line) with the 12-18um longwave radiation of a 288K (15°C) blackbody:
Just a little explanation of this graph and how to compare it to the solar version.
The average surface temperature of the earth is 15ºC, and it emits radiation very close to blackbody radiation (watch out for a dull post on Emissivity soon).
The proportion of radiation of a 288K blackbody between 12-18μm is 28%. What we want to do is enable a comparison between the CO2 absorption of solar radiation and terrestrial radiation.
Averaged across the globe and the year the incoming solar radiation at the top of atmosphere (TOA) is 239 W/m2 and the radiation from the earth’s surface is 396 W/m2. This works out to 65% higher, but so as not to upset people who don’t quite believe the earth’s surface radiation is higher than incoming solar radiation I simply assumed they were equal and scaled this section of the earth’s terrestrial radiation to about 28% of the solar radiation on the earlier graph. We are only eyeballing the two graphs anyway.
So with this information digested, the way to compare the two graphs is to think about the absorption spectra of CO2 simply being scaled by the amount of radiation shown overlaid in both cases.
As you can see the amount of absorption by CO2 of solar radiation is a lot less than the absorption of longwave radiation. Remember that we are looking at the log plot of absorption.
Is That the Complete Story?
Really, it’s more complicated, as always with atmospheric physics. There’s nothing wrong with taking a look at the approximate difference between the two absorption spectra, but luckily someone’s already done some heavy lifting with the complete solution to the radiative transfer equations using line by line calculations. For more on these equations, see the CO2 – An Insignificant Trace Gas series, especially Part Three, Four and Five.
The paper with the heavy lifting is Radiative forcing by well-mixed greenhouse gases: Estimates from climate models in the IPCC AR4 by W.D. Collins (2006). There’s a lot in this paper and aspects of it will show up in the long awaited Part Eight of the CO2 series and also in Models, On – and Off, the Catwalk.
Solving these equations is important because we can look at the absorption spectrum of CO2 in the 15μm band, but then we have to think about the absorption already taking place and what change in absorption we can expect from more CO2. Likewise for the solar spectrum.
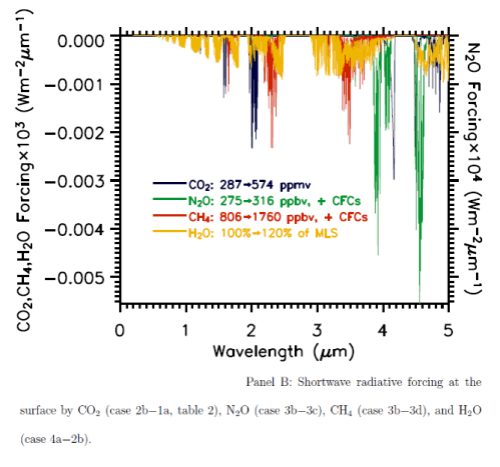
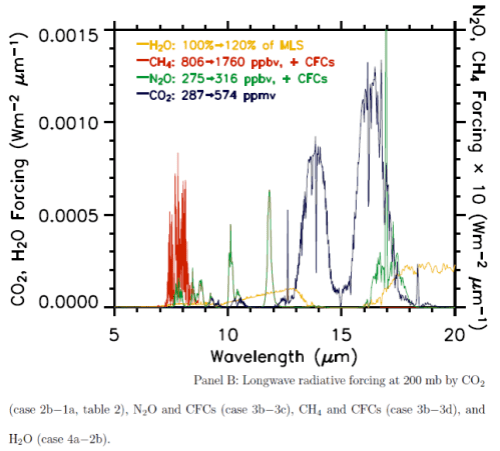
Here are the two graphs, which include other important trace gases, as well as the impact of a change in water vapor. Note the difference in vertical axis values – the forcing effect of these gases on solar radiation has to be multiplied by a factor of 1000 to show up on the graph. The blue lines are CO2.
You can also see that the CO2 absorption in shortwave is across quite narrow bands (as well as being scaled a lot lower than terrestrial radiation) – therefore the total energy is less again. The vertical scale is energy per μm..
From these calculations we can see that with a doubling of CO2 there will be a very small impact on the radiation received at the surface, but a comparatively huge increase in longwave radiation retained – “radiative forcing” at the tropopause (the top of the troposphere at 200mbar).
So Is That the Complete Story?
Not quite. If trace gases in the atmosphere absorb solar radiation, is that so different from the surface absorbing solar radiation?
Or to put it another way, if the radiation doesn’t strike the ground, where does it go? It’s still absorbed into the climate system, but in a different location (somewhere in the atmosphere).
But as one commenter said:
The other point [this one] you make is simply not true and/or also not proven. There is only so much energy that can be taken up by a molecule.
This is a theme that has arrived in various comments from various posts. So the concept of How much work can one molecule do? is worth exploring in a separate post.
Hopefully, it’s clear from what is presented here that increases in CO2 absorption of the solar radiation are very small compared with absorption of longwave radiation.


Science of doom, you say:
“For example, the solar radiation between 2 – 4μm is only 5% of the solar radiation, so any absorption by CO2 will be quite limited”.
According to various sources that I consulted the amount of energy coming onto earth is 46-47% from the infra red region of the sun. So this presentation is flawed from the beginning.
For example, when I stand here in the African sun, in the summer, I can only allow my skin a few minutes exposure, then I have to look for cover. The radiation on my skin gets too hot. But, if during the same day, the humidity rises, that same direct heat from the skin becomes less. You can feel this. This is the reason why the temperature on the coast (where humidity is higher) is always a few degrees less than more inland. So carbon dioxide, like water vapor does exactly the same thing….. it cools the atmosphere. My question was and is: by how much?
There is also no mention in your graphs of all the absorptions of CO2 including those in the UV region which have only been discovered lately.
You also say : “We are only eyeballing the two graphs anyway”.
When there is so much at stake here.?
You cannot do that.You have to come with an experiment and with actual test results. in W/m3 (0.04%-0.06% CO2)/m2/24 hours cooling and warming. We know that Svante Arhenius formula was wrong, so where is the right formula? Don’t come with stories that really is only a believe system. We have to have formulas like Newton made, real science that everyone can test and rely on.
No eyeballing.
Because the results that I want, proposing the correct formula, simply donot exist, there is really little point in continueing this debate.
scienceofodoom wrote
Without looking up the whole derivation this looks a bit low. The peak radiation figure at ground level on a perpendicular surface is to the sun – to my vague recollection – of the order of 1400-1500 watts/sqm. This is after some filtering by the atmosphere but discounting clouds which can both decrease incident ground radiation and also increase it above the base figure.
The earth disk is pi r^2 and the earth surface area is 4 pi r^2 so the average insolation should be (say) 1450 / 4 = 362 W/sqm at ground level and higher at TOA.
– actually I have just seen another figure of 1396 W/sqm (seems low to my recollection) so giving a TOA of 349 W/sqm.
Update to my post – I had a typo in the 1396 W/sqm – should be 1369 W/sqm – resulting in 342 W/sqm – this is using the solar constant and earth orbit geometry and applies at TOA.
My slightly higher figure – from recollection – may include back radiation from atmosphere (?) The figure was what I used as a base for my terrestrial measurements and was approx 1440 W/sqm.
Yes, please explain the 239 as many sources give a radiation power density of 1,370 W/m2 or so.
I’m really enjoying the journey and the guide and I’m excited about some of the foreshadowed adventures. 🙂
henry Pool
On the comment that “..2 – 4μm is only 5% of the solar radiation..”
Perhaps we could say, there is a discrepancy between your presentation and this presentation. So the question becomes Which one is flawed?.
In fact what is “infra red”?
Infra-red is >700nm and approximately half of the radiation from the sun is in the infra-red. But from 2-4um there is only 5% of the energy.
You can see this in any graph of solar energy. And it quite closely matches the Planck function of 5780K which is shown in the graph. You can do the calculation yourself, just take the Planck blackbody formula and plot it on a graph and measure the area under different parts of the curve.
The Hitrans database is the most comprehensive database of absorption, this is what spectralcalc draws on.
In the UV, almost no solar radiation makes it to the surface anyway, so CO2 – or any other trace gas – absorption in the UV area is irrelevant, it’s all absorbed by O3 and O2 in the stratosphere. Increasing CO2 means an change from around 0% to ?
Really not sure what you are saying here. You like Newton’s formulae?
You don’t like the Beer-Lambert law of absorption?
You don’t like the 1st law of thermodynamics?
You don’t like Kirchoff’s law of emissivity = absorptivity?
Why trust Newton?
For Jerry and Dave McK:
See The Earth’s Energy Budget – Part One for explanation of the energy budget basics.
TSI, total solar irradiance at top of atmosphere = 1367 W/m^2
Averaged over the surface area of the earth = TSI/4 = 342 W/m^2
Taking into account the earth’s albedo (reflected radiation) = 239 W/m^2
In fact, many of these numbers cannot yet be known accurately enough. Perhaps in The Earth’s Energy Budget – Part Four this might be explained further.. but even though we put in numbers like 1367W/m^2 there is some +/- here.
Likewise with the albedo. Anyway, the important thing is to understand the relationship between the numbers above, check out the original post..
Scienceofdoom.
Just to be picky, your statement was actually
Which is somewhat misleading without reading all your posts and filling in the missing bits.
Also, does the earth radiation include the albedo backscatter or is it addition to the backscatter radiation?
I’ve also read a variety of discussions about this topic, some of which state that the long-wave IR incoming is a significant fraction of long-wave IR going out – perhaps even equal?
henry Pool:
Well, the reason I said “eyeball the two graphs” is that the solar absorption of CO2 is so obviously less than the absorption of terrestrial radiation in the 15um band. If they looked slightly close we might have to try some maths.
But from your other comments, physicists post-Newton don’t get a look in, so evaluating the absorption via the Planck function (radiation from a black body that you question) using the Beer-Lambert law of absorption (see CO2 – An Insignificant Trace Gas? Part Three ) would be a wasted effort?
What experiment can we do to satisfy you? We can measure the radiation from the sun and the zero UV that we already measure at the earth’s surface due to absorption by ozone and oxygen on the way in shows that any trace gas absorption in the UV has no extra effect. Because it’s already almost zero:
Jerry:
It’s good to be picky. Picky means getting to the bottom of things.
Well, atmospheric physics is a big topic, not easily explained in a few words.. Not aimed at being misleading, sometimes I have to state the facts and refer people to other posts to avoid very long (20 page) explanations..
The earth’s radiation is completely and totally the energy radiated from the surface of the earth due to its temperature. So a 15’C surface radiates 390W/m^2. And the energy vs wavelength follows the well-known Planck function.
Any solar radiation reflected is in addition. Luckily, the solar radiation can be easily discriminated from terrestrial radiation due to the aforementioned Planck formula.
I have found many people stating: “50% of the solar radiation is infrared therefore we can’t tell which is terrestrial and which is solar..”
“Infrared” = >700nm. And so the comment is irrelevant.
“Longwave” as the shorthand climate scientists use is greater than 4um and terrestrial. “Shortwave” as the shorthand climate scientists use is less than 4um and solar.
The overlap is less than 1%. The “infra-red” is a red herring
Can you explain the last question? I don’t understand it.
Oh. I had to think what that really means.
It’s averaged over day and night side and the poles that get but a fraction so it’s the ‘whole ball of wax’ average, correct?
So at noon on the equator you’re really getting close to your 1300W/m2 hitting the ground, right?
I have to pay close attention.
Dave McK:
It’s the whole ‘ball of wax’ average.
If you stand at the equator at noon under a clear sky you get something close to the 1367W/m^2 so long as no aerosols and no surface reflecting any radiation.
If you are out on the water and it’s calm and there are minimal aerosols then you will get something like 1300 W/m^2.
Check out the average albedos in Positive Feedback, Albedo and The End of All Things
We all have to pay close attention. Atmospheric physics is a journey, not a destination..
I will read it now. Your course is advanced for me. I’m off the dean’s list due to this one but it’s very, very interesting.
Thank you for answering my questions.
Done with my assignment. I only comment to show if I got the main point.
There are other positive feedback systems, too. A grass or forest fire is an example. A storm is a couple at once. Fission is the classic.
All of these things have a terminal condition that’s arrived at through larger equilibria – burning up all the fuel, for example.
There never is a problem with local reversal of entropy.
Life is that.
Trivia- if you look at a satellite view of Africa (at night) during dry season- at any time there are fires all across the savannah. That’s a 5 million sq mi swath right across the continent where the equatorial red stain of heat always is shown.
I suppose it practically must have been geothermal or cometary heat to reverse an ice age once it was established, eh? A nice swarm of ice comets at 50kps would boil some atmosphere. Dirty ones would be even better if they sooted up the poles.
p.s.
When you do the topic of ‘what can one molecule do’, please include cellulose.
The CO2 absorption of incoming, has, for me, never been an issue for doubting the claims that CO2 is going to trigger a climate catastrophe.
To henry Pool:
You wanted an experiment, right?….. Refer to the Figure Science of Doom posted in these comments. Look at the spectrum of sunlight that arrives at sea level (through the zenith) for a “standard” atmosphere. We have the means to model this observed spectrum of sunlight.
1) We use our best spectral models for atmospheric opacity of the Earth’s atmosphere. At minimum these models include the molecules present as a function of height. It can also include such and such aerosols, water droplet column density, what have you, although for these specific purposes clouds are omitted. These computations produce cross section for absorption or scattering (units: m^2) vs. wavelength of light (or wave number), for a specific set of conditions (temperature, molecular density). These models are quite sophisticated feats of quantum mechanical mastery (from experimental measurements and theoretical quantum predictions), and include, for example, the effects of temperature and molecular density that affect the widths and peaks of the molecular cross sections.
2) At every wavelength interval we integrate the cross section by the run of absorber number density with height. To give you a more intuitive feel for whats’ being done, this quantity is approximately the product of the cross section (m^2) times the column density of the atmosphere overhead (integrated number of absorbers and scatterers per m^2 vertical column). The resulting dimensionless quantity is known as the optical depth, labeled as the Greek letter “tau”, as a function of wavelength.
3) Take the Sun’s light spectrum incident at the top of atmosphere and multiply it by e^(-tau), wavelength by wavelength. The result is a predicted detailed spectrum of (zenith) sunlight that reaches sea level.
Compare prediction to observation. Match up? Yes, almost in entirety, with some isolated mismatches (molecular physicists go back to their laboratories and to their molecular model computations). Done.
Note that the above ratio, I/I_o = e^(-tau), does not include scattered light or molecular emission of the atmosphere. That’s another, more elaborate, calculation, of radiative transfer.
So my question was: what is the new formula for warming caused by CO2? We know that Svante’s Arrhenius formula was incorrect, IF IT WERE CORRECT EARTH SHOULD HAVE BEEN A LOT WARMER. So, I am looking for the test results from the experiments that evaluate the warming and cooling properties.
I could have said that the warming of CO2 must be insignificant because water vapor also absorbs in the 214-15 um region. But I chose not to confuse issues.
If you did not understand the arguments I will give it again:
Here is the famous paper that confirms to me that CO2 is cooling the atmosphere by re-radiating sunshine (12 hours per day).
http://www.iop.org/EJ/article/0004-637X/644/1/551/64090.web.pdf?request-id=76e1a830-4451-4c80-aa58-4728c1d646ec
Note that they measured this radiation as it bounced back to earth from the moon. Follow the green line in fig. 6, bottom . Note that it already starts at 1.2 um, then one peak at 1.4 um, then various peaks at 1.6 um and 3 big peaks at 2 um.
This paper here shows that there is absorption of CO2 at between 0.21 and 0.19 um (close to 202 nm) (UV):
Click to access DUV-CO2.pdf
There are other papers that I can look for again that will show that there are also absorptions of CO2 at between 0.18 and 0.135 um and between 0.125 and 0.12 um.
We already know from normal IR that CO2 has big absorption between 4 and 5 um.
So, to sum it up, we know that CO2 has absorption in the 14-15 um range causing some warming (by re-radiating earthshine, 24 hours per day) but as shown and proved above it also has a number of absorptions in the 0-5 um range causing cooling (by re-radiating sunshine). This cooling happens at all levels where the sunshine hits on the carbon dioxide same as the earthshine. The way from the bottom to the top is the same as from top to the bottom. So, my question is: how much cooling and how much warming is caused by the CO2? How was the experiment done to determine this and where are the test results? If it has not been done, why don’t we just sue the oil companies to do this research? (I am afraid that simple heat retention testing will not work here, we have to use real sunshine and real earthshine to determine the effect in W/m3 [0.04%]CO2/m2/24hours)
I am going to state it here quite categorically that if no one has got these results then how do we know for sure that CO2 is a greenhouse gas?, i.e that the net effect of CO2 is warming rather than cooling?
blah, blah,blah, no test results, no formula’s
The reflection of the solar radiation reflected from the earth and back from the moon is a very interesting idea.
But we can actually just measure it at the earth’s surface before it bounces off. It makes the measurements more accurate and yet more interesting.
It’s not new information that the solar spectrum is absorbed by many trace gases – they are in the Hitrans database shown above. And you can see them in the solar radiation received at the earth’s surface I showed in the graphic in an earlier comment
Here is the CO2 capture cross-section (from the same location) between 1.0 and 1.5um, shown on a linear axis to make it clearer the relative importance:
and from 1.0 to 2.0, again on a linear axis:
Repeating this:
Shows that you haven’t absorbed any of the information above. The proportion of solar radiation greater than 4um is less than 1%. If CO2 absorbs everything between 4-5um it has a maximum 1% effect on solar radiation received.
Take a look at the CO2 – An Insignificant Trace Gas series. You’ll be pleased to find not a single mention of Arrhenius, but you will see the formulae that govern the absorption and re-radiation by any gases.
And as Spaceman Spiff explained we have an experiment already – we can measure exactly how much radiation is absorbed (not lost) by the atmosphere by every single gas – we just measure the solar radiation at the surface.
Unfortunately, I don’t think you want “test results” and “formula’s” because when Spaceman Spiff explained them you dismissed them as “no test results, no formula’s”.
The Beer-Lambert law of absorption you can find in Part Three of the CO2 series.
Spaceman Spiff
I asked a while ago on a different thread about the why of the varying visual/optical depth of the atmosphere dependent on atmospheric pressure… and i think you have in a round about way answered it… thanks 😉
Im still unsure whether its caused by absorption or scattering of visible light… (its just the ole weather prediction method o using distant geographical objects appearance for the incoming weather…during a high pressure they appear more distant, with noticeably less visible detail… )
scienceofdoom
Another random Q that this thread got me thinking about from reading spacemans post…
Are the short wave/sun light reaching surface calculated from the zenith always? I ask just because this would seem counter intuitive if there is scattering of short wave through atmospheric conditions… because it would be relativly constant at the zenith irrespective of variable scattering, but would be more noticeable at greater angles to the surface? Early or later in the day. It just seems like spaceman was saying that its calculated always from zenith…
Im just a farmer end o the day, but sunlight is variable even under clear skies(according to my skin anyways 😉 )… or are averages used from varying times o day to measure short wave reaching the surface.
It seems too me that satellite measurements of global brightness/dimming would be better in general for covering yer bases as far as sunlight measurements go.
Mike Ewing:
For a 1-d analysis of radiation and absorption the conditions are usually stated. So, for example, one paper I was just reading had the sun at 60% from the zenith for its analysis.
Also parameters like latitude and time of year will be stated.
The GCMs, of course, take into account the angle of solar radiation hitting each part of the earth, with the consequent scattering and absorption effect of aerosols and clouds – and the albedo effect on the ground.
Some of these factors are well understood – like the dependence of the albedo of the ocean on the angle of solar radiation.
Others are poorly understood, like aerosols and clouds. The great Ramanathan describes clouds as the “gordian knot” of climate prediction.
Henry@all there
try looking at a few good graphs that show the incoming solar radiation and the outgoing earth radiation simultaneously on the same scale..
You would note the difference between what is measured on top of the atmosphere and at sealevel.
Almost 30% of incoming radiation is re-radiated to out of space because of the combined efforts of oxygen/ozone, water vapor and carbon dioxide – (on a sunny day, – we exclude clouds and cloudformation -)
Now if you look carefully at the outgoing radiation (from earth) you would have noticed that only a tiny little corner of earth’s radiation at between 14-15 is cut off due to the presence of CO2. This is because water vapor also absorbs strongly in that region.
So if I “eyeball” these graphs, then it looks to me that the carbon dioxde cuts down less than 1% of earth radiation and if I look at how much it cools it looks a lot more than 1% of sun’s radiation. So I say that the net effect of CO2 is probably cooling rather than warming. At worst, I would say that it is probably evens or close to evens between the warming and cooling.
It is you who does not understand or do not want to understand…. You have to find empirical proof that would show us that the one is greater than the other. In units like W/m2/M3 (CO2 -0.03-0.06)/24 hours. So, if you have those results, why not just give it to me? How much cooling and how much warming is caused by the CO2 (in exact values and at what concentrations) ?
& how was the testing done?
What is wrong with this statement?
“Man-made CO2 doesn’t appear physically capable of absorbing much more than
two-thousandths of the radiated heat (IR) passing upward through the atmosphere.”
Made by this man:
http://www.middlebury.net/op-ed/global-warming-01.html#bio
In this paper:
http://www.middlebury.net/op-ed/global-warming-01.html
Mike Ewing (re. absorption line profile dependence on T and P):
At the risk of boring you and the other readers to death, here is a brief tutorial:
To a very good approximation the shape of the cross sectional profile of a transition (in units of m^2) as a function of the difference in wavelength measured from the central transition wavelength lambda(0) (call this difference “dl”), can be described as a “Voigt” function ( see, e.g., http://en.wikipedia.org/wiki/Voigt_profile ). This function is the result of convolving the collision/pressure-broadened natural line profile with a Gaussian function representing the Doppler shift distribution of the gas molecules in a thermal bath: cross-section = G*(f/D) *exp{- (dl/D)^2}). G is a constant, f is a measure of the quantum mechanical probability of the transition in question, D is a characteristic width of the function called the Doppler width D = (lambda(0)/c) * sqrt{2kT/m} and is a measure of the most probable Doppler shift in wavelength units, with lambda(0) = central wavelength of the transition, m = mass of molecule and k is Boltzmann’s constant (J/K). Smaller masses and larger temperatures result in larger characteristic Doppler shifts D.
The natural (i.e., always present) line profile of the cross section of the line transition ultimately derives from the Heisenberg Uncertainty Principle regarding uncertainties in the energies of the particular quantum states involved in the transition and the (expectation value) lifetimes of said states. Interactions between the molecules (e.g, CO2 — CO2, or CO2 — other) further increase the uncertainty in the energy of a transition between quantum states and/or makes accessible other nearby quantum transitions. The shape of this natural line cross-section vs. separation from the transition’s central wavelength (dl) is approximately Lorentzian: cross section = L*(f/w) * w^2/(w^2 + dl^2), where L is a constant, f is a measure of the quantum mechanical transition probability, w is the characteristic width of the function, here set by the inverse quantum mechanical lifetimes of the states for the transition and the rate/type of interactions with other molecules. Greater pressures/gas densities result in larger values of w.
Left to themselves and random collisions, gas molecules set up a distribution function in kinetic energies (Maxwellian in the classical limit). The quantity sqrt{2kT/m} is the peak speed in this distribution function, and the mean (translational) kinetic energy per particle is (3/2)kT.
Temperature affects the distribution of quantum levels occupied by the molecules — and therefore determining what transitions are energetically probable. It also affects the degree of Doppler broadening (molecules of a given mass move more rapidly at higher T) and therefore the cross section function. Temperature varies with height in the atmosphere.
The atmospheric pressure and the number density of each constituent of molecules as a function of height affect the absorption line’s cross section values for dl > 3D (recall D is the characteristic Doppler width), and at essentially all wavelengths if the temperature is sufficiently low and/or the pressure is sufficiently high (thus Doppler broadening rendered unimportant).
Figures 9-1 through 10-5 at this book’s website http://www.sundogpublishing.com/AtmosRad/resources/Figs/index.html will be helpful, although you’d benefit further by purchasing the book (I am not the author, but it is a wonderful text accessible to advanced undergraduate students of the physical sciences).
I hope this was of some help.
scienceofdoom,
How does the most ephemeral of variables- clouds- become so important to climate prediction?
The time scales involved seem completely exclusive.
Henry@Spaceman Spiff
“I hope this was of some help”.
Answer: No.
Henry@ Hunter.
It took me 3 months to figure this out, mostly because people got stuck into a type of conditioned thinking. You have to become a non lateral thinker. this is my final report on global warming
FOR MY CHILDREN, & FAMILY AND FRIENDS LIVING IN THE NORTHERN HEMISPHERE
You may not know this. For a hobby I did an investigation to determine whether or not your carbon footprint, i.e. carbon dioxide (CO2), is really to blame for global warming, as claimed by the UN, IPCC and many media networks. I guess I felt a bit guilty after watching “An inconvenient truth” by Al Gore, so I had to make sure for myself about the science of it all. If you scroll down to my earlier e-mails you will note that I determined that, as a chemist, I could not find any convincing evidence from tests proving to me that CO2 is indeed a major cause for global warming. As my investigations continued, I have now come to a point where I doubt that global warming is at all possible…. Namely, common sense tells me that as the sun heats the water of the oceans and the temperatures rise, there must be some sort of a mechanism that switches the water-cooling system of earth on, if it gets too hot. Follow my thinking on these easy steps:
1) the higher the temp. of the oceans, the more water vapor rises to the atmosphere,
2) the more water vapor rises from the oceans, the more difference in air pressure, the more wind starts blowing
3) the more wind & warmth, the more evaporation of water (evaporation increasing by many times due to the wind factor),
4) the more evaporation of water the more humidity in the air (atmosphere)
5) the higher the humidity in the air the more clouds can be formed
6) Svensmark’s theory: the more galactic cosmic rays (GCR), the more clouds are formed (if the humidity is available)
7) the more clouds appear, the more rain and snow and cooler weather,
8) the more clouds and overcast conditions, the more radiation from the sun is deflected from the earth,
9) The more radiation is deflected from earth, the cooler it gets.
10) This cooling puts a brake on the amount water vapor being produced. So now it is back to 1) and waiting for heat to start same cycle again…
Now when I first considered this, I stood in amazement again. I remember thinking of the words in Isaiah 40:12-26.
I have been in many factories that have big (water) cooling plants, but I realised that earth itself is a water cooling plant on a scale that you just cannot imagine. I also thought that my idea of seeing earth as a giant (water) cooling plant with a built-in thermostat must be pretty original….
But it was only soon after that I stumbled on a paper from someone on WUWT who had already been there, done that …. well, God bless him for that!
i.e. if you want to prove a point, you always do need at least two witnesses!
Look here (if you have the time):
But note my step 6. The Svensmark theory holds that galactic cosmic rays (GCR) initiate cloud formation. I have not seen this, but apparently this has been proven in laboratory conditions. So the only real variability in global temperature is most likely to be caused by the amount of GCR reaching earth. In turn, this depends on the activity of the sun, i.e. the extent of the solar magnetic field exerted by the sun on the planetary system. We are now coming out of a period where this field was bigger and more GCR was bent away from earth (this is what we, skeptics, say really caused “global warming”, mostly).
But apparently now the solar geomagnetic field is heading for an all time low.
Look here:
Note that in the first graph, if you look at the smoothed monthly values, there was a tipping point in 2003 (light blue line). I cannot ignore the significance of this. I noted similar tipping points elsewhere round about that same time, (e.g. in earth’s albedo, going up). From 2003 the solar magnetic field has been going down. To me it seems for sure that we are now heading for a period of more cloudiness and hence a period of global cooling. If you look at the 3rd graph, it is likely that there wil be no sun spots visible by 2015. This is confirmed by the paper on global cooling by Easterbrook:
In the 2nd graph of his presentation, Easterbrook projects global cooling into the future. These are the three lines that follow from the last warm period. If the cooling follows the top line we don’t have much to worry about and the weather will be similar to what we had in the previous (warm) period. However, indications are already that we have started following the trend of the 2nd line, i.e. cooling based on the 1880-1915 cooling. In that case it will be the coldest from 2015 to 2020 and the climate will be comparable to what it was in the fifties and sixties. I survived that time, so I guess we all will be fine, if this is the right trendline.
Note that with the third line, the projection stops somewhere after 2020. So if things go that way, we don’t know where it will end. Unfortunately, earth does not have a heater with a thermostat that switches on if it gets too cold. Too much ice and snow causes more sunlight to be reflected from earth. Hence, the trap is set. This is known as the ice age trap. This is why the natural state of earth is that of being covered with snow and ice. This paper was a real eye opener for me:
However, man is resourceful and may find ways around this problem if we do start falling into a little ice age again. As long as we are not ignorant and listen to the [—–moderator’s snip—-]
Obviously: As Easterbrook notes, global cooling is much more disastrous for humans than global warming.
Note that in Easterbrook’s projection graph, the line showing the increase and decrease in global temperatures of the northern latitude is dashed. It looks like the northern hemisphere is always getting the brunt of the extreme weather.
So if you get tired of all that ice and snow, you may know that you are always most welcome to come and stay with us here, in the southern hemisphere!
Blessings from your child, brother, dad, friend,
date: 24 Jan. 2010
hunter:
Rather than say “doesn’t appear physically capable”?
He should say “without any experiments and theory I was able to deduce (guess?) that CO2 has very little effect”
There’s no mention of any atmospheric physics in his “treatise”:
– absorption equation integrated across all wavelengths
– re-emission of energy by these trace gases throughout the atmosphere
– the integration of these equations vertically through the atmosphere
I.e, the radiative transfer equations, well-proven by the way. He didn’t disprove them, just ignored them.
Skeptical people might say he isn’t even aware of them..
Or evidence:
– how can the top of atmosphere OLR only be around 240 W/m^2 when the radiated energy from the ground is around 396 W/m^2?
– how can the downward longwave radiation in the CO2 band be so high (see CO2- Part Six, Visualization )
Then later:
Won’t? Can’t?
Perhaps the writer is unaware that N2 has almost zero absorption. And the O2 only “absorbs” incoming UV solar radiation by breaking apart into atomic oxygen – then forming ozone, etc. But absorbs almost no longwave radiation.
People can write any old stuff, it’s fascinating.
But hunter, surely you didn’t think it was good? Did I do such a bad job on the CO2 series ?
Hunter,
This webpage provides a (bit dated) overview of the effects of clouds on climate:
http://www-das.uwyo.edu/~geerts/cwx/notes/chap09/rossow.html
NASA has articles here:
http://earthobservatory.nasa.gov/Features/Clouds/
If you need more meat with that:
http://isccp.giss.nasa.gov/climanal.html and
http://isccp.giss.nasa.gov/role.html and
http://ams.allenpress.com/perlserv/?request=get-abstract&issn=1520-0477&volume=80&page=2261
In a nutshell:
low, thick clouds cool in net
high, thin clouds warm in net
Also: indirect effects of aerosol injection, by providing more potential condensation nuclei for cloud droplets to form. Net effect: in the lower troposphere the SW cloud albedo increases.
hunter:
Because it doesn’t matter how long one cloud stays in the atmosphere. What matters is how much cloud.
Generally clouds provide a cooling effect of about -18W/m^2. Compare with CO2 radiative forcing under a doubling of CO2 of around 4W/m^2.
What effect does warming have on clouds? What are the feedbacks?
I think Roy Spencer sees clouds as the major influencer on the last 100 years of temperature (don’t quote me on that last point).
Thanks spaceman and science o doom..
Henry, i myself am skeptical o many many things, i have to be able to test em or observe em myself before i buy into much o anything(especially if the person telling me stands to gain outta my belief)
But in regards yah hypothesis up there… i know the hydrological cycles are as science o doom puts it the gordian knot o climate prediction(and unlike Alexander, yah cant just hack it with a sword) So more co2= heat=water vapor= heat = wind +water vapor=cloud(what kind? rain? high low cloud? etc) So sure, uncertainty, but where are you getting certainty in the result?
But ok, ive spent a bit o time kicking around the jungles in the tropics in my younger days, and those massive thunder heads undoubtedly cause cooling… but the mercury can still hit 50C before they do… Or as cool as mid 30s. And sure it can happen here at the mid latitudes, although less frequently… But surly it crossed your mind that adding a persistent climate forcing “could” expand the climate bands? Essentially growing the tropics, and sub tropics etc. And still be inline with that reasoning….
I personally find paleoclimatolgy fascinating, and what we know from that is the globe can certainly be warmer than it is today, i dont think co2 is the only factor determining climate(continental drift certainly shows a correlation with hot house/ ice house phases) But climate can/has and does change.. pretty much constantly.
henry Pool:
You have the mistaken impression that WattsUpWithThat is a repository of scientific knowledge. If you’re truly interested in understanding the behavior of the natural world (just about everything outside of the “social world”), then you will (a) stop repeating the above mistake, (b) make a serious effort to understand for yourself what science currently understands by reading, well, the science.
Radiation transfer is a multidisciplinary science, understood and applied successfully over the past century by:
-terrestrial climate, atmospheric physicists
-Astrophysicists (stellar atmospheres, stellar interiors, stellar winds, interstellar medium absorption, intergalactic absorption, Solar System Planetary atmospheres, exo-planet atmospheres, accretion disks,…. the list is nearly endless)
-laser physicists
-X-ray imaging scientists
-the list is very, very long…
Does it really seem all that likely to you that you (or perhaps Mr. Watts?) actually understands something so foundationally fundamental to matter-light interactions that 10s of thousands of scientists in multiple sciences, in multiple subfields within a scientific field, through ~100s of thousands of publications over the past century somehow did not and do not?
One would think that out of zillions of spectral observations of nature over the past century, nature would have told us a few times (at least) how very, very mistaken we are.
That said, one should not mistake science’s not knowing everything for knowing next to nothing.
scienceofdoom,
Actually you did pretty well.
I am poking around this with sharp sticks, so to speak, and like to see where extraordinary claims get us.
The main take away I have in this is that CO2 is very limited in its over all impact on the energy budget.
Your answer irt clouds is what I thought it would be.
Thanks,
Henry Pool –
Peek at the last page if you have to know the ending – but don’t spoil it for anyone who is enjoying the process of getting there…lol
I don’t want to disrupt a smooth narrative and I appreciate the fine scholarship and here comes the disjuncture:
two statements seem rough:
” how can the top of atmosphere OLR only be around 240 W/m^2 when the radiated energy from the ground is around 396 W/m^2?” (duration not specified)
“Because it doesn’t matter how long one cloud stays in the atmosphere. What matters is how much cloud.” (Hard to characterise a knot by one thread)
I trust you to resolve everything as the plot unfolds. That confidence means I stay on my hinges better and don’t jump from my seat and burst out…lol – the carpet doesn’t get so much of my coffee; I get more. The matrimonial models have a negative sign on the consequences of the HostileSpouseCleaning oscillation to account for a GloriousMeanTemper. One forgets at one’s peril. Calm is good.
Dave McK:
Because not all of it makes it to the top.
Water vapor, H2O, CH4, N2O and various other little trace gases absorb radiation and warm up, and so warm up the atmosphere around them and then – any gases that are able, radiate energy in all directions.
Some of this “all directions” is down.
See:
CO2 – An Insignificant Trace Gas? Part Six – Visualization
CO2 Can’t have that Effect Because..
The Earth’s Energy Budget – Part Two
On clouds I think I was answering another question. “How could they be so important if so ephemeral?”
scienceofdoom said:
“Generally clouds provide a cooling effect of about -18W/m^2. Compare with CO2 radiative forcing under a doubling of CO2 of around 4W/m^2.”
For clarity, this statement could use a bit of context. The *total* contribution of CO2 to earth’s energy budget is significant — all by itself amounting to over 30 W/m^2 (without the resultant feedbacks with water vapor or other greenhouse gas sources, etc). The value of ~4 W/m^2 is for a doubling of CO2 from 280 ppm to 560 ppm. The value quoted for the cooling by clouds is their total contribution.
Geophysicist David Archer has a fine web page demonstrating a radiative atmosphere, with all of the more important greenhouse gases whose concentration can be varied by the user, keeping track of temperature and gas concentration gradients vertically through the atmosphere. One can view the spectrum from any altitude, either upward or downward. It can even model simple water vapor feedback. Try it out here. These are not the results of the best radiative transfer calculations (aka, “Line by Line”), and so should serve for illustrative/educational purposes only.
Better than computing the TOA fluxes with this tool is the learning one can do by comparing the relatively detailed spectra as one varies the concentrations of greenhouse gases. See here for a nice tutorial (geared to an undergraduate student), Chapter 4 of his excellent textbook (errata).
Well, I actually stickled over that wattmeters is a measure of intensity – equivalent to a temperature- where a quantity is really the valid measure, e.g. watthourmeters – equivalent to heat. Sorry to make it a distraction because I know it all gets sorted.
Spaceman Spiff
Sorry your posts keep getting caught by the filter, no idea why.
Henry@ Spaceman Spiff
You don’t need all of those experts. really. Come here to Africa and then you measure the light on a sunny day at a specific time and also the temperature. Then wait for a cloudy day and measure the light again and the temperature at around the same time.. Then you know the “anomaly” caused by clouds. And when you look at that difference, you too will begin to understand the importance of clouds and cloud formation and how I came to my (own) 10 steps.. I mean what is 0,6 or 0,7 degrees warming over 100 years? I doubt even if the thermometers were that accurate 100 years ago. BTW it was not me who started the question here about clouds. I did not hear about Svensmark from WUWT. I stumbled on WUWT when I was just looking for some keywords on earth’s albedo. The quotes and theories that I have from WUWT were all figured out by myself, even before, but it does come in handy to have everything together.
Actually, I wished you were right. I think global warming is infinitely better than global cooling. But I know global cooling is coming so I have to start warning you people to clean up your act. Last year winter was a lot cooler here in the SH than the year before and this year the winter in the NH was a lot cooler than last year. All indicators are that global cooling is coming. Now people have changed global warming into “climate change”. That way you can even “explain” the cooler weather. But is that going to help when we all start freezing up? (I don’t think the cut by the moderator was justified)
Spaceman Spiff@henry Pool:
1) I was addressing your criticisms of the radiative transfer and molecular opacities, not the role of clouds.
2) All climate scientists will tell you that clouds remain a significant source of uncertainty. I was posing no argument with this. But a heck of a lot has been done in the past 20 years in the relationship between clouds and climate. And it’s not as if Svensmark hasn’t been heavily criticized in the peer-reviewed literature. The following web site compiles this literature, with topical organization — this one on “debunked” papers:
Search for Svensmark.
It is also true that Svensmark’s cosmic ray/cloud-albedo idea is just that — a speculative hypothesis without (yet) a coherent physical mechanism.
henry@spaceman spiff
1) I did not really see the proof from you that CO2 is a greenhouse gas and you also did not prove to me that the 70 odd ppms (0.0075%) that were added to the atmosphere since 1960 are indeed significant – I need to see actual test results that show a progression when you use atmospheres containing different amounts of CO2 ( at the relevant concentrations) taking into account that CO2 also causes cooling….(see my initial posts).
Question:
Also, I think we need to investigate the role of water – which apparently is a much stronger greenhouse gas – or so I hear.
The average water vapor content in the atmosphere is 1% which is a lot more than the 0.04% of CO2. All burning, cooling and energy processes by humans add more water vapor, even nuclear power…. (for cooling). Then we also have many dams, ponds and pools made by humans which surely must add more water vapor (shallow water easily warms up). So my question is: why does no one talk about that?
Singling out CO2 is short sighted. I believe more CO2 is better, it is like fertilizer. Everything you eat today depends on CO2. What people must complain about is the CO (from incomplete combustion) and gases like SO2 (from impurities in the fuel). CO2 is good.
2) The proof of the pudding is in the eating. My 10 steps make a lot more sense than all your complicated stories.
You want to believe your stories, that it is fine with me. I already predicted flooding of the rivers in the US and Europe because of all that extra snow and ice in the NH winter. It is already coming true. I predict further that more global cooling is on hand.
Maybe the picture on top of your site is an omen. It looks cold. Better make sure you prepare for the big freeze.
henry Pool
They do. Maybe you are looking in the wrong places. Water vapor is a major focus of research. There are many 100’s of papers on it (I’m sure 1000’s).
Without understanding the basics, any story will sound attractive. If you want to understand the basics, including the relative role of water vapor vs CO2 –
check out the CO2-An Insignificant Trace Gas? series and by Part Five you will see how the role of water vapor is calculated.
I’m sure your story will resonate with a lot of people. We’ll stay with the complicated stuff here.
henry@scienceofdoom
Come off it. I cannot believe you. If you want to prove a point or a rule or a law you must be able to produce a formula. Where is it?
There is no formula. I studied the IPCC reports. What they did is look at the global warming values. Someone decided that CO2 or greenhouse gases must be to blame (let us have a planet, add some CO2 , see if the temp. goes up, it did, so that must be it. What else can it be?) and then they looked at the concentrations of the gases at 1750 compared to 2005. Then they assigned radiative forcings attributed to the individual gases that would explain the warming. It is really just a weighting. That is the only formula I could find. But what is this? That is looking at a solution for a problem if you know what the cause is of your problem. You can only do that if you are 100% sure of the cause of your problem. It is an error that many doctors and scientists have made, myself including.
If you want to be a good scientist you must first prove your theory with a formula – then we can begin to look for the solution of the problem – in this case – global warming – if it it still happening. I see many people are beginning to doubt whether global warming is still happening.
henry Pool:
In Part Three – as previously explained.
In Part Five you will see Ramanathan and Coakley’s 1978 paper discussed with a link to their paper. Why not take a look? 25 pages of equations, here’s a small extract:
The IPCC report has references. In the references are papers like this one. For example, chapter 2 of the 2007 report: “Changes in Atmospheric Constituents and in Radiative Forcing” has 17 pages of references.
So where does it show the warming and cooling of CO2 in W/m2/m3 {CO2 0.03-0.05%}/24 hours?
henry Pool:
You have been convinced that no one has any equations, “the whole thing is all made up”.
Why not take some time to read at the least the series on CO2 on this blog? And think about it. And look up some of the references in the IPCC report. And then come back and ask some questions.
Your convictions are strong but you have demonstrated to anyone reading here that some of those convictions are based on lack of information, and some are based on false information. The fact that there is a large body of work – with equations – to support various ideas about the possible effect of CO2 on climate should make you pause and rethink. You’ve just confidently asserted that that isn’t the case. Take some time to review the information provided.
You think you have some answers, but you don’t. This problem is really very simple. Took me only 3 months to figure it out and I was a staunch Al Gore supporter! you should remove your censorship in my post as people will be wondering what you took out….
henry Pool
Next time you are at WUWT at a sun based thread, read and follow Leif’s comments and links… he knows his stuff. And look at it objectively. Im not saying it has no credibility, but look hard at the evidence for and against.
You are showing a conformation bias, we can all be guilty o that..(i have designed a positive displacement turbine thats gonna make me a billionaire!!!! muhahaha 😉 )
Like i said in my previous post to you, i am a skeptical individual by nature. And certainly find some of the more apocalyptic AGW claims questionable… And i realize it can be a question o trust as far as sourcing information… But the basic physics regarding the absorption properties of co2 is/and has been well understood for quite some time. And this blog here has to my knowledge the best threads breaking this down, and showing it that i have come across so far.
How they interact with other factors in a chaotic system may have areas of less certainty. But the basic greenhouse system is what makes this planet habitable. And is really beyond question, i can observe this by noticing that the average global temperature isnt -18oC or so.
Id urge you read dooms co2 series, it is well written. Yah can still be skeptical o AGW and understand the physics of the greenhouse effect.
henry Pool said:
—-
“…Singling out CO2 is short sighted. I believe more CO2 is better, it is like fertilizer. Everything you eat today depends on CO2. What people must complain about is the CO (from incomplete combustion) and gases like SO2 (from impurities in the fuel). CO2 is good.”
and
“2) The proof of the pudding is in the eating. My 10 steps make a lot more sense than all your complicated stories.
You want to believe your stories, that it is fine with me. I already predicted flooding of the rivers in the US and Europe because of all that extra snow and ice in the NH winter. It is already coming true. I predict further that more global cooling is on hand.”
and
“Come off it. I cannot believe you. If you want to prove a point or a rule or a law you must be able to produce a formula. Where is it?
There is no formula. I studied the IPCC reports. What they did is look at the global warming values. ”
and
“…If you want to be a good scientist you must first prove your theory with a formula – then we can begin to look for the solution of the problem…”
and
“You think you have some answers, but you don’t. This problem is really very simple. Took me only 3 months to figure it out and I was a staunch Al Gore supporter!”
—-
You’re putting us on, right? April Fools’ Day and all that? You really had me going there until you insinuated Al Gore. (Am I right? Or…?Or….?) Either way (IMO) your recent statements above have effectively quashed any further attempts of carrying on a fruitful, science-grounded discussion.
Poe’s Law
an anti-AGW parody blog
henry@spaceman spiff
My point of departure was Al Gore’s movie. At the time, I believed that the correlation given there was correct i.e that CO2 causes global warming. But I determined that it was the other way around. Global warming causes more CO2 in the atmosphere. As any good chemist knows: CO2 dissolves in water and if you want it out of the water you just have to make the water warmer.
I am glad you think you are more clever than me. But at least someone greater than Al Gore was here. Read my posts carefully again and you too will soon understand that clouds and rain and colder weather is already upon is. It all starts there where we know it must start: at the sun. Study the processes of the sun and you will soon realize that global cooling is coming. Make sure you are not left outside in the cold.
If you see the ice creeping up on you at your front doors, you know:
global warming is over. we now have to seriously look at global cooling and the consequences of that for every day life. No time for playing games and funny science.
especially for henry Pool-
from Etiquette : “..I am trying to keep posts and comments on topic. If the topic [the article] is the future of the world, go right ahead, otherwise try and stay roughly on the topic.”
I will be moderating or removing comments which move too far from the topic. Too far is totally subjective. The two comments above are “too far”. They can stay as illustrations.
Henry@the moderator and @Mike Ewing
I will show the moderator that my above comments are still on the topic but I can understand that you lost my train of thought.
I think after the last two winters, the vast majority of people in the Northern Hemisphere (with perhaps the exception of Eastern Canada and West Greenland) are fully aware that we have entered a period of global cooling.
So I am saying: perhaps it is the other way around: i.e. there being a relationship between increased CO2 and global cooling —
If by now you have understood what I have written here, you will follow that in my mind this might not be such a far fetched idea…
::::
My theory might be true after all, the cooling properties of CO2 might be greater than the warming properties. The net effect of more CO2 may cause cooling, not warming….Without proper testing proving the opposite, this is what I have been suspecting all along…..:
We know that CO2 has absorption in the 14-15 um range causing some warming (by re-radiating earthshine, 24 hours per day) but as shown and proved it also has a number of absorptions in the 0-5 um range causing cooling (by re-radiating sunshine). This cooling happens at all levels where the sunshine hits on the carbon dioxide same as the earthshine. The way from the bottom to the top is the same as from top to the bottom. So, my question was: how much cooling and how much warming is caused by the CO2? How was the experiment done to determine this and where are the test results? If it has not been done, why don’t we just sue the oil companies to do this research? (I am afraid that simple heat retention testing will not work here, we have to use real sunshine and real earthshine to determine the effect in W/m3 [0.04%-0.06%]CO2 /m2/24hours).
When they analysed the spectra, did they look at all the absorptions, namely also at those of CO2 in the UV – that have only been discovered recently? I also doubt that spectra analysis would work here – you have to come up with a more real time experiment.
Namely, for example, I think especially the cooling of CO2 caused at 4.3 um might be considerable because this is where the sun’s radiation is at its hottest (on your skin). Note that the temp. on the coast on a sunny day (no wind) is always a few degrees cooler than more inland. This is due to same cooling caused by water vapor in the sun’s solar spectra – as you know, I am saying CO2 does exactly the same thing. Without CO2 in the atmosphere more (hot) IR radiation would be slammed on top of our heads.
So what is the net effect of CO2? How do we all know for sure that CO2 is a greenhouse gas when clearly Svante Arrhenius formula has long been proven wrong and nobody has come to me with the right formula?
henry Pool
I will add to the etiquette that continual repetition of points, again at the discretion of the moderator, will also be removed.
You are very keen to the make the same points then ignore any answers.
The solar radiation >4um is <1% of the total solar energy. As has already been noted.
And "no one has come to you with the right formula".
We'll let current and future readers decide for themselves if your questions have merit. And if the various answers provided have merit.
I would have more interest in trying to answer specifics if you had shown any interest in absorbing any answers so far.
First henry Pool post deleted for repetition.
henry Pool – if you want to be part of a discussion here, show that you have attempted to grasp the content of answers to your comments.
If you ask a question that shows that you have actually read and thought about earlier responses, your comment will stay up.
I hate to put this comment here, but your RSS feeds appear to be broken.
Thanks, I appreciate you letting me know. It’s now fixed according to the great people at wordpress.
CO2 does not cause less sunlight to hit the earth only by absorption, it can also do so by Rayleigh scattering. Has anyone considered this effect? Is it included in the IPCC calculations? References would be greatly appreciated.
Sound like someone grasping at straws especially since the measured temperature data shows the temperatures have been flat for 15 years or so.
Stanley,
What is the relationship between the first clause in your sentence and the remainder of the sentence?
Are journals like the Journal of Quantitative Spectroscopy and Radiative Transfer going to shut down now? They suddenly find that those absorption lines they have measured are all wrong? Because of something to do with global temperature.. I’m struggling to see the relationship..
Can you explain what was wrong with the measurements of absorption lines?
here are my latest results
(that prove there is no man made global warming)
http://blogs.24.com/henryp/2013/02/21/henrys-pool-tables-on-global-warmingcooling/
Try to stay on top of it,
Dr. S
Henry: Try reading these links:
http://berkeleyearth.org/about
http://berkeleyearth.org/summary-of-findings
frank, CO2 is a red herring.
First of all, there are giga tons of bi-carbonates in the oceans and any type of heating would cause it to be released
Heat + HCO3- => CO2 (g)+ OH-
So, CO2 follows the heat, not the other way around. It does not cause heat.
2nd your sample of global stations must be properly balanced,
like I explained in my earlier link.
If you do it right,
getting your own sample of 50 odd stations, properly balanced, you should be getting the same results as I did
Click to access henryspooltableNEWc.pdf
Your chemistry is wrong. It’s 2 HCO3- → CO2 + CO3= + H2O. And it takes quite a bit of heat. At sea surface temperature, a change of a few degrees only affects the solubility of CO2 slightly. We also know from ice core data that the effect of ocean temperature on atmospheric CO2 is less than 20 ppmv/degree.
Maybe your reaction is correct for sea water but for making standard solutions we had to boil water to remove any dissolved CO2 and in the end [10 minutes, I think] there were no carbonates left. But [sometimes] we had to neutralize the water afterwards.So, I think my reaction is correct as well.
Either way, it is neither here nor there: the CO2 follows the heat, not the other way around.
http://joannenova.com.au/global-warming-2/ice-core-graph/
There is no man made global warming, as apparent from my own results, which you can easily duplicate.
Henry: Your temperature records show that there has been a 0.55 degC (-0.01375 degC/yr) fall in minimum temperature in the SH over the last 40 years. However, the 95% confidence interval for the mean of these 27 records is -0.13 to -0.97 degC. In other words, IF these 27 stations were representative of the SH and IF they were accurate, you could barely conclude that minimum temperatures in the SH have fallen. However, you’ve got the big picture partly right: There has been more warming in the NH (especially the arctic) than the SH and a hiatus in warming since about 2000.
http://betterfigures.files.wordpress.com/2014/04/blwhnwbcaaaa02alarge.png?w=636&h=310&crop=1
A more robust answer requires more data. Since the mean temperature at a particular location varies about one degree from year to year and you are interested in TENTHs of at degree, you want to analyze all the data, just not the endpoints. When you look at all the data, you’ll find that it is very noisy and contains obvious artifacts, because it has not been collected under controlled conditions where one can accurately detect changes of a few tenths of a degC in the raw data. The Berkeley Earth project (“BEST”) – started by a vocal critic of the climate science establishment with some funding from the Koch brothers – is working on an independent analysis. They have records from 40,000 stations, all of which is transparently available at their website and linked to maps. If you look up the station that showed the great cooling in your table (Tandil, Argentina), you can see the raw data for this station AND select from a list of nearby stations. You will find that nearby stations are generally warming, not cooling. BEST has created a regional composite of all of the nearby stations, showing periods when the relationship between one station and its neighbors was stable and when the relationship suddenly changed (presumably due to some change at the station). BEST shows how well the station data and the regional composite agree after the breakpoints were corrected. The large number of corrections and the impact of these corrections on overall warming are distressing (they contribute about 25% of overall warming), but this is the best answer we have got.
Go to the BEST website, collect some more data, make sure it is somewhat representative of the location and doesn’t contain too many discontinuities, see how BEST chose to correct it, and calculate an answer that is good enough to share on the web. Unless you deliberately cherry-pick (which BEST will make easy for you), you will probably find some warming, even in the SH. You might also begin to feel you are wasting your time when other critics of the climate science establishment have already done a far more sophisticated and transparent job than you can do. However, it never hurts to replicate the work of others. The problem occurs when you don’t understand enough about a particular issue and use an inadequate analysis to proclaim that scientists are wrong.
@Frank,

I did not look around Tandil (where they chopped the trees) for the same reason as why I did not look around Las Vegas (where they turned a desert into a paradise). The sample of weather stations must be random.
[It was just interesting to note that apparently there is a link between vegetation and minimum temperatures.]
You must understand that my sample was random but I followed a special sampling technique that you have to try and understand…. The correlation coefficients for the deceleration of warming can be described as very high. It will pass any statistical test for significance. As a chemist I was always happy if my 4 points for analysis (AAS, UV-Visible spectro-photometry, etc) gave a me a correlation of more than 0.99.
You cannot say that my Rsquare= 1 for the deceleration of minimum temps could be co-incidental?
No Frank. The reality is that it has been cooling globally, as the major data sets are now also showing.
http://www.woodfortrees.org/plot/hadcrut4gl/from:1987/to:2015/plot/hadcrut4gl/from:2002/to:2015/trend/plot/hadcrut3gl/from:1987/to:2015/plot/hadcrut3gl/from:2002/to:2015/trend/plot/rss/from:1987/to:2015/plot/rss/from:2002/to:2015/trend/plot/hadsst2gl/from:1987/to:2015/plot/hadsst2gl/from:2002/to:2015/trend/plot/hadcrut4gl/from:1987/to:2002/trend/plot/hadcrut3gl/from:1987/to:2002/trend/plot/hadsst2gl/from:1987/to:2002/trend/plot/rss/from:1987/to:2002/trend
Antarctic ice has been increasing significantly.
We are cooling from the [top] latitudes downward, as these 10 samples from Alaska show:
(-0.055K/annum, since 1998, average results for 10 stations in Alaska)
A natural consequence of global cooling is a small (?) shift of cloud formation and precipitation, more towards the equator, on average. This will amplify the cooling effect even further. Whilst maximum temperatures will still be dropping, average temperature around the equator remains more or less unchanged, largely due to more condensation energy coming free.
At the higher latitudes >[40] especially in the America’s it will become progressively drier, from now onward, ultimately culminating in a big drought period similar to the dust bowl drought 1932-1939. My various calculations all bring me to believe that this main drought period on the Great Plains will be from 2021-2028. It looks like we have only 7 “fat” years left…..
I want to challenge you. I hope you see the importance of my analysis and that you will take the time to try and duplicate, even when using Berkely results; just make sure that you balance the sample exactly as I have done, as described here,
http://blogs.24.com/henryp/2013/02/21/henrys-pool-tables-on-global-warmingcooling/
You can take more samples [than the 54] if you want to, but if you see such high correlations appearing as described in my graphs setting the speed of warming out against time, you will probably also stop and just sit in wonder.
Henry wrote: “The correlation coefficients for the deceleration of warming can be described as very high. It will pass any statistical test for significance.”
Learn some statistics. Each of the points you plotted should have a large error bar (of up to +/-0.5 degC/40 years). A proper correlation analysis would plot all data points for each time period (not just the mean) and then you look for correlation. You will get the same fit, but the correlation coefficient will be near zero.
With three adjustable parameters available in a quadratic fit, you don’t prove anything by finding a reasonable fit to four data points.
Try plugging x=1 or x=100 into one of your quadratic equations and explaining what the answer means. Hint: The 40-year warming rate should have been plotted over the year 1984 (the midpoint of the 40-year period to which you have fit a linear trend) not over the number 40, the 34-year warming rate should have been plotted over the year 1987, etc. Then you might be able to tell us something about how the rate of warming has slowed over the last 40 years.
The recent weather in Alaska, sea ice in Antarctica, logging in Argentina, and speculation about the past or future Dust Bowl have nothing to do with GLOBAL warming. Why we should believe your error-ridden analysis rather than the far more sophisticated analysis by the skeptics at BEST?
@frank
forget about the error bars. we are looking at the average change from the average yearly temperature in degreesK/annum. I wonder if you know how to do linear regression? Now to determine the speed of warming in degrees K/annum, I can chose any 4 intervals going back in time. I happened to chose 1974-2014, 1980-2014, 1990-2014 and 2000-2014, My results are very reliable and comparable with those of others over comparable periods. For example, Spencer reports 0.13/K decade for the past three decades and I get about the same (means table).
Having obtained those values, as stated in my tables, I can proceed to set the speed of warming out against time, which gives me K/annum square. It is just like setting the speed of a thrown ball in m/s out against time, which gives you a curve in m/second square. It shows the acceleration and deceleration.
Of those 3 curves I can exactly see what is happening.
Note that your best BEST does not even have minima or maxima. Useless.
Having obtained a perfect curve for the decline in Minima, I conclude there is no man made warming. Otherwise, of those three curves that I did find, the last one should have shown the greatest chaos (i.e. a lower Rsquare value)
HenryP writes: “forget about the error bars”.
That is exactly the problem. No one cares about the trend in the mean of the temperature at the 50+ stations (the sample) you have selected. We want to use the data from these 50+ stations to tell us what is happening on land as a whole (the population). If you selected a second groups of stations, how close would the mean of the second group be to the first? If you had evenly spaced stations in every km^2, what would be trend be? The standard error of the mean and confidence intervals are used to answer these questions. Your analysis needs to take into account these uncertainties. You need to recognize the difference between your 50 station sample (which is hopefully accurate and representative of global land surface temperature) and what you want to learn from this sample – what is happening on land as a whole.
@frank
don’t worry about the “error”
that is a different subject on its own.
e.g how could you people ever think that you can compare data from 50 years ago and further back with those from now? (both considering collection of data and accuracy?)
never mind not [even] looking at global maxima and -minima, as stated before,
the incompetence of you and your (“sophisticated”) ilk goes even further.
For example, although most official data sets are showing some global cooling now from 2002,
http://www.woodfortrees.org/plot/hadcrut4gl/from:1987/to:2015/plot/hadcrut4gl/from:2002/to:2015/trend/plot/hadcrut3gl/from:1987/to:2015/plot/hadcrut3gl/from:2002/to:2015/trend/plot/rss/from:1987/to:2015/plot/rss/from:2002/to:2015/trend/plot/hadsst2gl/from:1987/to:2015/plot/hadsst2gl/from:2002/to:2015/trend/plot/hadcrut4gl/from:1987/to:2002/trend/plot/hadcrut3gl/from:1987/to:2002/trend/plot/hadsst2gl/from:1987/to:2002/trend/plot/rss/from:1987/to:2002/trend
none of the official global data sets are actually properly balanced, taking
a) equal amount of stations NH and SH,
b) all stations balanced at close to zero latitude
c) 70% of stations near or at sea
d) 30% of stations inland
e) stations from all continents
So, like I said, you can trust my data set, more than anyone else’s because I kept the right balance.
Anyway, the proof of the pudding is in the eating. You can take another sample of 50 or 54 stations and keep this balance, and you will find the same results as I get
But you won’t do it, because that would open the can of worms.
All the best,
Henry
Henry,
Which way the net flow of CO2 goes between the oceans and atmosphere is a matter of balance. Increasing the temperature moves the balance towards release from the oceans to the atmosphere. Increasing the CO2 concentration of the atmosphere leads to an opposite change.
The changes balance when both the temperature and the atmospheric concentration change in some specific ratio. Many factors influence the ratio, which is therefore not precisely known, only approximately. The value is roughly 10 ppm/C, certainly not much higher. Therefore it’s known for sure that warming of oceans has not contributed more than a few percents of the increase in the atmospheric CO2 concentration.
That there’s so much more carbon in the oceans results from chemistry, which tells also, how little that situation changes from warming as long as temperatures do not differ really much from the present ones.
@Pekka
My latest results clearly show there is no warming as a result of GHG increase. Namely AGW proposes/implies that minimum temperatures should be rising, pushing up means. That does not happen. Minimum temperatures follow a natural path downward.
Last graph, bottom of the last table, looking at deceleration of warming.
Click to access henryspooltableNEWc.pdf
HenryP: Before you say that there has been no warming as a result of the CO2 increase, don’t you first need to know how much warming we expect to see and how accurately you are able to measure warming?
Your data for mean global temperature at 52 sites shows 0.49 degC (0.26-0.72 degC 95% ci) of warming over 40 years.
CO2 has increased from 329 to 398 ppm over the last 40 years, a 21% increase or about 1/4 of a doubling of CO2. (1.21^4 = 2.14). More accurately, 27% of a doubling.
The IPCC tells us each doubling of CO2 likely will raise temperature by 1.0-2.5 degC/doubling of CO2. (This is transient climate response, TCR, the warming expected at the end of a period of gradual forcing, not the higher equilibrium climate sensitivity, ECS, expected for total warming many decades after forcing has stabilized.) This change in CO2 should produce a warming of 0.27-0.68 degC.
Your mean global temperature data is completely consistent with what the IPCC tells us to expect. (For simplicity, I have ignored changes in other GHGs and other forcings. To a first approximation, the forcing from the increase in other GHGs has been been offset by other changes in aerosols and other forcing.)
For global minimum temperature, you identified a warming of 0.17 degC (-0.19 to +0.53 degC 95% cI) at 52 stations over the last 40 years. While your central estimate for the increase in minimum temperature is slightly lower than the IPCC projects, given the scatter in your data, the result is still completely consistent with the IPCC’s expectations.
Warming has certainly paused (or slowed) since 2000. When there is no change in forcing, climate (like weather) fluctuates chaotically. One possible cause is chaotic fluctuation in the currents exchanging water between the cold deep ocean and the surface. For example, the rise of cold deep water off the west coast of South America (among many other things) is interrupted during El Nino events. Longer fluctuations in such currents may have enhanced warming during 1930-1945 and 1978-1998 and suppressed warming during 1955-1975 and since 2000. Multi-decadal oscillations such as the PDO and AMO may be associated with such fluctuations, but we haven’t observed these oscillations long enough to quantify their behavior. “Unforced variability” of this type – which is common in chaotic systems – makes it very difficult to interpret the meaning of discrepancies of a few tenths of a degC in the relationship between CO2 and temperature.
Frank says
Before you say that there has been no warming as a result of the CO2 increase,
Henry says
Let us stop right there
\
why don’t you prove to me from an [designed] experiment that the increase of CO2 from 0.03 to 0.04 % of CO2 in the atmosphere would cause any warming at all?
http://blogs.24.com/henryp/2011/08/11/the-greenhouse-effect-and-the-principle-of-re-radiation-11-aug-2011/
[which would bring us back to the original argument of the post here]
[I think] It would be easier to simply repeat my experiment that shows there is no influence of CO2 on the climate. (Table 3, correlation 100% for a natural decline of minimum temps.)
Namely, I figure that the proposed mechanism for AGW implies that more GHG would cause a delay in radiation being able to escape from earth, which then causes a delay in cooling, from earth to space, resulting in a warming effect.
It follows naturally, that if more carbon dioxide (CO2) or more water (H2O) or more other GHG’s were to be blamed for extra warming we should see minimum temperatures (minima) rising faster, pushing up the average temperature (means) on earth.
I subsequently took a sample of 54 weather stations, analysed all daily data, and determined the ratio of the speed in the increase of the maximum temperature (maxima), means and minima. Here you can see the results.
http://blogs.24.com/henryp/2013/02/21/henrys-pool-tables-on-global-warmingcooling/
What you say could be happening is not happening.
Temperatures are going down. That will cause climate change. Live with it.
http://blogs.24.com/henryp/2013/04/29/the-climate-is-changing/
HenryP asked: Why don’t you prove to me from an [designed] experiment that the increase of CO2 from 0.03 to 0.04 % of CO2 in the atmosphere would cause any warming at all?
In general, I agree with the detailed explanations you can find at this site, which are summarized here with links to other posts:
As a chemist doing spectroscopy, you understand how you can use a spectrophotometer and Beer’s law to gather data that will allow you to predict the absorption of radiation by a sample under a variety of conditions (concentration, path length). For the atmosphere, we need to be concerned with both absorption and emission. The Schwarzschild equation tells us how to do this:
dI/ds = emission – absorption = n*o*(B(lamba,T) – n*o*I_0
dI/ds = n*o*[(B(lamba,T) – I_0]
where dI is the incremental change in intensity of the incoming radiation, I_0, at a given wavelength, lamba, as it travels an incremental distance, ds, through a sample; n is the concentration of GHG molecules; o is the absorption coefficient at that wavelength; and B(lamba,T) is the Planck function. This equation reduces to Beer’s law when emission is negligible (the usual situation in the lab at 295 degK using a light source with a lamp filament at several thousand degK) and when n, o, and T are constants. None of these qualifications apply to the atmosphere. So, we need to numerically integrate this equation over the path to space (or the surface) and over the full range of wavelengths that allow radiation to escape to space (or reach the surface). You can read about the details at this website. Or you can try the online calculator at http://forecast.uchicago.edu/modtran.html – which uses this equation, a database of absorption coefficients (initially compiled for aerospace engineers before global warming became an issue), and typical profiles of the atmosphere (composition and temperature) at various latitudes and altitudes.
Proof: When you increase the amount of a GHG (n), the change in a radiative flux depends on whether B(lamba,T) is greater or less than I_0. Since upward traveling radiation usually has been emitted where the temperature is higher than where it is absorbed, increasing GHGs will decrease the outward flux. This must lead to some warming. How much warming will occur is a very complicated question that depends on feedbacks. Since I personally don’t have much faith in climate models, I used the IPCC’s very wide estimate for TCR to predict for you how much warming you might expect to observe – before the complications from unforced variability. There are very large uncertainties in climate science, but not in the radiative forcing expected for GHGs.
So why does BEST find a slightly greater increase in maximum temperature than minimum temperature over the past few decades despite rising GHGs? It don’t know. It is trivial to show that the amount of night time cooling that occurs is greater when there is less water vapor (a GHG) in the air or less total GHG to pass through (at higher elevations). The principle is obviously correct in general, but we are looking for a very small effect in a complicated phenomena that is being monitored with inadequate equipment. If you check out Table 1 of this paper, you’ll see that the NCDC found (in 2004) that minimum temperatures had risen more than maximum temperatures, contradicting BEST. More importantly, models predicted that this diurnal range should have shrink only 0.08 degC over 54 years!
ftp://ftp.orbit.nesdis.noaa.gov/pub/smcd/spb/lzhou/AMS86/PREPRINTS/PDFS/100659.pdf
It doesn’t make any sense to me to discarding the predictions of established physics and chemistry (the Schwarzschild eqn) because of discrepancies this small and unreliable – unless your motivations are political, not scientific. Doing so without a reasonable understanding of the uncertainty and confidence intervals is a sign of hubris or worse. That doesn’t mean that we shouldn’t be skeptical about many other aspects of climate science. Many followers of scienceofdoom are skeptics who appreciate the generally unbiased presentation of science.
I tried the modtran calculator to see the diffrence in temperatures between 300 and 400 ppm co2. And I got the same temperatures. Is there any calculation that can show the temperature effect of an increase in co2 from 300 to 400 ppm?
nobodyknows,
You can determine the temperature effect by adjusting Ground T offset and requiring that Upward IR Heat Flux is the same as with the other CO2 concentration. To get most meaningful results the comparison should be made for radiation looking down at 17 km, which represents well enough the tropopause height for all atmospheres considered. When I tried that for the default case clear sky tropical atmosphere, the required offset was 0.49 C. This is the no-feedback warming for the atmosphere considered.
A corresponding comparison done at much higher altitudes is distorted by stratospheric effects that UChicago MODTRAN cannot determine in the way required for this calculation.
Thank you Pekka. That was most helpful. I have been wondering about this for some time. If you use statistics then for the 100 years from ca 1913 to 2013, there should have been a co2 warming of 0,5 C, and the warming has actually been 0,8 C, which gives an additional forcing of 0.3 C. (heat uptake, water vapor, clouds, insolation etc.) Would not that be the best statistic estimate? It is much less mystifying than using 20 models to get the overall picture, I think.
Frank says
Proof: When you increase the amount of a GHG (n), the change in a radiative flux depends on whether B(lamba,T) is greater or less than I_0. Since upward traveling radiation usually has been emitted where the temperature is higher than where it is absorbed, increasing GHGs will decrease the outward flux.
Henry says

The proof has to start with the testing showing that the net effect of [more of] a GHG in the atmosphere is that of warming or cooling. You have not done that.
Unfortunately, in their time, Tyndall and Arrhenius could not see the whole picture of the spectrum of a gas which is why they got stuck on seeing only the warming properties of a gas (i.e. the closed box experiments). If people would understand this principle, they would not singularly identify green house gases (GHG’s) by pointing at the areas in the 5-20 um region (where earth emits pre-dominantly) but they would also look in the area 0-5 um (where the sun emits pre-dominantly) for possible cooling effects. If you really want to understand what happens in the atmosphere, this rough graph / representation (on a cloudless day) is very important
[never mind the fact that the amounts of radiation heat from the sun’s 5525K and 210-310K from earth displayed, are completely out of proportion ]
just see how the absorptions that are apparent in the spectra of the individual components of the atmosphere affect the outgoing radiation of earth and see how they affect the incoming radiation. For example, let us look at the absorption of ozone at between 9-10 um? It makes a dent in earth’s outgoing radiation at 9-10. In other words what happens: Radiation from earth of 9-10 goes up, hits on the ozone, most of which is high up in the sky and which is already absorbed to capacity, and therefore a great percentage (at least 50%, probably more) is sent back to earth, leading to entrapment of heat, leading to delay in cooling, leading to a warming effect. Also look at water vapor and CO2 around 2 um and see how that makes a dent in the incoming solar radiation. Notice that the ozone shields us from a lot of sunlight by absorbing and re-radiating in the UV region. In fact, if you really grasp what you are seeing in this graph/ representation (from a cloudless day), you would realize that without the ozone and CO2 and H2O and other GHG’s you will get a lot more radiation on your head. In fact, you would probably fry.
so the first question that must be asked: is the net effect of more of a particular GHG that of cooling or warming, or is it more or less neutral?
e.g. For comprehensive proof that CO2 is (also) cooling the atmosphere by re-radiating sunshine, see here:
http://www.iop.org/EJ/article/0004-637X/644/1/551/64090.web.pdf?request-id=76e1a830-4451-4c80-aa58-4728c1d646ec
They measured this re-radiation from CO2 as it bounced back to earth from the moon. So the direction was sun-earth (day)-moon(unlit by sun) -earth (night). Follow the green line in fig. 6, bottom. Note that it already starts at 1.2 um, then one peak at 1.4 um, then various peaks at 1.6 um and 3 big peaks at 2 um. You can see that it all comes back to us via the moon in fig. 6 top & fig. 7. Note that even methane cools the atmosphere by re-radiating in the 2.2 to 2.4 um range.
This paper here shows that there is absorption of CO2 at between 0.21 and 0.19 um (close to 202 nm):
Click to access DUV-CO2.pdf
Hence we can identify it as being present on other planets.
There are other papers that I can look for again that will show that there are also absorptions of CO2 at between 0.18 and 0.135 um and between 0.125 and 0.12 um.
We already know from the normal IR spectra that CO2 has big absorption between 4 and 5 um.
So, to sum it up, we know that CO2 has absorption in the 14-16 um range causing some warming (by re-radiating earthshine) but as shown and proved above it also has a number of absorptions in the 0-5 um range causing cooling (by re-radiating sunshine). This cooling happens at all levels where the sunshine hits on the carbon dioxide same as the earthshine. The way from the bottom to the top is the same as from top to the bottom. So, my question is: how much cooling and how much warming is caused by the CO2? How was the experiment done to determine this and where are the test results? (I am afraid that simple heat retention testing might not work here, we have to use real sunshine and real earthshine to determine the effect in W/m2/m/ [0.03%- 0.06%]CO2/time period).
In all of this we are still looking at pure gases. The discussion on clouds and the deflection of incoming radiation by clouds is still a completely different subject.
Note that CO2 also causes cooling by taking part in the life cycle. Plants and trees need warmth and CO2 to grow – which is why you don’t see trees at high latitudes and – altitudes. It appears no one has any figures on how much this cooling effect might be. There is clear evidence that there has been a big increase in greenery on earth in the past 4 decades.
From all of this, you should have figured out by now that any study implying that the net effect of more CO2 in the atmosphere is that of warming, must exhibit a balance sheet in the right dimensions showing us exactly how much radiative warming and how much radiative cooling is caused by an increase of 0.01% of CO2 that occurred in the past 50 years in the atmosphere. It must also tell us the amount of cooling caused by the increase in photosynthesis that has occurred during the past 50 years.
There are no such results in any study, let alone in the right dimensions. For example, consider the fact that time must be in the dimensions.
For more on why it is considered highly unlikely that CO2 is a contributory cause to global warming, see here:
http://blogs.24.com/henryp/2013/04/29/the-climate-is-changing/
HenryP wrote: “The proof has to start with the testing showing that the net effect of [more of] a GHG in the atmosphere is that of warming or cooling … If people would understand this principle, they would not singularly identify green house gases (GHG’s) by pointing at the areas in the 5-20 um region (where earth emits pre-dominantly) but they would also look in the area 0-5 um (where the sun emits pre-dominantly) for possible cooling effects.”
First, the spectra Henry provides show that CO2 has a strong absorption (15 um) in the center of the outgoing LWR, two weak absorptions on the edge of incoming SWR, and an intermediate absorption (4-5 um) where both SWR and LWR are negligible. The figure Henry has provided has been constructed so that the areas under the blackbody curves curves are equal, so the relative importance of any wavelength to the planetary energy balance is obvious. The weaker absorbance of incoming SWR effects far smaller percentage of total SWR than the stronger absorbance of outgoing LWR of total LWR.
Second, SWR absorbed by the troposphere, rather than the surface, still warms the planet! In fact, 1/3 of incoming post-albedo SWR is absorbed by the troposphere and never reaches the surface. When more SWR is absorbed by the atmosphere, less convection is needed to carry heat away from the surface and produce a stable lapse rate. The radiative balance for the planet – which determines whether the planet warms, cooling, or remains the same – depend on the total radiation absorbed (not reflected) by the surface and atmosphere and the total radiation escaping from the surface and atmosphere.
HenryP also complains: “For comprehensive proof that CO2 is (also) cooling the atmosphere by re-radiating sunshine, see here …”
“Re-radiation”, “radiative cooling”, and other synonyms for emission have not been ignored. HenryP apparently doesn’t realize that the Schwarzschild eqn QUANTITATIVELY takes into account both absorption AND emission. The n*o*(B(lamba,T)) term deals with emission.
dI/ds = emission – absorption = n*o*(B(lamba,T)) – n*o*I_0
In the laboratory – where emission is negligible and samples are homogeneous – HenryP uses extinction coefficients (chemistry’s term for absorption cross-section) and Beer’s Law to calculate how much radiation is lost on its path through a sample. In the atmosphere – where emission is significant and density, temperature and humidity vary with altitude – climate scientists use the same parameters and the Schwarzschild equation to calculate how much radiation is lost and gained on a path from the surface to space.
The predictions of the Schwarzschild eqn are consistent with observations of the earth’s atmosphere. See: https://scienceofdoom.com/2010/11/01/theory-and-experiment-atmospheric-radiation/
None of HenryP’s other distractions change this conclusion: earthshine (ie OLR) bouncing off the moon, ozone (an interesting, but complicated topic), deep-UV absorption by CO2 (wavelengths at which the sun emits a negligible fraction of its energy), detection of habitable planets (the subject of HenryP’s first reference), the greening of the planet, the [non]-missing dimension of time (radiation intensity in the Schwarzschild eqn is measured in units of energy per time per area), cooling by photosynthesis, clouds (another complicated topic), and mischaracterizing a 24% increase in CO2 as an 0.01% increase.
I’ve already shown above how much warming we expect to have occurred, how unforced variability complicates detection of that warming, and why HenryP’s amateur temperature analysis is inadequate for detecting warming. The length of the current hiatus certainly casts doubt upon the ability of climate models to produce realistic unforced variability and/or climate sensitivity; but it doesn’t invalidate the Schwarzschild eqn. Questioning climate models makes sense; ignoring the implications of the Schwarzschild eqn does not.
Arrrrgh
Frank, I am sure I am wasting my time with you, but yet, I will try [only] one more time.
Basically all of your arguments are faulty. I am only going to pick one.
Frank says:
Second, SWR absorbed by the troposphere, rather than the surface, still warms the planet! In fact, 1/3 of incoming post-albedo SWR is absorbed by the troposphere and never reaches the surface. When more SWR is absorbed by the atmosphere, less convection is needed to carry heat away from the surface and produce a stable lapse rate. The radiative balance for the planet – which determines whether the planet warms, cooling, or remains the same – depend on the total radiation absorbed (not reflected) by the surface and atmosphere and the total radiation escaping from the surface and atmosphere.
Henry says
There is no mass in the atmosphere “to absorb” much heat. The water in the oceans have the ability to absorb and convert to heat, because there is mass.
This “absorption” was an unfortunate term that was used in relation to gases. What the so-called GH gases can do is re-radiate. For example: let us say sunshine 1-2 um hits on a CO2 molecule. Where must it go?? All gases around except water [vapor] are transparent to 1-2?
So, at least 50% of that radiation [that hits on the CO2 molecule] is going back to space. Hence the reason why we can measure it, even bouncing off from the moon. Cooling the earth.
I have explained this all in detail, here
http://blogs.24.com/henryp/2011/08/11/the-greenhouse-effect-and-the-principle-of-re-radiation-11-aug-2011/
Henry says:
Henry helpfully demonstrates to everyone except himself that he has no idea about anything related to atmospheric physics.
The mass of the atmosphere is not zero.
Why does pressure decrease with height?
The atmosphere has mass and a specific heat capacity. You can find this in many atmospheric physics textbooks.
Henry, are they all wrong?
Why is the surface pressure 1000mbar instead of zero?
Not much point explaining where you have gone wrong with the rest of your “explanation”.
Start with the simplest.
This is definitely true. You are wasting your time with all of us. Trying promoting your theories on a blog where no one has any idea about basic physics and you will have more success.
Tell them no one was prepared to take the blinkers off here. No one ready to open their mind. Or whatever.
@nobodyknows
you cannot calculate that which nobody has tested first.
HenryP,
Are you completely unaware that it’s possible to calculate atmospheric emission spectra that are in very good agreement with measured spectra if you know the temperature and humidity profile at the time? And that, even though the inverse problem is ill-posed, you can retrieve atmospheric temperature and humidity profiles from a spectrum measured at the surface using a line-by-line radiative transfer program that are in fair agreement with measured profiles?
http://rtweb.aer.com/
Note that the RMS residual of 4E-08 in graph 9(b) means that the error is less than 1% of the measured radiance on average.
As far as ghg’s cooling the atmosphere rather than making the surface warmer than it would be with a transparent atmosphere:
But I’m confident that you can hand wave all that away.
As far as correlation coefficients by themselves meaning anything, you can plot three random points on a plane and fit them to the equation for a circle with a correlation coefficient of identically 1. It doesn’t mean that the next random point you plot will lie anywhere close to that circle.
DeWittPayne says
As far as correlation coefficients by themselves meaning anything, you can plot three random points on a plane and fit them to the equation for a circle with a correlation coefficient of identically 1. It doesn’t mean that the next random point you plot will lie anywhere close to that circle.
Henry says
I could go with that (intelligent) argument, but remember that I don’t have just one data set.
I have three data sets that show that temperature is decelerating downwards at coefficients between 0.96-1.000.
That means something. In probability theory, it means that I figured out that the average of throwing the dice will be 3.5
[hint: Gleissberg)
Anyway, to settle the matter, why don’t you repeat my experiment, preferably taking different samples, using my own unique sampling technique.
http://blogs.24.com/henryp/2013/02/21/henrys-pool-tables-on-global-warmingcooling/
If you have a class of stats 1 students available, who are learning about linear regression, it would not take you a long time, now would it? Give them each one station to do. Use BEST/ or you can use my source.
Remember that my forward and backward projection is just for illustration. However, within my range, I can tell you EXACTLY what the speed of warming/cooling was at any time between 2014 and 1974 [as far as minimum temperatures are concerned]
With some help I try to find the best estimates. And for now it looks like that a hundred years of global warming by 0,8 C can directly be attributed to co2 with 0.5 C and indirectly with 0.3 C, if we assume that natural variation has been smoothed out. It cuold be a working hypothesis.
@nobody knows
from the seventies we switched to continuous recording (every second) with thermo couples as opposed to human observation recording a few times per day (4-6 times, if you were lucky and nobody got sick or had to go on leave) with a thermometer.
Now interestingly, the first evidence of a re-calibrated thermometer I could find is 1948. It would seem that before that time thermometers were manufactured and never re-calibrated…..
So how do you know that the difference you think you are seeing is not simply because of the difference in the collection of data?
I just think that I have to rely on hardcrut data. I don`t know any better.
Sea level has become 20 cm higher in 100 years. It fits closely with temperature.
“the remarkably close link of global temperature and the rate of sea-level rise we find for 1880–2000. In particular, it shows that the rate of sea-level rise increased up to 1940 in line with rising temperatures, then stagnated up to the late 1970s while global temperature also remained nearly level, followed by another rise that continues until today”
http://www.pnas.org/content/106/51/21527.full
I think this is a good indication of the increased temperatures.
0.8 C higher temperatures give 20 cm higher ocean. And most interesting: It is calculated that this warming of oceans and ice melting is in balance with air temperature. There has been an energy balance between ocean and air for 100 years. Net OLR energy imbalance has got into ocean as well as air, like physical laws wuold predict it. As I interpret it.
Correction: I meant net TOA energy imbalance.
Henry writes above:
There is no mass in the atmosphere “to absorb” much heat. The water in the oceans have the ability to absorb and convert to heat, because there is mass.
This “absorption” was an unfortunate term that was used in relation to gases. What the so-called GH gases can do is re-radiate. For example: let us say sunshine 1-2 um hits on a CO2 molecule. Where must it go?? All gases around except water [vapor] are transparent to 1-2?
So, at least 50% of that radiation [that hits on the CO2 molecule] is going back to space. Hence the reason why we can measure it, even bouncing off from the moon. Cooling the earth.
I have explained this all in detail, here
Henry is suffering from several delusions:
1) The atmosphere has mass: 14.7 pounds above every square inch or 10,000 kg above every square meter. That is the same mass as the top 10 meters of the ocean. All thermal infrared radiation is absorbed and emitted by the top 1 mm of the ocean, and the vast majority of SWR is absorbed by the top 10 m. The heat capacity of the atmosphere is equal to that of the top 2.5 m of the ocean. Unlike the atmosphere, the ocean is stably stratified. The temperature of the top 10 m of the ocean is determined exchange with the atmosphere, not the bulk of the ocean.
2) More importantly, ALL of the photons that escape to space from the earth pass through at least part of the atmosphere. 90% of the photons escaping to space are EMITTED by molecules in the atmosphere! (Only 7% come from the top 1 mm of the ocean which is in equilibrium with the atmosphere immediately above.) Both the emission and transmission (non-absorption) of these photons is governed by the Schwarzschild eqn. That equation predicts additional that GHGs will reduce the rate at which radiation escapes to space.
3) If the portion of the atmosphere that emits most of the photons to space were not in local thermodynamic equilibrium, then we would have to supplement the Schwarzschild equation with a term for “re-radiation” (properly known as photoluminescence). Many phenomena (fluorescence, phosphorescence, lasers, and LEDs) involve emission of photons from molecules or materials that are not in local thermodynamic equilibrium. However, in the lower atmosphere and on the surface (which together emit the vast majority of the photons escaping to space), collisions occur several orders of magnitude faster than re-radiation of an absorbed photon. The vast majority of absorbed photons are “thermalized”, not re-radiated. The n*o*B(lamba,T) term therefore properly accounts for the vast majority of the radiative cooling (emission) by the atmosphere and surface. Nothing significant has been ignored.
4) Henry may be familiar with two-photon spectroscopy, where intense radiation passes through a sample promoting significant fraction of molecules to an excited electronic state capable of absorbing a second photon. But he probably doesn’t worry about this phenomena during ordinary lab experiments because the excited state in his experiments is relaxed by collisions much faster than a second photon can be absorbed. Likewise, vibrationally excited molecules in the atmosphere are relaxed by collisions before they can emit a photon.
5) Since collisions occur so frequently in the lower atmosphere, it is impossible to discuss what happens to the energy from any one photon. It becomes part of the internal energy (temperature) of the molecules near where it was absorbed. Those molecules emit thermal radiation, but their rate of thermal emission depends on their temperature and absorption/emission coefficients – terms that are found in the Schwarzschild eqn. When Henry says that “50% of that [solar] radiation [that hits on the CO2 molecule] is going back to space”, he is talking nonsense. Essentially all photons are converted to thermal (internal) energy, before they can return to space. As long as we know the temperature of the atmosphere, we can predict how much radiation it will emit. We don’t need to know if the internal energy of a group of gas molecules arrived by radiation, convection (especially condensation of water vapor) or conduction.
Frank,
You know you aren’t going to get anywhere. Anyone who is sure that the atmosphere has neither mass nor heat capacity is not someone you can educate. HenryP is not even as interesting as a certain NN who refuses to accept that there are over 3 m³ of CO2 (at STP) above every square meter of the planet. He considers that fact absurd. He was also, at one time and may still be, unwilling to accept the fact that a surface pressure of 1013.25 hPa at mean sea level translates to 10,000kg of mass. People pay him to teach. Needless to say, he, like a certain DC are banned at most serious blogs.
HenryP
Are you the same Henry Pool who posted comments to this article a few years ago?
I reproduce a bit of my comments from that era..
Whether or not you are the same Henry P – and I would like to know – this is not the blog for you.
If you have some evidence that the atmosphere has no mass it will be very interesting but please check the Etiquette.
We accept basic physics found in textbooks as a given. If you want to disprove basic physics you have somewhere between zero and almost zero latitude.
1. The surface pressure is not zero because?
2. The mass of the atmosphere as found in all textbooks is wrong because?
Henry, stay on topic. Answer the questions, with evidence, or your comments will be deleted.
And then, you, in company with just a very few elite soldiers, can have confirmed status as people this blog has banned.
I think I must be that same person as my e-mail was linked to comments on this blog here. It [this blog] must have been dead and gone for such a long time until you or somebody here started it up again and I started receiving the comments made here in my e-mail inbox again.
Mr. Doom, I think you [and others] are perhaps misunderstanding my comment about there being no mass in the atmosphere. Or it was my poor explanation.
I actually think that Frank does understand it. What I said was that basically only GHG’s are able to absorb and re-radiate in certain areas of the spectrum. The rest of the atmosphere is transparent to almost all radiation coming from the sun and earth. This can be easily proven from the spectra of oxygen and nitrogen. The GHG’s make about 0.5 or 0.6 % of the atmosphere. That is a small amount of mass? That means that if there is absorption in the sun’s spectrum the molecule will start re-radiating that particular radiation in all directions, including 50% back to space, the other to earth.
It [i.e. the back radiation from GHG’s ] cannot “heat” the other 99.5% of the gases in the atmosphere at all. Hence almost all radiation and the 50% re-radiation from GHG falls in the oceans and on earth. Water has some absorption in the UV and more so in the IR. Because the oceans have a lot of mass, the incoming radiation eventually will be re-radiated and re-radiated and subsequently turned into heat.
The “heat” to the atmosphere is supplied mostly by the condensing of water vapor in the atmosphere, from the bottom up.
I hope that clarifies this point.
TOA is another subject altogether because it is like a chemical reaction vessel. We will speak about that some other time, perhaps.
Henry,
You are missing many important factors:
1) When radiation is absorbed by a molecule, the energy is in almost all cases (something like 99.99% of all cases) released collisions to other molecules, most often to the N2 molecules. It’s an exception that it’s emitted by the same molecule.
2) While a molecule radiates as often up than down, the atmosphere does not. The atmosphere radiates much more to the surface than to the space, because lower atmosphere is warmer than the upper atmosphere.
These are perhaps the two most important ones, but there are certainly many other issues you have missed as well.
@Pekka
You are wrong on both counts.
But this aspect is misunderstood by most people so I will try to explain once more:
1) There is no or almost no heat transfer because the neighboring molecules N2 and O2 are all transparent to the re-radiation from any of the the so-called GHG….
2) Assuming the molecule acts more or less like a sphere you will get 62.5% back in the direction where the light came from….That means in the 0-5 um region actually more light [of the absorption region of the molecule] will be deflected back to space than will hit on earth….
I have tried to explain this in my earlier work. You have to try and understand the principle.
http://blogs.24.com/henryp/2011/08/11/the-greenhouse-effect-and-the-principle-of-re-radiation-11-aug-2011/
Henry,
Can’t help myself.
Pekka is correct and you’re the one who misunderstands the fundamentals of local thermodynamic equilibrium and its effect on molecular radiation, for example Kirchhoff’s Law. Molecules can collide with other molecules or surfaces and exchange energy with no radiation involved. If you have nitrogen or oxygen in a closed container and raise the temperature of the container walls, do you think that the gas inside doesn’t change temperature because it’s transparent to radiation? Collisions between molecules at different energies can also result in energy changes. That’s what happens to the vast majority of molecules that have sufficient energy to emit radiation. The radiative half life of a free excited CO2 molecule, which is related to the Einstein A21 coefficient, is on the order of 1 second, but the collisional half life at normal atmospheric pressure and temperature is on the order of 10 microseconds.
What you don’t know about molecular spectroscopy would fill a book. Most of what you think you know is wrong.
HenryP: A molecule in an excited state usually is “relaxed” by one of two processes: 1) colliding with other molecules or 2) emitting a photon. When a molecule excited by absorbing a photon relaxes, we say that the energy from the photon has been: 1) “thermalized” or 2) “re-radiated”. Since an excited state can be created by collision or absorption and since the excited state retains no information as to which process created it, there is no practical difference between “re-radiation” and ordinary [thermal] emission. For vibrationally and electronically-excited molecules, relaxation usually means to the lowest electronic or vibrational state, but many different rotational states are populated at terrestrial temperatures.
Proof that thermalization occurs: A fluorometer is a UV-Vis spectrophotometer with two detectors: the usual detector that collects transmitted radiation and a second detector that collects photons “re-radiated” perpendicular to the path of the beam. Most absorbing substances emit no radiation towards the second detector, because virtually ALL the absorbed radiation has been thermalized. Fluorescent and phosphorescence molecules do “re-radiate” towards the second detector, but that radiation has a longer wavelength than the radiation that was absorbed. The excited state of such molecules is relaxed by collisions, but in this case only part of the energy is lost before a meta-stable excited electronic state is produced. This meta-stable state sometimes lasts long enough to emit a photon. In solution, thermalization is too fast and re-radiation is undetectable.
http://en.wikipedia.org/wiki/Fluorescence_spectroscopy
Which process happens faster in the atmosphere, “thermalization” or “re-radiation”? Unfortunately, we can’t detect thermal infrared in an ordinary fluorometer because the walls of the device emit thermal radiation. The paper below (“Vibrational relaxation of CO2(n2) by atomic oxygen”) investigates the contribution of atomic oxygen to the collisional de-excitation of CO2 around 100 km above the surface. (Earlier papers investigated collisional relaxation by nitrogen and oxygen molecules.) At this altitude, the density – and therefore collision rate – are about 10^6 lower than at the surface. The introduction of the paper begins:
“The upper atmospheres of Earth, Venus, and Mars all cool by radiative emission from vibrationally excited CO2, particularly from the [0110] bending mode at 15 mm. In the terrestrial upper mesosphere–lower thermosphere (UMLT), roughly 80 – 110 km, emission by CO2 is essentially the only mechanism by which cooling occurs. At these altitudes, the relatively low gas collision frequency, about 1000 s-1 at 100 km, results in INCOMPLETE collisional coupling between the populations of the various CO2 vibrational levels. Because of this phenomenon, the vibrational populations of CO2 depart from local thermodynamic equilibrium (LTE).”
http://onlinelibrary.wiley.com/store/10.1029/2006JA011736/asset/jgra18417.pdf?v=1&t=hzvonem8&s=6de14d6f5f5e51acf76a80446334a3ff4d173390
Even when the collision rate is 1/1,000,000 the rate at the surface, relaxation by collision still competes with re-radiation! In the lower and middle atmosphere (where increasing GHGs decrease OLR), re-radiation is negligible.
At altitudes where the vast majority of vibrationally-excited GHGs are created and relaxed by collisions, the atmosphere is said to be in local thermodynamic equilibrium (LTE). At those altitudes, emission is given by the n*o*B(lamba,T) term of the Schwarzschild eqn. The following lecture notes assert that 99.5% of the atmosphere (up to 0.05 mb or 37 km) is in LTE with OLR. Another source says LTE with OLR extends to 50 km.
Click to access LTE_and_Kirchoffs.pdf
UNLESS you are willing to challenge the expertise of scientists measuring the rate at which collisions relax excited states of CO2 (or challenge my interpretation of their work), it is time to STOP talking about “re-radiation”. Virtually all the photons emitted by the lower atmosphere come from a GHG molecule that was excited by a collision, not by absorption of a photon. Those photons are emitted, not “re-radiated”. The tiny fraction of the upper atmosphere that is not in LTE with OLR has negligible effect on OLR.
Yes, I know that a ridiculous number of explanations for the GHE refer to “re-radiation” of absorbed OLR. Such explanations are wrong to imply that re-radiation differs from ordinary thermal emission (the process that produces blackbody radiation). The sloppy explanations of the GHE provided by many sources provide HenryP an excellent excuse for his misunderstanding of this subject.
You can purchase Grant Petty’s “A First Course in Atmospheric Radiation” used for $36. It has everything one needs to know.
nobody says
http://www.pnas.org/content/106/51/21527.full
henry says
ja, I would be careful with that as well. First of all, you have to be sure why the sea levels are rising. In a number of countries, like Sweden, it is because the the land is simply sliding downward, because of tectonic movement. I think this applies to Florida as well. It does not seem the writers of this paper even know or acknowledge this.
2nd my data sets imply that it has been getting warmer, naturally, for at least the past 45 years. [with a correlation coefficient of one on the minima graph I think I am entitled to a little backward projection?],
so yes, I would think that this would influence the water level somewhat.
But it does not change the fact that [now] we are busy cooling and that it will only be a matter of time before the whole trend reverses again.
In any case, I do not think that sea level is something to worry about. A book that I have on the [South-African] shoreline says that in the “interglacial periods temperatures were occasionally warmer than they are at present, resulting in the melting of the ice sheets and a sea level higher than that of today. During such times sea levels around the South African coast were up to 30 meters higher than present levels and the Cape Peninsula was transformed into a string of islands”.
30 meters? Natural variation? How does that compare with your 20cm?
HenryP,
Here’s a thought experiment question for you: Take one cubic meter of air at 1 atmospheric pressure and 300K temperature and enclose it in a perfectly transparent container. Then transport that container to intergalactic space. What would happen next and why?
De witt Paiyn says
Molecules can collide with other molecules or surfaces and exchange energy with no radiation involved.
henry says
Yes, I realize that, but we were not talking about that movement.
We were only talking about the GH effect (a natural warming effect due to bounce back from radiation absorbed by GHG in the atmosphere to earth) and the anti-GH effect ( a natural cooling effect due to back radiation from radiation absorbed by GHG in the atmosphere to space)
Now, it has been alleged by Frank and you and others that the GH effect is stronger or greater, if you prefer, than the anti-GH effect.
My results seem to suggest that the net effect appears to be neutral,
as can be shown by the the natural decline in minimum temperatures,
Click to access henryspooltableNEWc.pdf
(last table)
if you were prepared to study it. So more CO2 is fine.
I don’t think I can help you much further. You can bring a horse to the water, but you cannot make him drink. All the best.
In fact something you wrote implied exactly that. Let’s see if I can find it.
No, it won’t. The temperature is too low for significant radiation from CO2 at wavelengths shorter than 5 μm. Kirchoff’s Law is that emissivity equals absorptivity, not emission equals absorption. To get emission, you multiply the emissivity by the Planck function for that wavelength and temperature. Hence, only a very tiny fraction of any energy absorbed by CO2 at short wavelengths is emitted again by CO2 molecules. The rest will be transferred by collisions to the surrounding oxygen and nitrogen molecules. The vast majority of emission by CO2 is in the range around 15 μm.
Once again proving that irony always increases. Since your water is poison, the horse would be smart not to drink it.
Don’t let the door hit you on the way out. Don’t come back.
And, as expected, you didn’t answer the question.
DeWittPayne says
Since your water is poison, the horse would be smart not to drink it.
Henry says
Actually I predict there will be no water at all on the Great Plains, due to the global cooling.Remember that.
We will talk again 7 years from now.
Or not.
Umm, Dust Bowl 1932-36 also 1887-1897 and 2012-. Drought in the Great Plains is cyclical. Your prediction could be correct, but not for the reason you stated.
You still haven’t answered the question. Worse, you didn’t leave like you said you would.
bye,
bye
OK, quick review of the vibrational spectroscopy of CO2. There are four vibrational modes for CO2, but you only see two fundamental bands at 666 and 2350cm-1. That’s because the two bending modes at 666cm-1, in plane and out of plane are degenerate and cannot be distinguished. The symmetric stretch vibration does not change the dipole moment so it doesn’t have a significant IR band. The asymmetric stretch mode is at 2350 cm-1 or 4.3μm. The band near 5000 cm-1 or 2μm is an overtone, i.e. a quantum number change of 2 instead of 1, of the 2350cm-1 band. As a result, it’s orders of magnitude weaker than the 2350cm-1 band.
In order to emit radiation, there have to be molecules at energies greater than or equal to the energy of the emitted radiation. One can calculate this fraction using the Boltzmann distribution. To do that, one needs to calculate hν/kT where h is Planck’s constant, ν is the frequency, k is Boltzmann’s constant and T is the temperature in Kelvin. hν = Joules = 2350/5.034E22 = 4.67E-20 . At 298K, kT = 298*1.38066E-23 = 4.11E-21. hν/kT = 11.4. The fraction of molecules in the ground state is then 1-exp(-hν/kT) = 0.999988 or ~0.001% of the molecules are in an excited state. The fraction in the first excited state is (1-exp(-hν/kT)*exp(-hν/kT) = 1.12E-05. The fraction in the second excited state, i.e. the molecules that could emit at ~2 μm is (1-exp(-hν/kT)*exp(-2hν/kT) ~1E-10.
By comparison, for 666cm-1, hν/kT = 3.22 and the fraction in the ground state is 1-2exp(-3.22) {times two because there are two degenerate excited states} = 0.92. That’s why you get significant emission from CO2 at 666 cm-1 but very little at 2350cm-1 and much less than that at 5000cm-1. And that’s not counting the effect of the Einstein A21 coefficients for the different transitions.
As I said above, the amount of solar radiation emitted at 2μm is insignificant compared to the amount absorbed. To a very good approximation, all the solar energy absorbed by CO2 is thermalized. No cooling there. The majority of emission from CO2 is in the band centered at 666cm-1.
DeWitt: Doesn’t the derivation of the Planck function assume a radiation field in equilibrium with quantized “oscillators” with a Maxwell-Boltzmann distribution of energy? All of your math presumably reduces to the n*o*B(lamba,T) term of the Schwarzschild eqn – without having to go into fractional occupancy of various energy levels. For HenryP – if he were listening – the simpler, the better.
The relationship between the macroscopic world of a) absorption cross-section and the Planck function and b) the microscopic world of Einstein coefficients and the MB occupancy of energy levels isn’t crystal clear to me. However, I think I now understand now why re-radiation is negligible where LTE prevails. Also that TLE depends on the rate of collisional relaxation of populated excited states, not some fundamental property of the system like collision frequency. The atmosphere is not in LTE with the SWR passing through it, though it is with respect to LWR. Since neither you, nor Pekka, nor our host have jumped on a mistake (which I appreciate), I may be learning.
As you predicted, Henry tuned out when confronted with information that challenged his religious belief about the GHE. I’ll never understand why so many people with some knowledge of science fall into the trap of challenging the GHE, rather than the real weaknesses of AGW: feedbacks, climate models (especially parameter uncertainty), unforced variability, etc. Somehow people go through the motions in school of proposing a hypothesis, conducting an experiment, and analyzing data without ever realizing that the outcome is supposed change what they believe. However, this problem may reach far beyond the “den^ers”, since it is increasingly hard to reconcile the observed hiatus, the high climate sensitivity of climate models, and their low unforced variability. Perhaps my prejudices are clouding my scientific judgment.
Frank,
You can make it too simple.
The weakness of climate models pales into insignificance when you start looking at the hand waving going on about damages from warming and mitigation costs in the IPCC WGII and WGIII reports. Germany is in the process of showing exactly how costly and damaging to the economy is an attempt to reduce CO2 emissions with current wind and solar technology without nuclear power. Electricity costs are already skyrocketing and they’ve barely started.
More sensibly, Australia repealed their carbon tax. Any hope the Greens had that Peak Oil would force a reduction in CO2 emissions is also gone. An economy powered by relatively inexpensive fossil fuels is going to crush an economy powered by wind and solar. Which is why the Chinese are building coal fired power plants as fast as they can. Of course, their economy is currently on thin ice for other reasons. They’re pouring money into less and less productive projects. It can’t last.
In terms of the Planck equation at 298K assuming an emissivity of 1, which is barely achievable for some narrow frequency ranges near 5000cm-1 for an 8km path length at 1 atm and a volumetric mixing ratio of 0.0004 for CO2:
Peak frequency = 584 cm-1
radiance at:
584 cm-1= 1.5043E-01 W/(m² cm-1 sr)
2350 cm-1 = 1.8264E-03
5000 cm-1 = 4.8835E-08
You don’t need to tell me about the %^*# in WGII and WGIII, but there is little science there that interests me. WGI is the “only game in town” I want to play. Your and my opinions about policy (and those of the other side) are often based value judgments and opinions, not science. The few facts available are interesting, but soft. China will emit double the CO2 of the US in 2014. When will they reach triple? X% economic growth per year minus y% improvement in carbon efficiency (3% target). Planning and construction are already underway. 2030 is my estimate, but I don’t know much about how other booming Asian economies grew (or crashed) after such booms.
@frank
LOL
you can “calculate” as much as you want, but eventually observation will show you that there is no man made global warming.
Click to access henryspooltableNEWc.pdf
You guys are a piece of art.
But then again, if there is no art, life is a defect.
Still want the last word, do we?
In other words, you’ve made up your mind. We shouldn’t confuse you with the facts. The principal fact being that your hypothesis that CO2 acts to cool the atmosphere by radiating 50% of all solar energy absorbed in the 2 μm back to space is wrong. The radiance of CO2 at that wavelength is insignificant at 298K and most of the atmosphere is colder than that. And that’s not to mention that the absorption at that wavelength isn’t very large either. It is an overtone band, after all.
There’s also ocean heat content, which is increasing. That means there’s a radiative imbalance at the top of the atmosphere, as would be expected for increasing ghg concentration in the atmosphere.
It gets better. If CO2 acts to cool the planet instead of warm it because it radiates solar energy away, then what about water vapor? Water vapor absorbs far, far more solar energy than CO2. If your hypothesis were correct, life as we know it on Earth wouldn’t be possible as all the water would be frozen.
So how do you explain this paper:
http://www.iop.org/EJ/article/0004-637X/644/1/551/64090.web.pdf?request-id=76e1a830-4451-4c80-aa58-4728c1d646ec
They measured this re-radiation from GHG’s as it bounced back to earth from the moon. So the direction was sun-earth (day)-moon(unlit by sun) -earth (night). Follow the lines in fig. 6, bottom. Note that for CO2 it already starts at 1.2 um, then one peak at 1.4 um, then various peaks at 1.6 um and 3 big peaks at 2 um. You can see that it all comes back to us via the moon in fig. 6 top & fig. 7. Note that even water vapor and methane cools the atmosphere due to the back radiation
there is no doubt that GHG’s radiate back to space (in the absorptive regions):
it has been proven.
My guess from my results in the case of CO2 is that the heat entrapment (of earthshine) is the same as the back radiation from sunshine. More or less.
That paper looks at the reflected radiation. The total amount of reflected solar radiation (albedo) is 30% of all solar radiation that hits the Earth. That’s not absorbed and re-emitted, that’s reflected or scattered without being absorbed at all.
I am not sure if I understand you correctly. Are you saying that we already “counted” the back radiation of the GHG’s in absorptive regions as part of the albedo of earth, so if more of GHG is added to the atmosphere, this cannot change [increase] the albedo?
Henry,
I’m saying that moonshine is reflected and scattered solar radiation, not radiation emitted by Earth atmosphere or the surface. That part of the solar radiation is reflected or scattered out of the atmosphere is taken into account in the albedo. there’s nothing more to explain.
so, then
please explain
how the radiation specific only to the absorptive regions of earth’s GHGs can be measured coming back from the moon? Are you saying that the moon has GHG?
Henry,
You referred to a paper, but it seems that you haven’t read even the abstract. It states clearly that are discussing radiation reflected by the Earth atmosphere, not emitted.
Near infrared is still dominated by the solar radiation. Emission from the Earth surface and atmosphere gets important at wavelengths longer than 4 µm as you can see from the figure you linked to yourself.
Makes me wonder, whether you understand a word of what you are reading or writing on these subjects.
it seems to me that it is you who simply does not [want to] understand that you cannot not singularly identify green house gases (GHG’s) by pointing at the areas in the 5-20 um region (where earth emits pre-dominantly) but that you must also look in the area 0-5 um (where the sun emits pre-dominantly) for possible cooling effects.
HenryP,
Apparently you don’t understand the difference between reflectance, transmssion, absorption and emission spectra either.
Figure 6 bottom are transmission spectra of water vapor, CO2, methane, oxygen and ozone. A value of 1 means that there is no absorption there, it’s transparent. A value of zero means that there is no transmission, because all the radiation is absorbed and it’s opaque. If there were strong emission, it wouldn’t be opaque. But there isn’t and it is. H2O is by far the strongest absorber, with CO2 a distant second.
Looking at Figure 7, peaks are where the atmosphere and surface reflect and dips are where it absorbs. Note that almost all the dips are from water vapor that match the dips in the transmission spectrum with only a few dips from CO2. But they are all dips. There is no significant emission from the atmosphere at those wavelengths. There is only absorption.
And all the absorption is indeed included in the albedo. An increase in CO2, all other things being equal, would lower the albedo slightly because of increased absorption. But only slightly because the absorption by CO2 is small compared to water vapor and the incident radiant intensity is small too. Only about 1.5% of the total TOA solar radiation is in the frequency range of 4700-5300cm-1 (1.89-2.13 μm) where CO2 absorbs strongly. And the absorption is well beyond the linear range, so total absorption would increase by approximately the logarithm of the concentration ratio.
been there
done that
HenryP: Why the insults, which will simply get you banned here? You had a chance to learn something new about “re-radiation”, local thermodynamic equilibrium, the Schwarzschild eqn., and why scientists believe that the GHE exists and will be enhanced by CO2. If you are a scientist, you should be interested in the physics/chemistry of how radiation interacts GHGs. You can confirm the accuracy of what you have heard in Grant Petty’s book. It’s a book for meteorologists about Atmospheric Radiation, not global warming. According to the index, global warming is mentioned on only two pages and the GHE on a third.
For the record, reliable observations can’t tell us whether there has or has not been man-made global warming. Observations simply tell us about total warming (or cooling). In addition to AGW, we must consider solar forcing, volcanic forcing and unforced natural variability. The only thing that can tell us about unforced variability is centuries of accurate temperature data (which we currently lack) and climate models (which some of us don’t trust).
Henry wrote: “there is no doubt that GHG’s radiate back to space (in the absorptive regions): it has been proven. My guess from my results in the case of CO2 is that the heat entrapment (of earthshine) is the same as the back radiation from sunshine. More or less.
The idea that CO2 traps heat or radiation is a simple-minded misrepresentation that alarmists use to make the public think they understand the GHE. Everyone here knows about radiative cooling (earthshine). Your reference concerning how astronomer can detect Earth-like planets doesn’t add anything new. You are correct to be skeptical about “trapping” and ask why “earthshine” hasn’t been mentioned. Likewise, the idea that “trapped” radiation is “re-radiated” in all directions is another over-simplification foisted on the public and confuses those who wish to understand more. The energy from essentially all absorption in the troposphere and most of the stratosphere is turned into heat, not directly re-radiated.
GHGs both emit and absorb LWR. Emission doubles when CO2 doubles, but the distance each photon travels is roughly cut in half. The 3.5 W/m2 radiative forcing (reduced flux to space) produced by 2XCO2 is the very small difference between two much larger opposing changes. The outgoing flux at the surface is around 400 W/m2 at the surface and only 40 W/m2 escapes directly to space through the atmospheric window (where GHGs don’t absorb). Eventually 238 W/m2 reaches space. The real numbers are even bigger because we haven’t accounted for the energy transferred within the atmosphere by photons that travel only 1 km, or 100 m, or even 10 m between locations they are emitted and absorbed.
Our intuitions aren’t useful for reasoning about how a small difference between two large numbers changes when both large numbers change significantly. The truth is that increasing CO2 warms the troposphere and cools the stratosphere – simple-minded “hand waving” about trapping and re-radiating can not explain that.
The Schwarzschild equation is used to calculate how the intensity of upward radiation decreases as CO2 increases. It say the outward LWR flux will decrease by about 3.5 W/m2 after an instantaneous doubling of CO2.
There is one intuitive way to understand the right answer. When the atmosphere contains more GHGs, the average photon escaping to space will have been emitted from a higher altitude, where it is colder. That means less energy will escape to space and the earth will warm until 238 W/m2 of outgoing LWR again balances 238 W/m2 of post-albedo incoming SWR. This is explained in more detail by Lindzen, a vocal critic of the IPCC:
www-eaps.mit.edu/faculty/lindzen/230_TakingGr.pdf
The stratosphere behaves differently from the troposphere because it gets warmer, not colder, with altitude. Unfortunately, little radiation escaping to space originates in the stratosphere.
HenryP,
Since you still seem to be here in spite of your promise to leave, I suggest you consider the temperature profile of the stratosphere. The stratosphere is not complicated by convection, so it’s a good test of radiative transfer calculations.
Temperature in the stratosphere increases with altitude because the greenhouse effect is reversed there. Unlike the troposphere, the stratosphere is more opaque to incident solar radiation because oxygen and ozone absorb strongly in the UV than in the LW IR. In the thermal IR from 4-50μm, the stratosphere is nearly transparent, average transmittance from 100-1500cm-1 is 0.915 for the Tropical Atmosphere, because the molecules that absorb and emit in that region are too dilute. In order to maintain a steady state energy balance, the stratosphere must warm so that the molecules emitting in the LW IR band, ghg’s, will emit more and balance the rate of energy absorption. This is exactly what’s calculated by radiative transfer models, even simple single layer non-reflecting atmosphere models.
In the last 50 some odd years, the stratosphere has cooled because CFC’s reduced ozone and CO2 has increased. CO2 does cool the stratosphere, but not by absorbing and re-emitting in the near IR, but by increasing emission in the thermal IR while absorbing an insignificant amount of incident solar radiation. Again, this is precisely what is calculated.
The field of atmospheric radiative transfer benefited greatly from Defense research. The military wanted heat seeking missiles to actually work. So they needed to know how IR radiation was transmitted through the atmosphere to be able to design the detector in the warhead. That provided a lot of data that eventually resulted in the HITRAN database used by line-by-line radiative transfer programs.
DeWittPayne says
In the last 50 some odd years, the stratosphere has cooled because CFC’s reduced ozone
Henry says
now, honestly, you don’t think that I believe in that CFC nonsense story anymore than I believe in the nonsense that the net effect of more CO2 causes warming?
That ozone story is interesting [and it can be linked to the output of the sun] and maybe I will tell you some more about it,
if you want me to.
I get the last word.
Nice deflection to avoid acknowledging that you have no explanation for the fact that temperature increases with altitude in the stratosphere while it decreases in the troposphere Variation in solar output has absolutely nothing to do with that.
Of course you don’t believe that CFC’s reduce ozone. That was to be expected given your lack of understanding of chemistry and physics. However, it doesn’t matter whether the observed decline in ozone concentration in the stratosphere was caused by CFC’s or not. It has declined, but it’s starting to recover, and the temperature in the stratosphere also declined as expected.
You should get together with Bryan. You seem to have a lot in common.
DeWitt or others: Could you point me to a reference that clearly shows that stratospheric ozone outside Antarctica has unambiguously recovered? The only references I could find show that natural variability at individual sites is high due to seasonal changes in UV, the QBO, volcanic aerosols, and perhaps stratospheric “weather” and relied on unfamiliar statistical methods to detect a very modest “significant” change. Do any of the stratospheric chemistry modules do a good job in reproducing this natural variability?
To demonstrate the importance of anthropogenic forcing, the IPCC shows us a graph of predicted temperature vs time with and without anthropogenic forcing. I’d like to see the same thing for ozone with and without change in halogen sources. Unfortunately, I’m not sure we have the ozone analog of a “mean global temperature” and I’d really like to separate the poles (where polar stratospheric clouds amplify and complicate the problem) from regions outside the poles (where people might be harmed).
As for the ozone “hole”, there appears to be limited, but adequate, evidence that a hole of the current magnitude didn’t develop in the 1960s and 1970s, even though we lacked the satellite observations to fully characterize whatever change did occur each spring.
No, because it hasn’t. The concentrations of some chlorine compounds have either peaked or aren’t increasing as rapidly, but it will take decades before results will be unambiguous. Because of the transport time, the peak will be later in the SH than in the NH.
That being said, the satellite lower stratosphere temperature anomaly reported by UAH and RSS has been pretty flat since the last big volcano.
Henry,
You don’t understand a thing about the subject you write about.
This is a science blog.
You have had the latitude of 25 or so comments recently on this article. I’m going to block all further comments from you.
The wonderful thing about the internet is it allows people to demonstrate to the entire world how little they know, rather than confine this just to friends and family.
Thanks for giving it your all. But rewriting basic physics isn’t what this blog is about. The good news for you is that there are many blogs out there that will welcome your approach to science.
Reblogged this on Climate- Science.